Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the Biocontrol of Wheat Head Blight
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Co-Culture and Pathogen Inhibition Assay
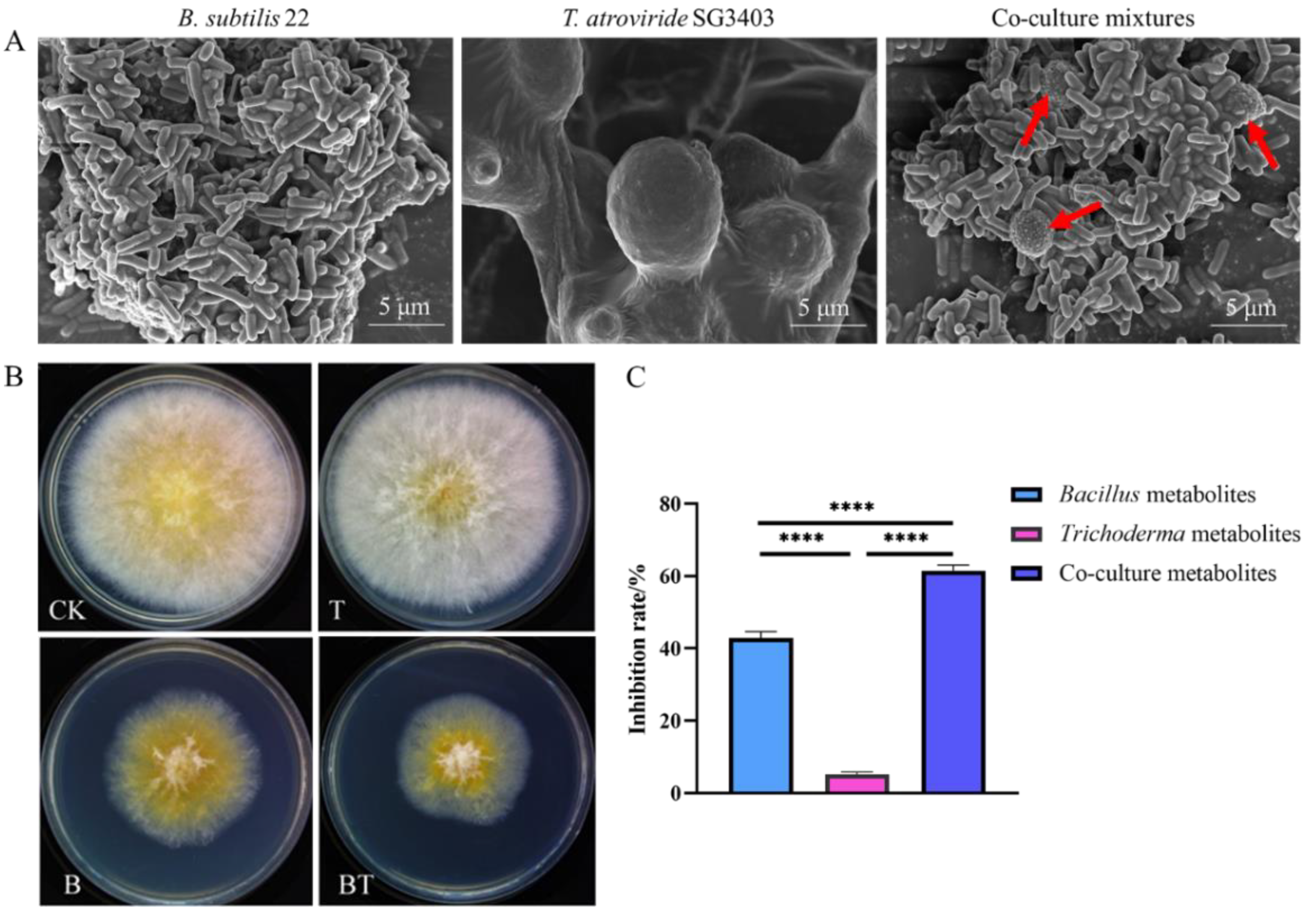
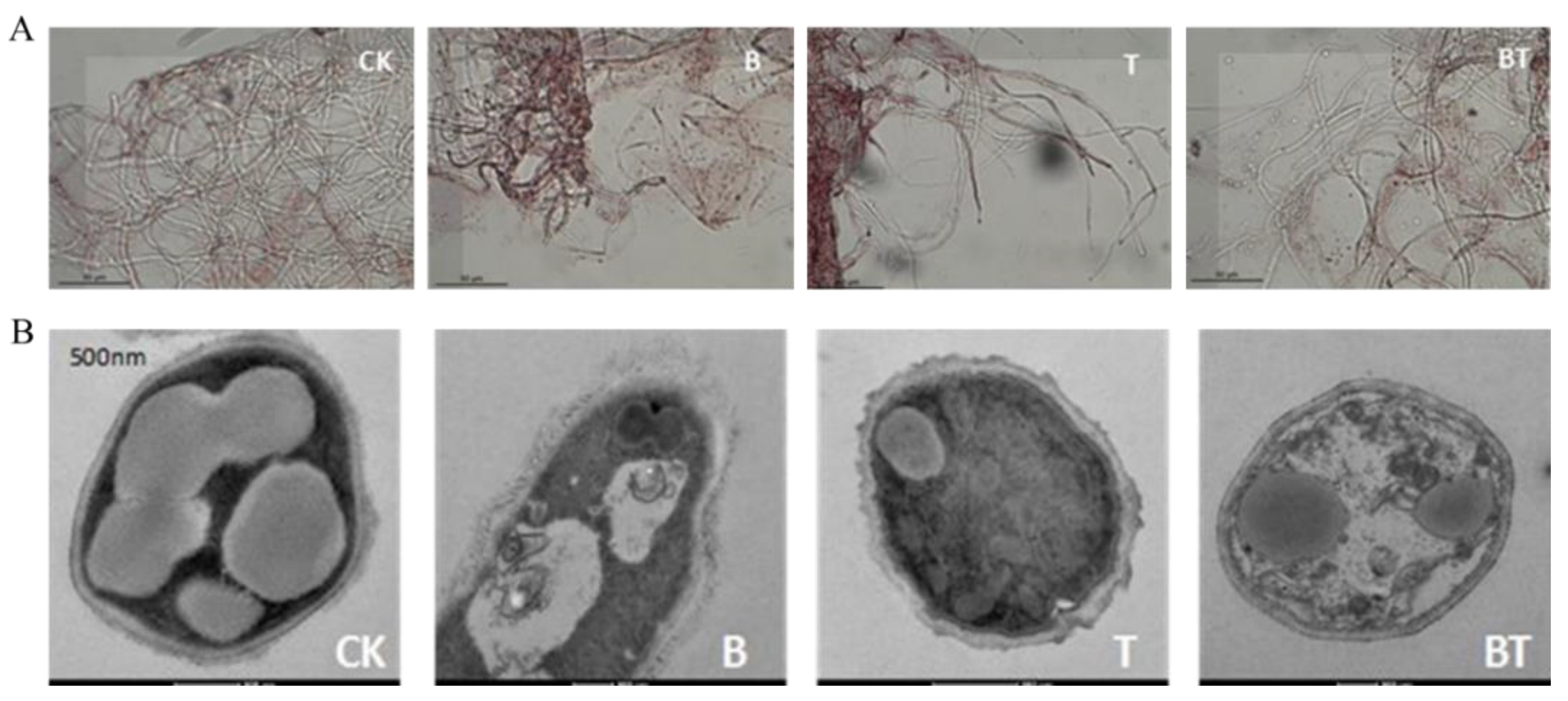
2.3. Microscopy Observation
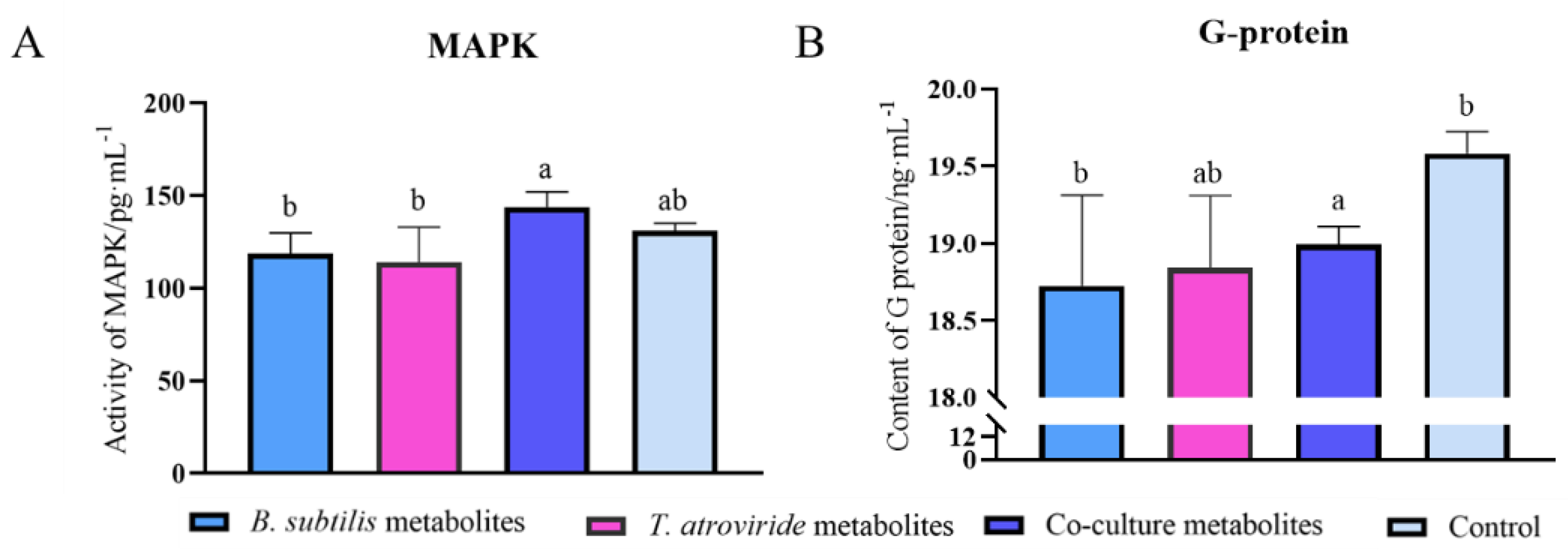
2.4. Assays of G Protein and MAPK in Fusarium graminearum
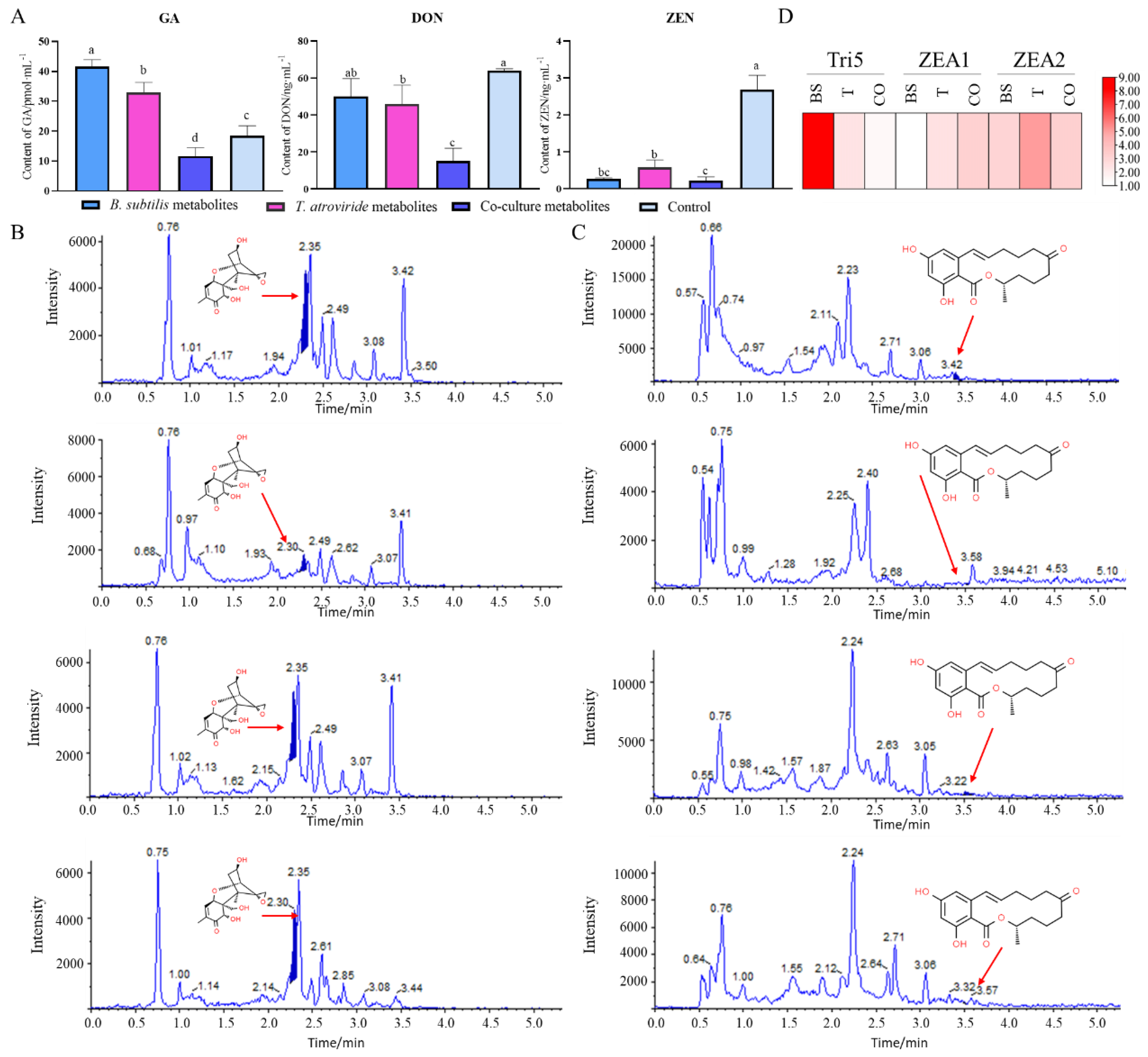
2.5. Assays of GA and Toxins Production in Fusarium graminearum
2.6. Seed Treatments with Biocontrol Microbial Culture
2.7. Gene Expression Study
2.8. Field Assay
3. Results
3.1. Inhibition of Fusarium graminearum Growth by Co-Culture Metabolites
3.2. Effects of Co-Culture Metabolites on the Mycelium Structure of Fusarium graminearum
3.3. Effects of Co-Culture Metabolites on the Activity of MAPK and G Protein of F. graminearum
3.4. Effects of Co-Cultured Living Cells on the Production of GA and Mycotoxins by F. graminearum
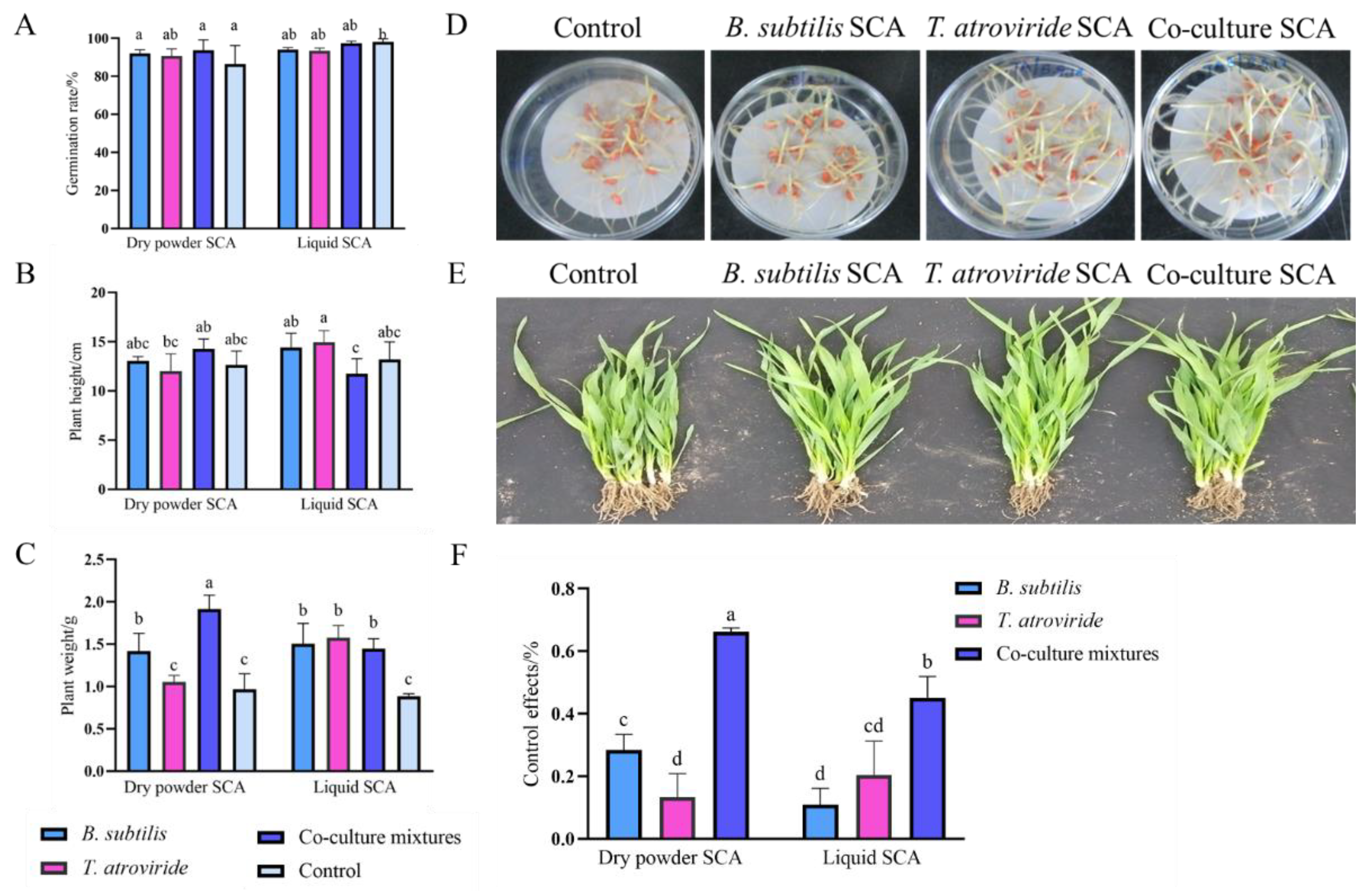
3.5. Effects of Bio-Seed Coating Agent on Wheat Seed Germination and Plant Growth
3.6. Effects of Bio-Seed Coating Agent against Wheat Head Blight
3.7. Effects of Seed Treatment on the Expression of Defense Genes in Wheat
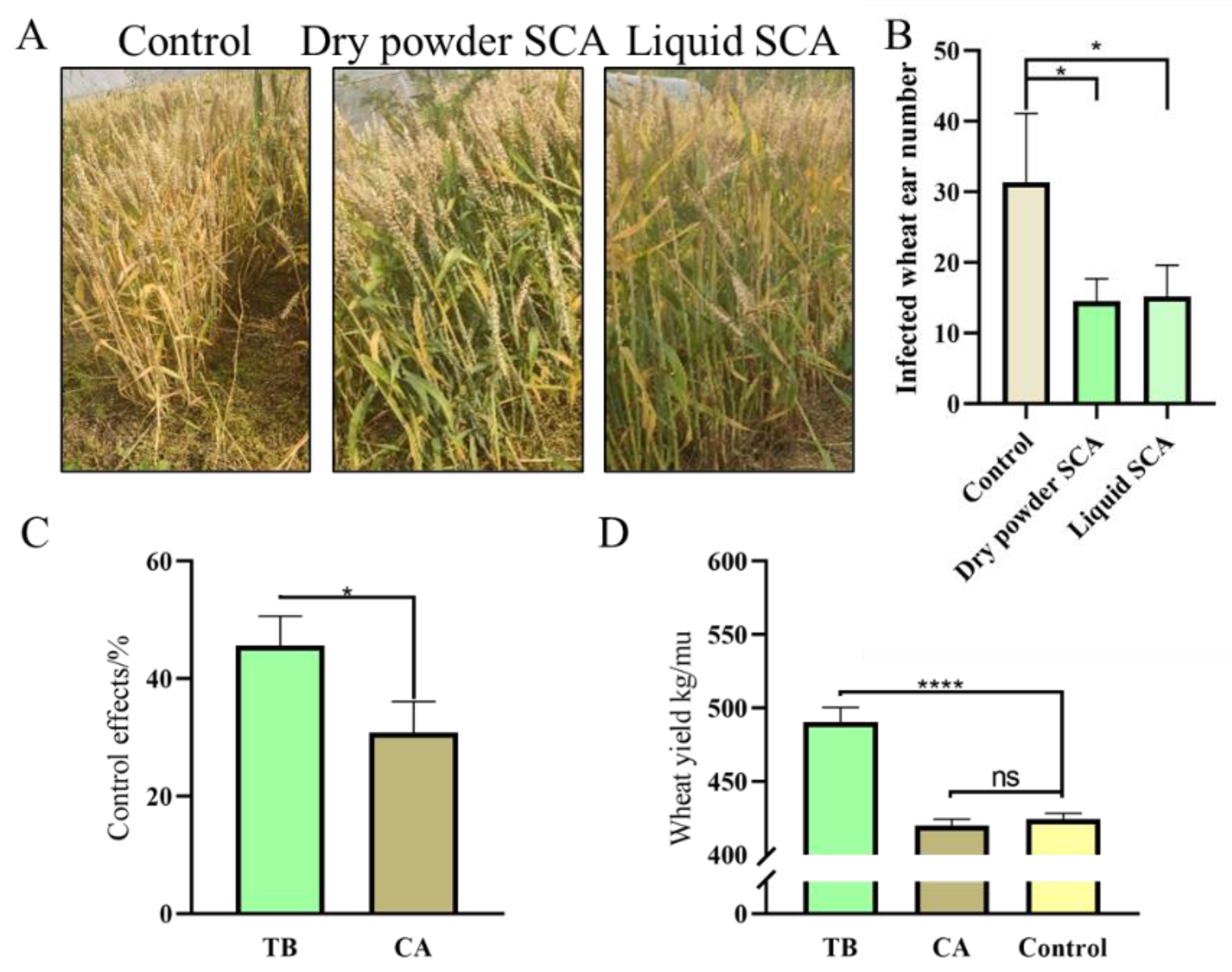
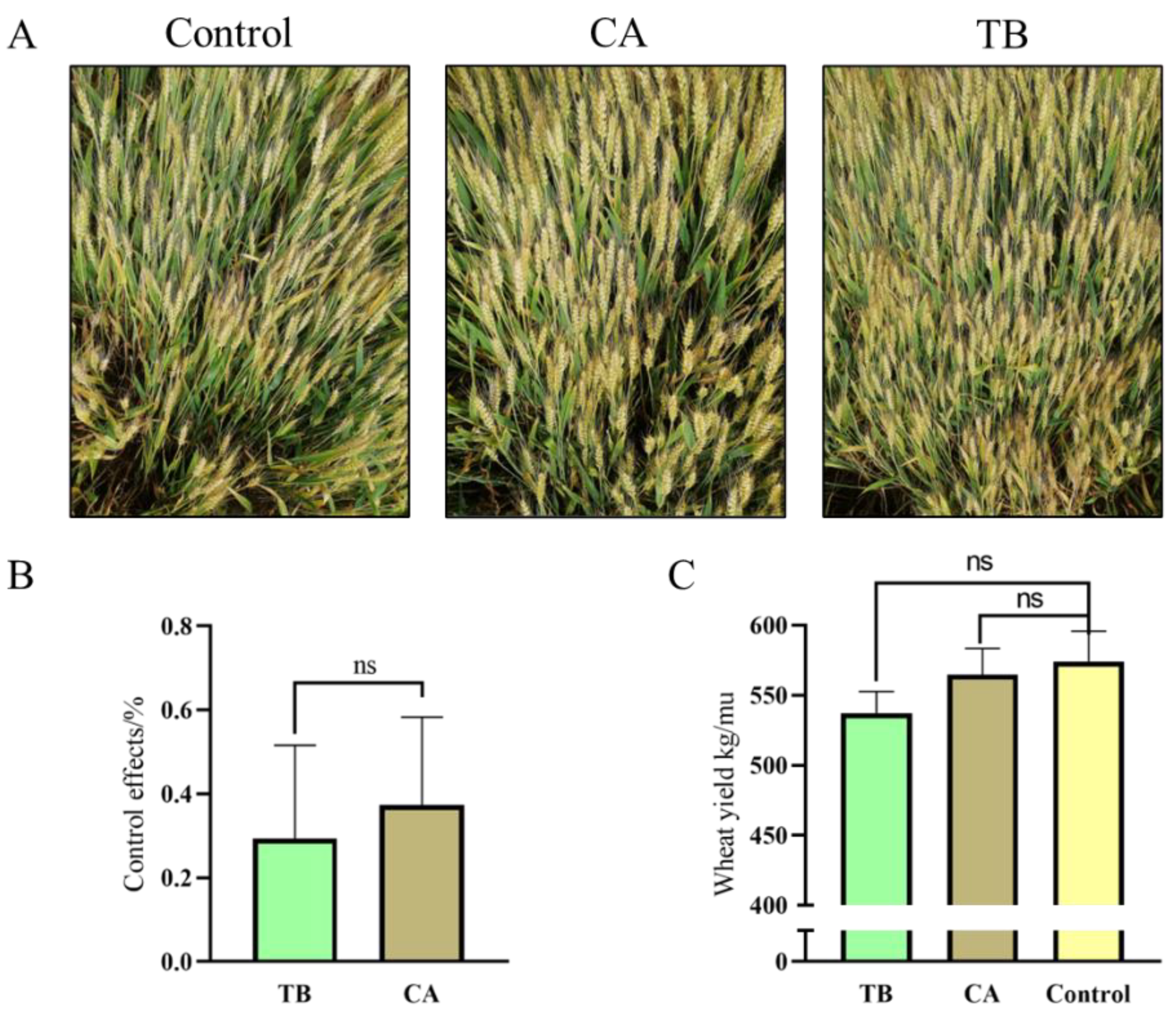
3.8. Effects of Seed-Coating Agents on Yield and Control of Wheat Head Blight in Field Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuels, G.J. Trichoderma: A Review of Biology and Systematics of the Genus. Mycol. Res. 1996, 100, 923–935. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, N.; Kabadwal, B.C.; Tewari, A.K.; Kumar, J. Review on Plant-Trichoderma-Pathogen Interaction. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- Gajera, H.; Domadiya, R.; Patel, S.; Kapopara, M.; Golakiya, B. Molecular Mechanism of Trichoderma as Bio-Control Agents against Phytopathogen System—A Review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus Amyloliquefaciens S76-3 from Wheat Spikes against Fusarium Graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.; Backhouse, D.; Ponte, E.M.D. Climate Change Impacts on the Ecology of Fusarium Graminearum Species Complex and Susceptibility of Wheat to Fusarium Head Blight: A Review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Ben Mahmoud, A.; Ali, H.; Sellami, S.; Nasfi, Z.; Tounsi, S.; Jamoussi, K. Antagonist Effects of Bacillus Spp. Strains against Fusarium Graminearum for Protection of Durum Wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2016, 192, 148–158. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the Chemical Diversity through Microorganisms Co-Culture: Current Status and Outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.-L. Metabolite Induction via Microorganism Co-Culture: A Potential Way to Enhance Chemical Diversity for Drug Discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Slattery, M.; Rajbhandari, I.; Wesson, K. Competition-Mediated Antibiotic Induction in the Marine Bacterium Streptomyces Tenjimariensis. Microb. Ecol. 2001, 41, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Stahl, U. The Influence of Co-Cultivation on Expression of the Antifungal Protein in Aspergillus Giganteus. J. Basic Microbiol. 2003, 43, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, R.; Ni, M.; Yu, J.; Li, Y.; Yu, C.; Dou, K.; Ren, J.; Chen, J. Identification of a Novel Fungus, Trichoderma Asperellum GDFS1009, and Comprehensive Evaluation of Its Biocontrol Efficacy. PLoS ONE 2017, 12, e0179957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, C.; Abbas, N.; Mao, Z.; Zhang, Y. Multiple Heavy Metal Tolerance and Removal by an Earthworm Gut Fungus Trichoderma Brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mehetre, S.T.; Sherkhane, P.D.; Muthukathan, G.; Ghosh, A.; Kotasthane, A.S.; Khare, N.; Rathod, P.; Sharma, K.K.; Nath, R.; et al. A Novel Seed-Dressing Formulation Based on an Improved Mutant Strain of Trichoderma Virens, and Its Field Evaluation. Front. Microbiol. 2019, 10, 1910. [Google Scholar] [CrossRef]
- Jones, R.K. Assessments of Fusarium Head Blight of Wheat and Barley in Response to Fungicide Treatment. Plant Dis. 2000, 84, 1021–1030. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial Communication Leading to the Activation of Silent Fungal Secondary Metabolite Gene Clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Johnson, K.B.; Sugar, D.; Loper, J.E. Mechanistically Compatible Mixtures of Bacterial Antagonists Improve Biological Control of Fire Blight of Pear. Phytopathology 2011, 101, 113–123. [Google Scholar] [CrossRef]
- Brunner, K.; Zeilinger, S.; Ciliento, R.; Woo, S.L.; Lorito, M.; Kubicek, C.P.; Mach, R.L. Improvement of the Fungal Biocontrol Agent Trichoderma Atroviride to Enhance Both Antagonism and Induction of Plant Systemic Disease Resistance. Appl. Environ. Microbiol. 2005, 71, 3959–3965. [Google Scholar] [CrossRef]
- Xie, S.; Jiang, H.; Ding, T.; Xu, Q.; Chai, W.; Cheng, B. Bacillus Amyloliquefaciens FZB42 Represses Plant MiR846 to Induce Systemic Resistance via a Jasmonic Acid-Dependent Signalling Pathway. Mol. Plant Pathol. 2018, 19, 1612–1623. [Google Scholar] [CrossRef]
- Wu, Q.; Ni, M.; Dou, K.; Tang, J.; Ren, J.; Yu, C.; Chen, J. Co-Culture of Bacillus Amyloliquefaciens ACCC11060 and Trichoderma Asperellum GDFS1009 Enhanced Pathogen-Inhibition and Amino Acid Yield. Microb. Cell Fact. 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, V.; Sun, J.; Li, T.; Vallikkannu, M.; Chen, J. Co-Cultivation of Trichoderma Asperellum GDFS1009 and Bacillus Amyloliquefaciens 1841 Causes Differential Gene Expression and Improvement in the Wheat Growth and Biocontrol Activity. Front. Microbiol. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The Current Status of Research on Gibberellin Biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Atanasoff-Kardjalieff, A.K.; Studt, L. Secondary Metabolite Gene Regulation in Mycotoxigenic Fusarium Species: A Focus on Chromatin. Toxins 2022, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X.; Xu, H.; Cui, G.; Chen, L. Action of Bacillus Natto 16 on Deoxynivalenol (DON) from Wheat Flour. J. Appl. Microbiol. 2021, 131, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Tinyiro, S.E.; Wokadala, C.; Xu, D.; Yao, W. Adsorption and Degradation of Zearalenone by Bacillus Strains. Folia Microbiol. 2011, 56, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; de Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma When Confronted with Fusarium Graminearum. Toxins 2016, 8, 335. [Google Scholar] [CrossRef]
- Yang, S.B.; Zheng, H.C.; Xu, J.Y.; Zhao, X.Y.; Shu, W.J.; Li, X.M.; Song, H.; Ma, Y.H. New Biotransformation Mode of Zearalenone Identified in Bacillus Subtilis Y816 Revealing a Novel ZEN Conjugate. J. Agric. Food Chem. 2021, 69, 7409–7419. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of Four Major Mycotoxins by Eight Manganese Peroxidases in Presence of a Dicarboxylic Acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef]
- Karuppiah, V.; He, A.; Lu, Z.; Wang, X.; Li, Y.; Chen, J. Trichoderma Asperellum GDFS1009-mediated Maize Resistance against Fusarium Graminearum Stalk Rot and Mycotoxin Degradation. Biol. Control. 2022, 174, 105026. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, P.; Ma, Y.; Jiang, R.; Jiang, C.; Wang, G. Insights into Intracellular Signaling Network in Fusarium Species. Int. J. Biol. Macromol. 2022, 222, 1007–1014. [Google Scholar] [CrossRef]
- Brown, N.A.; Schrevens, S.; van Dijck, P.; Goldman, G.H. Fungal G-Protein-Coupled Receptors: Mediators of Pathogenesis and Targets for Disease Control. Nat. Microbiol. 2018, 3, 402–414. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.-R. Mitogen-Activated Protein Kinase Signaling in Plant Pathogenic Fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef] [PubMed]
- Kipngeno, P.; Losenge, T.; Maina, N.; Kahangi, E.; Juma, P. Efficacy of Bacillus Subtilis and Trichoderma Asperellum against Pythium Aphanidermatum in Tomatoes. Biol. Control. 2015, 90, 92–95. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Adie, B.A.T.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.-J.; Schmelz, E.A.; Solano, R. ABA Is an Essential Signal for Plant Resistance to Pathogens Affecting JA Biosynthesis and the Activation of Defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Xu, C.; Hao, B.; Sun, G.; Mei, Y.; Sun, L.; Sun, Y.; Wang, Y.; Zhang, Y.; Zhang, W.; Zhang, M.; et al. Dual Activities of ACC Synthase: Novel Clues Regarding the Molecular Evolution of ACS Genes. Sci. Adv. 2021, 7, 8752. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2020, 87, 421–466. [Google Scholar] [CrossRef]
- Ma, Y. Seed Coating with Beneficial Microorganisms for Precision Agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef] [PubMed]

| Name | Forward Primer (5′-3′) | Description |
|---|---|---|
| PR1 | F-CTACGACTACGGGTCCAACA R-GCTTATTACGGCATTCCTTT | Salicylic acid Pathway |
| PR3 | F-AGAGATAAGCAAGGCCACGTC R-GGTTGCTCACCAGGTCCTTC | Jasmonic acid Pathway |
| PR4 | F-CGAGGATCGTGGACCAGTG R-GTCGACGAACTGGTAGTTGACG | Jasmonic acid Pathway |
| PR5 | F-ACAGCTACGCCAAGGACGAC R-CGCGTCCTAATCTAAGGGCAG | Salicylic acid Pathway |
| SOD | F-GCCTTTTGGCCTCTTTATCC R-AACCTCAAGCCCATCAGCG | Antioxidant enzyme gene |
| ACS | F-CTCTCGCTGGACCTGATCG R-GTCCTGGAAATTGGCGATCC | Ethylene Pathway |
| β-actin | F-CTCTGACAATTTCCCGCTCA R-ACACGCTTCCTCATGCTATCC | Housekeeping gene of wheat |
| Tri5 | F-TCTATGGCCCAAGGACCTGTTTGA R-TGACCCAAACCATCCAGTTCTCCA | DON biosynthesis-related gene |
| ZEA1 | F-GGCACTTTGACAACCGCTTC R-TTGCGCCGTCTGAGTACCC | ZEN biosynthesis-related gene |
| ZEA2 | F-ACATGCGTGTCGTTGTTGTAAG R-TGCACCGTGAGAAGCCGT | ZEN biosynthesis-related gene |
| GAPDH | F-CTACATGCTCAAGTACGACTCTTCC R-GCCGGTCTCGGACCACTTG | Housekeeping gene of Fusarium graminearum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, T.; Li, Y.; Wang, X.; Chen, J. Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the Biocontrol of Wheat Head Blight. J. Fungi 2022, 8, 1250. https://doi.org/10.3390/jof8121250
Liu H, Li T, Li Y, Wang X, Chen J. Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the Biocontrol of Wheat Head Blight. Journal of Fungi. 2022; 8(12):1250. https://doi.org/10.3390/jof8121250
Chicago/Turabian StyleLiu, Hongyi, Tingting Li, Yaqian Li, Xinhua Wang, and Jie Chen. 2022. "Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the Biocontrol of Wheat Head Blight" Journal of Fungi 8, no. 12: 1250. https://doi.org/10.3390/jof8121250
APA StyleLiu, H., Li, T., Li, Y., Wang, X., & Chen, J. (2022). Effects of Trichoderma atroviride SG3403 and Bacillus subtilis 22 on the Biocontrol of Wheat Head Blight. Journal of Fungi, 8(12), 1250. https://doi.org/10.3390/jof8121250







