Screening, and Optimization of Fermentation Medium to Produce Secondary Metabolites from Bacillus amyloliquefaciens, for the Biocontrol of Early Leaf Spot Disease, and Growth Promoting Effects on Peanut (Arachis hypogaea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
Inoculum Suspension and Mode of Fermentation
2.2. Screening of Key Factors in Fermentation Medium of BAM Strain by Single-Factor Experiment
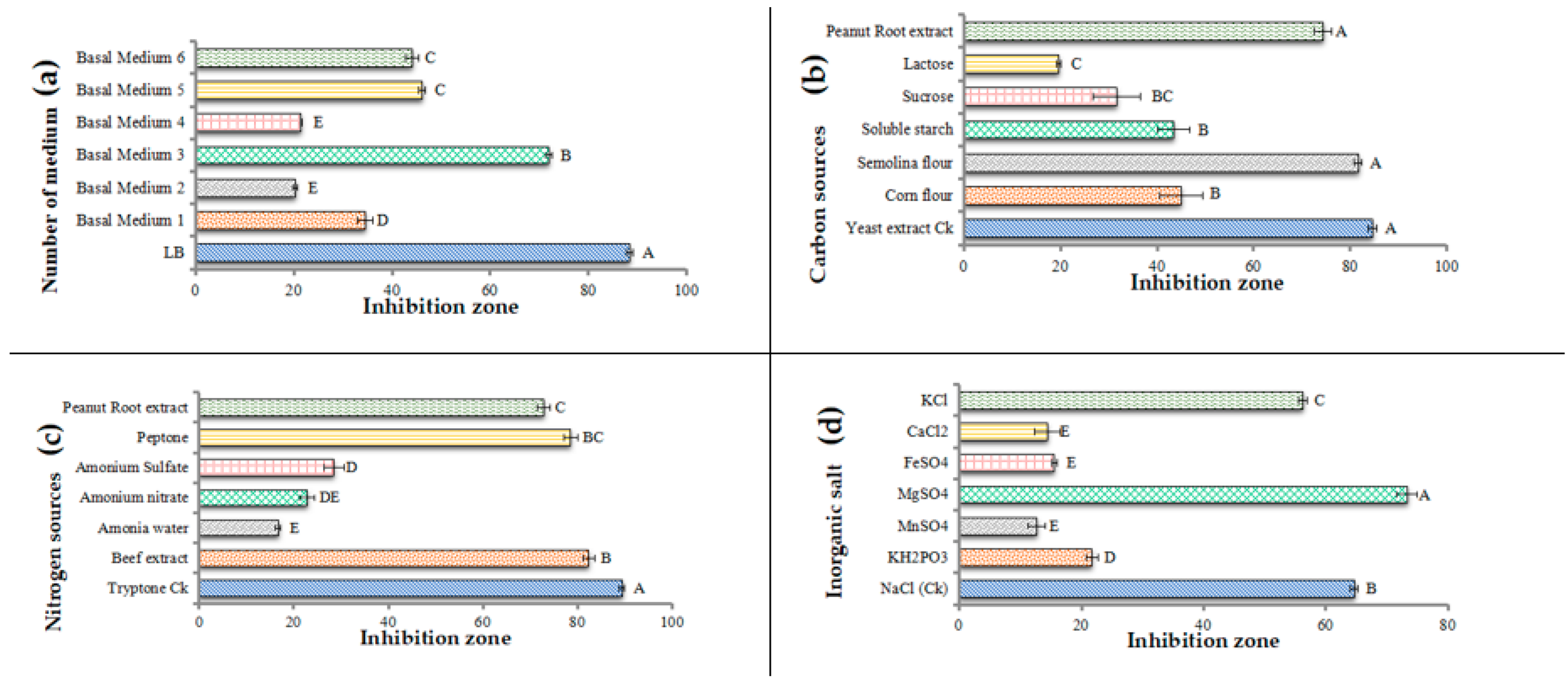
2.2.1. Different Carbon Sources
2.2.2. Different Nitrogen Sources
2.2.3. Different Inorganic Salts
2.2.4. Experimental Design for Optimization of Nutrient Medium by Central Composites Design (CCD)
2.2.5. Metabolic Profile in the Fermentation Batch
2.2.6. Antagonistic Activity of BAM Strain against C. arachidicola In Vitro
2.2.7. Disease Control Effects of BAM Strain on Peanut Plants in Pot Experiments
2.2.8. Determination of Growth and Physiological Parameters of Peanut Plants by the Effect of BAM Strain
2.2.9. Statistical Analysis
3. Results
3.1. Single-Factor Screening of Fermentation Medium for B. amyloliquefaciens Strain
3.2. Optimization of Fermentation Medium through CCD
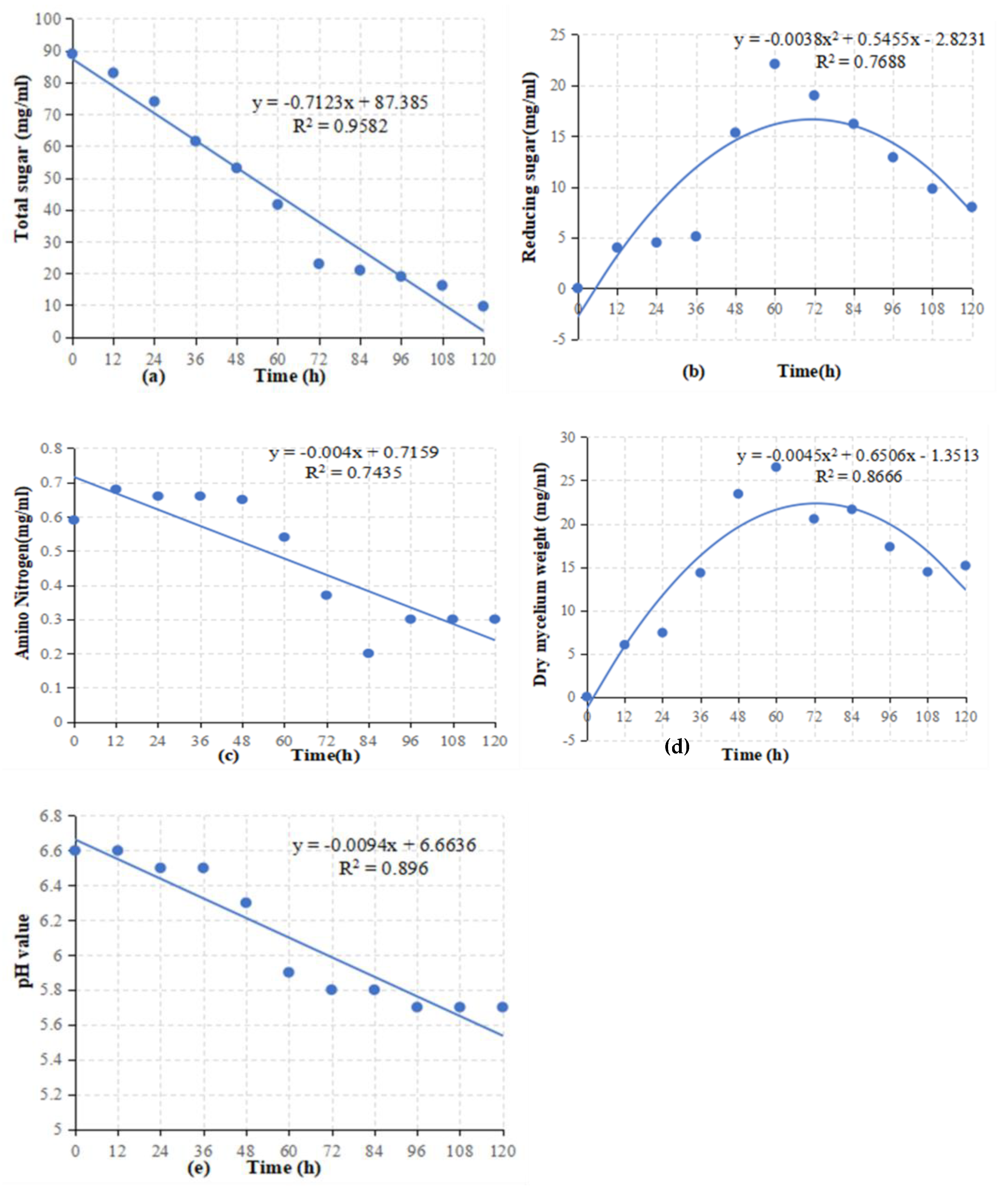
3.3. Metabolism of BAM Strain in Submerged Fermentation
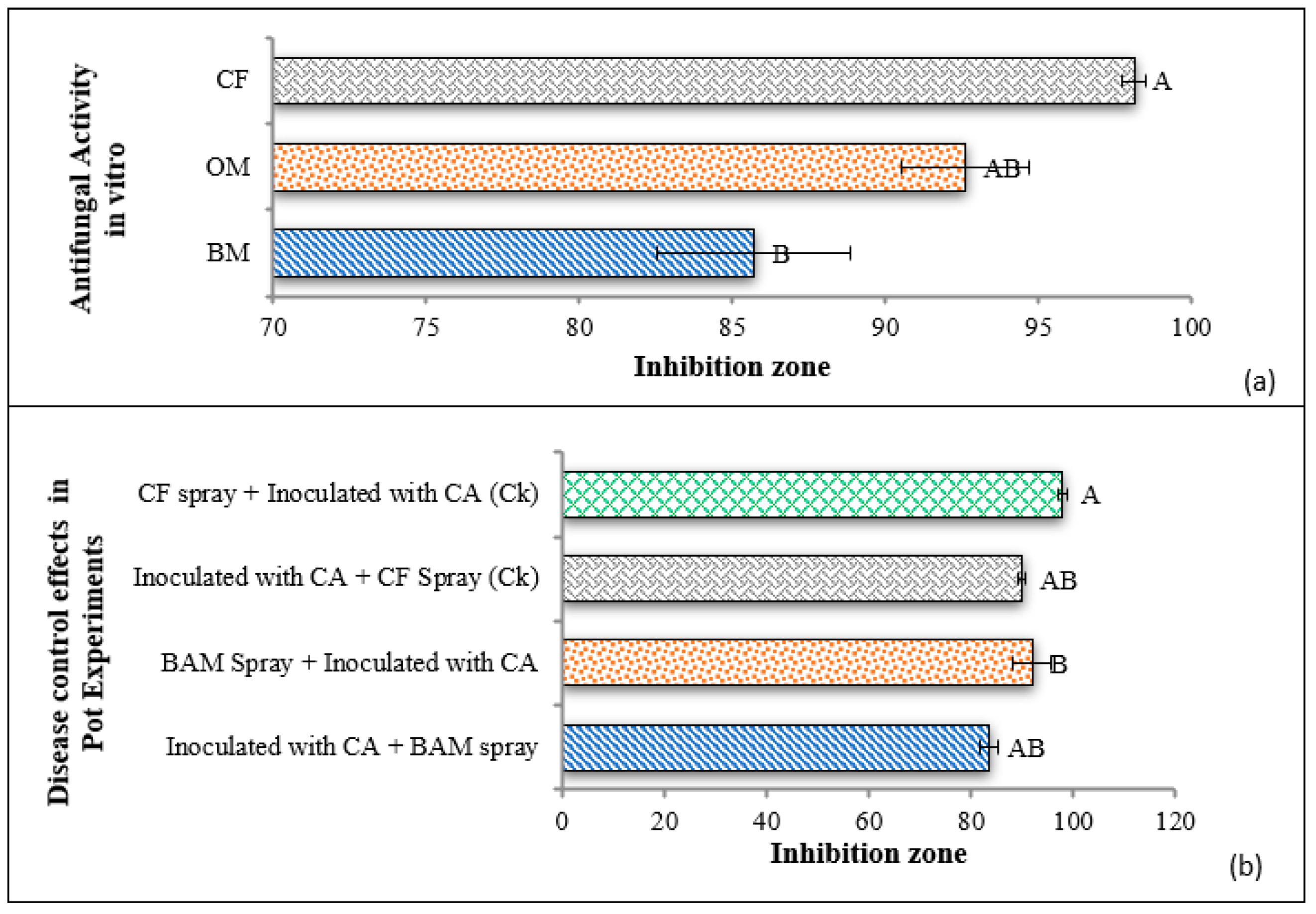
3.4. Antagonistic Activity of BAM Strain against C. archidechola In Vitro
3.5. Disease Control Efficiency of BAM Strain in Pot Experiments
3.6. Effects of BAM Strain on the Growth and Physiology of Peanut Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, X.; Gao, M.; Wang, N.; Cui, X.; Sang, S.; Fan, W.; Wang, Z. Genome resource for peanut web blotch causal agent Peyronellaea arachidicola strain YY187. Plant Dis. 2021, 4, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Han, S.; Yuan, M.; Ma, X.; Hagan, A.; He, G. Transcriptomic analyses reveal the expression and regulation of genes associated with resistance to early leaf spot in peanut. BMC Res. Notes 2020, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Nakashima, C.; Crous, P.W. Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA Fungus 2013, 4, 265–345. [Google Scholar] [CrossRef]
- Braun, U.; Crous, P.W.; Nakashima, C. Cercosporoid fungi (Mycosphaerellaceae) 5. Species on dicots (Anacardiaceae to Annonaceae). IMA Fungus 2016, 7, 161–216. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Amin, A.; Akbar, M.; Khalil, T.; Akram, W.; Ahmad, A. Antifungal activity of Alternanthera philoxeroides organic solvent extracts against plant pathogenic fungi. Pak. J. Bot. 2022, 54, 337–344. [Google Scholar]
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Akbar, M.; Amin, A.; Khalil, T.; Iqbal, M.S.; Nazir, A.; Taswar, A. Antibacterial activity of Alternanthera philoxeroides (Mart.) Griseb. against bacterial phytopathogens: Erwinia carotovora, Ralstonia solanacearum and Xanthomonas axonopodis. Allelopath. J. 2021, 53, 83–92. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for novel biocontrol agents applicable in plant disease management–A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Du, Y.; Wang, T.; Jiang, J.; Wang, Y.; Lv, C.; Sun, K.; Sun, J.; Yan, B.; Kang, C.; Guo, L.; et al. Biological control and plant growth promotion properties of Streptomyces albidoflavus St-220 isolated from Salvia miltiorrhiza rhizosphere. Front. Plant Sci. 2022, 13, 976813. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, D.; Jin, R.; Li, E.; Li, P. Bioactivities evaluation of an endophytic bacterial strain Bacillus velezensis JRX-YG39 inhabiting wild grape. BMC Microbiol. 2022, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Kobayashi, M.; Tanaka, F.; Takeuchi, K.; Oo, A.Z.; Jiang, C.-J. Identification of Pseudomonas strains for the biological control of soybean red crown root rot. Sci. Rep. 2022, 12, 14510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Duan, Y.; Jiang, W.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The Endophytic Strain Trichoderma asperellum 6S-2: An Effificient Biocontrol Agent against Apple Replant Disease in China and a Potential Plant-Growth-Promoting Fungus. J. Fungi 2021, 7, 1050. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, J.; An, M.; Li, B.; Xie, Y.; Xu, C.; Jiang, L.; Yan, F.; Wang, Z.; Wu, Y. Bacillus velezensis SYL-3 suppresses Alternaria alternata and tobacco mosaic virus infecting Nicotiana tabacum by regulating the phyllosphere microbial community. Front. Microbiol. 2022, 13, 840318. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; AbuQamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using streptomycete and non-streptomycete actinobacteria in the United Arab Emirates. Front. Microbiol. 2018, 9, 829. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Kessentini, S.; Tounsi, S.; Jamoussi, K. Optimization of Bacillus amyloliquefaciens BLB369 Culture Medium by Response Surface Methodology for Low Cost Production of Antifungal Activity. Microorganisms 2022, 10, 830. [Google Scholar] [CrossRef]
- Koim-Puchowska, B.; Kłosowski, G.; Dróżdż-Afelt, J.M.; Mikulski, D.; Zielińska, A. Influence of the Medium Composition and the Culture Conditions on Surfactin Biosynthesis by a Native Bacillus subtilis natto BS19 Strain. Molecules 2021, 26, 2985. [Google Scholar] [CrossRef]
- Gomes, R.J.; Ida, E.I.; Spinosa, W.A. Nutritional supplementation with amino acids on bacterial cellulose production by Komagataeibacter intermedius: Effect analysis and application of response surface methodology. Appl. Biochem. Biotechnol. 2022, 194, 5017–5036. [Google Scholar] [CrossRef] [PubMed]
- Vlajkov, V.; Anđelić, S.; Pajčin, I.; Grahovac, M.; Budakov, D.; Jokić, A.; Grahovac, J. Medium for the production of Bacillusbased biocontrol agent effective against aflatoxigenic Aspergillus flavus: Dual approach for modelling and optimization. Microorganisms 2022, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, J.; Bharathiraja, B.; Varjani, S.; PraveenKumar, R.; Kumar, S.M. Anaerobic biobutanol production from black strap molasses using Clostridium acetobutylicum MTCC11274: Media engineering and kinetic analysis. Bioresour. Technol. 2022, 346, 126405. [Google Scholar] [CrossRef] [PubMed]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef]
- Santra, H.K.; Maity, S.; Banerjee, D. Production of bioactive compounds with broad spectrum bactericidal action, bio-film inhibition and antilarval potential by the secondary metabolites of the endophytic fungus Cochliobolus sp. APS1 isolated from the Indian medicinal herb Andrographis paniculata. Molecules 2022, 27, 1459. [Google Scholar] [PubMed]
- Venkateswarulu, T.C.; Prabhakar, K.V.; Kumar, R.B. Optimization of nutritional components of medium by response surface methodology for enhanced production of lactase. 3 Biotech. 2017, 7, 186. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Xu, S.; Wang, B.; Ju, J.; Tan, H.; Li, W. Activation and enhancement of Fredericamycin A production in deepsea-derived Streptomyces somaliensis SCSIO ZH66 by using ribosome engineering and response surface methodology. Microb. Cell Fact. 2015, 14, 64. [Google Scholar] [CrossRef]
- Amid, A.; Ismail, N.A.; Yusof, F.; Salleh, H.M. Expression, purification, and characterization of a recombinant stem bromelain from Ananas comosus. Process Biochem. 2011, 46, 2232–2239. [Google Scholar] [CrossRef]
- Almeida, D.G.; da Silva, R.D.F.S.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Response surface methodology for optimizing the production of biosurfactant by Candida tropicalis on industrial waste substrates. Front. Microbiol. 2017, 8, 157. [Google Scholar] [CrossRef]
- Sa, R.-B. The Antimicrobial Activity Substances and Disease Prevention Mechanism of the Endophytic Antagonistic Bacterium N6–34 from Poplar. Doctoral Dissertation, Shandong Agricultural University, Tai’an, China, 2018. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Vu, T.; Le, V. Using fed-batch fermentation in high-gravity brewing: Efects of nutritional supplementation on yeast fermentation performance. Int. Food Res. J. 2010, 17, 117–126. [Google Scholar]
- Wen, Z.; Liu, Z.; Hou, Y.; Liu, C.; Gao, F.; Zheng, Y.; Chen, F. Ethanol induced astaxanthin accumulation and transcriptional expression of carotenogenic genes in Haematococcus pluvialis. Enzym. Microb Technol 2015, 78, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; He, Q.; Jiang, Y.; Huang, L. Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae Xjy and its biocontrol efcacies against Fulvia fulva and Botryosphaeria dothidea. J. Phytopathol. 2016, 164, 155–165. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Wu, Y.; Irfan, M. Application of response surface methodology for optimization of medium components for the production of secondary metabolites by Streptomyces diastatochromogenes KX852460. AMB Express 2017, 7, 96. [Google Scholar] [CrossRef]
- Jensen, D.F.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys rosea to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W.J., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 429–471. [Google Scholar]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases—What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Purama, R.K.; Goyal, A. Screening and optimization of nutritional factors for higher dextransucrase production by Leuconostocmesenteroides NRRL B-640 using statistical approach. Bioresour. Technol. 2008, 99, 7108–7114. [Google Scholar] [CrossRef]
- Tays, C.; Guarnieri, M.T.; Sauvageau, D.; Stein, L.Y. Combined Effects of Carbon and Nitrogen Source to Optimize Growth of Proteobacterial Methanotrophs. Front. Microbiol. 2018, 9, 2239. [Google Scholar] [CrossRef]
- Grant, C.; Cubadda, F.; Carcea, M.; Pogna, N.E.; Gazza, L. Chapter 7—Vitamins, minerals, and nutritional value of durum wheat. In American Associate of Cereal Chemist International, Durum Wheat, 2nd ed.; Sissons, M., Abecassis, J., Marchylo, B., Carcea, M., Eds.; AACC International Press: Washington DC, USA, 2012; pp. 125–137. [Google Scholar]
- Dai, Y.; Wang, Y.H.; Li, M.; Zhu, M.L.; Wen, T.Y.; Wu, X.Q. Medium optimization to analyze the protein composition of Bacillus pumilus HR10 antagonizing Sphaeropsis sapinea. AMB Express 2022, 12, 61. [Google Scholar] [CrossRef]
- Yoon, H.; Warshel, A. Simulating the fdelity and the three Mg mechanism of pol and clarifying the validity of transition state theory in enzyme catalysis. Proteins 2017, 85, 1446–1453. [Google Scholar] [CrossRef]
- Tian, Z.; Hou, L.; Hu, M.; Gao, Y.; Li, D.; Fan, B.; Wang, F.; Li, S. Optimization of Sporulation Conditions for Bacillus subtilis BSNK-5. Processes 2022, 10, 1133. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.; Jia, R.; Hu, L.; Wang, Y. Response Surface Methodology (RSM) Mediated Optimization of Medium Components for Mycelial Growth and Metabolites Production of Streptomyces Alfalfae XN-04. Microorganisms 2022, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Nouby, M.; Mathivanan, D.; Srinivasan, K. A combined approach of complex eigenvalue analysis and design of experiments (DOE) to study disc brake squeal. Int. J. Eng. Sci. Technol. 2009, 1, 254–271. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Li, X.M.; Li, Y.; Li, P.R. The relations among reducing sugar, pH, dry weight of mycelium and production during the liquid fermentation of Marasmius androsaceus. Edible Fungi China 2002, 21, 37–38. [Google Scholar]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Tsalgatidou, P.C.; Thomloudi, E.-E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated Genomic and Metabolomic Analysis IlluminatesKeySecreted Metabolites Produced by the Novel Endophyte Bacillus halotolerans Cal.l.30 Involved in Diverse Biological Control Activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seififikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Effificient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef]
- Majumdar, S. Evaluation of Jute Rhizospheric Bacteria for Plant Growth Promotion and Disease Suppression. Ph.D. Thesis, University of North Benga, Bharatisar, India, 2017. [Google Scholar]
- Gohil, R.B.; Raval, V.H.; Panchal, R.R.; Rajput, K.N. Plant Growth-Promoting Activity of Bacillus sp. PG-8 Isolated from Fermented Panchagavya and Its Effect on the Growth of Arachis hypogea. Front. Agron. 2022, 4, 805454. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis BERA 71 improves salt tolerance in chickpea plants by regulating the plant defensemechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Naveed, M.; Hafeez, S.; Rafique, M.; Mumtaz, M.Z.; Subhani, Z.; Holatko, J.; Hammerschmiedt, T.; Malicek, O.; Mustafa, A.; Kintl, A.; et al. Plant-endophytemediatedimprovement in physiologicaland bio-protective abilities of marigold (Tagetes patula). Front. Plant Sci. 2022, 13, 993130. [Google Scholar] [CrossRef]
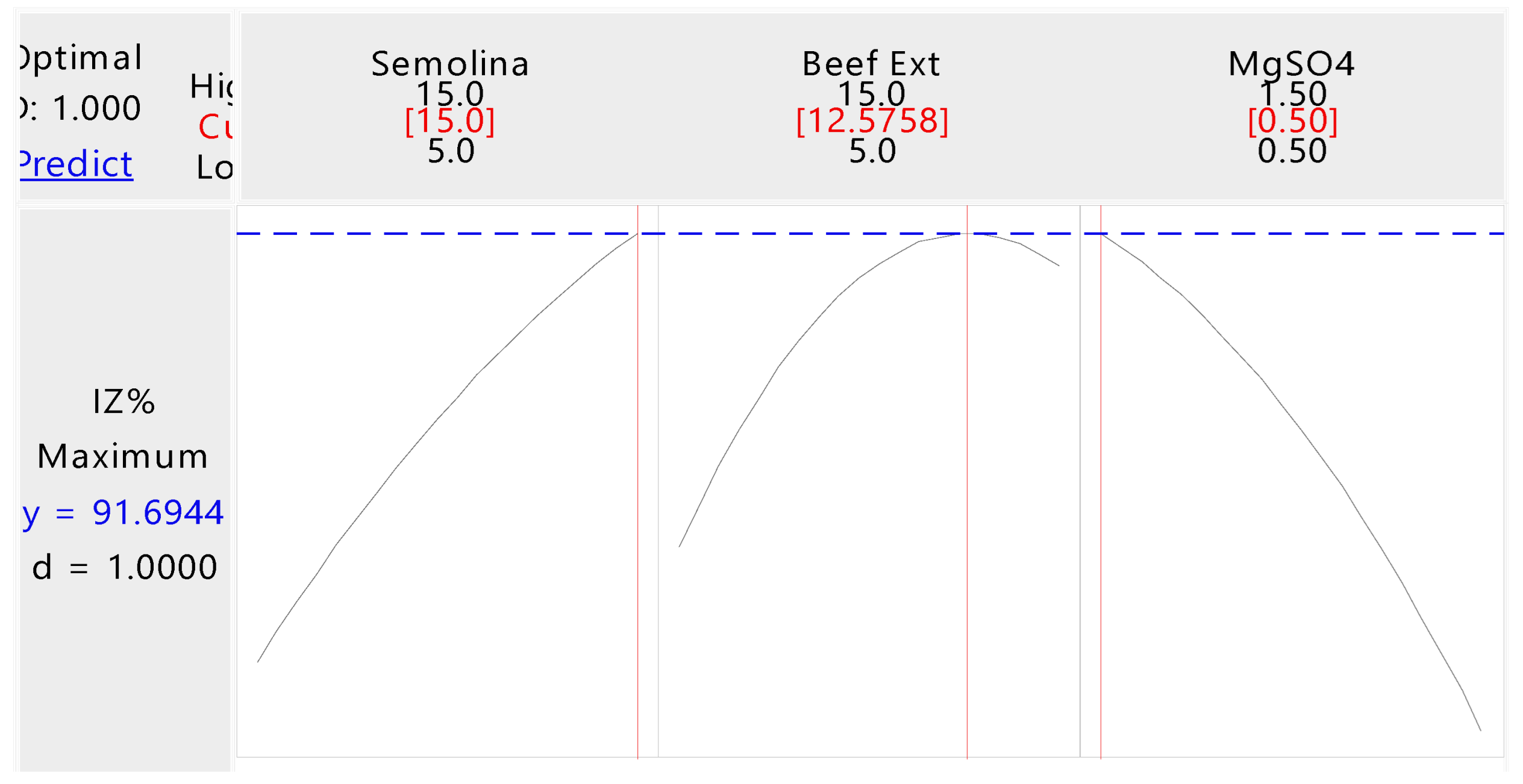


| StdOrder | RunOrder | PtType | Blocks | X1 | X2 | X3 | Observed Values |
|---|---|---|---|---|---|---|---|
| 5 | 1 | 2 | 1 | −1 (5) | 0 (10) | −1 (0.5) | 55.61 |
| 9 | 2 | 2 | 1 | 0 (10) | −1 (5) | −1 | 53.90 |
| 2 | 3 | 2 | 1 | 1 (15) | −1 | 0 (1) | 54.98 |
| 3 | 4 | 2 | 1 | −1 | 1 (15) | 0 | 56.45 |
| 7 | 5 | 2 | 1 | −1 | 0 | 1 (1.5) | 51.76 |
| 10 | 6 | 2 | 1 | 0 | 1 | −1 | 71.54 |
| 6 | 7 | 2 | 1 | 1 | 0 | −1 | 90.55 |
| 15 | 8 | 0 | 1 | 0 | 0 | 0 | 78.66 |
| 8 | 9 | 2 | 1 | 1 | 0 | 1 | 51.65 |
| 11 | 10 | 2 | 1 | 0 | −1 | 1 | 45.32 |
| 1 | 11 | 2 | 1 | −1 | −1 | 0 | 41.51 |
| 13 | 12 | 0 | 1 | 0 | 0 | 0 | 68.54 |
| 4 | 13 | 2 | 1 | 1 | 1 | 0 | 73.54 |
| 12 | 14 | 2 | 1 | 0 | 1 | 1 | 47.54 |
| 14 | 15 | 0 | 1 | 0 | 0 | 0 | 65.65 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 2512.13 | 279.126 | 10.59 | 0.009 |
| Linear | 3 | 1599.72 | 533.240 | 20.23 | 0.003 |
| X1: Semolina | 1 | 534.48 | 534.482 | 20.28 | 0.006 |
| X2: Beef Extract | 1 | 355.91 | 355.911 | 13.50 | 0.014 |
| X3: MgSO4 | 1 | 709.33 | 709.326 | 26.91 | 0.004 |
| Square | 3 | 542.57 | 180.856 | 6.86 | 0.032 |
| X12X1 | 1 | 39.15 | 39.150 | 1.49 | 0.277 |
| X22X2 | 1 | 452.78 | 452.780 | 17.18 | 0.009 |
| X32X3 | 1 | 103.77 | 103.766 | 3.94 | 0.104 |
| 2-Way Interaction | 3 | 369.85 | 123.282 | 4.68 | 0.065 |
| X12X2 | 1 | 3.28 | 3.276 | 0.12 | 0.739 |
| X12X3 | 1 | 307.13 | 307.126 | 11.65 | 0.019 |
| X22X3 | 1 | 59.44 | 59.444 | 2.25 | 0.193 |
| Error | 5 | 131.81 | 26.361 | ||
| Lack-of-Fit | 3 | 38.46 | 12.821 | 0.27 | 0.842 |
| Pure Error | 2 | 93.34 | 46.671 | ||
| Total | 14 | 2643.94 | |||
| Model Summary | S = 5.13432 | R2 = 95.01% | R2 (adj) = 86.04% | R2 (pred) = 68.78% |
| Term | Coef | SE Coef | T-Value | p-Value | VIF |
|---|---|---|---|---|---|
| Constant | 70.95 | 2.96 | 23.93 | 0.000 | |
| X1: Semolina | 8.17 | 1.82 | 4.50 | 0.006 | 1.00 |
| X2: Beef Extract | 6.67 | 1.82 | 3.67 | 0.014 | 1.00 |
| X3: MgSO4 | −9.42 | 1.82 | −5.19 | 0.004 | 1.00 |
| X12X1 | −3.26 | 2.67 | −1.22 | 0.277 | 1.01 |
| X22X2 | −11.07 | 2.67 | −4.14 | 0.009 | 1.01 |
| X32X3 | −5.30 | 2.67 | −1.98 | 0.104 | 1.01 |
| X12X2 | 0.91 | 2.57 | 0.35 | 0.739 | 1.00 |
| X12X3 | −8.76 | 2.57 | −3.41 | 0.019 | 1.00 |
| X22X3 | −3.86 | 2.57 | −1.50 | 0.193 | 1.00 |
| Treatment | Shoot Fresh Weight (g/plant) | Shoot Dry Weight (g/plant) | Root Fresh Weight (g/plant) | Root Dry Weight (g/plant) | Shoot Length (cm) | Root Length (cm) |
|---|---|---|---|---|---|---|
| CF (Ck) | 10.25 ± 0.48 c | 8.75 ± 0.25 ab | 3.08 ± 0.13 ab | 0.95 ± 0.08 ab | 15.00 ± 0.41 ab | 3.98 ± 0.23 ab |
| 200 μL BAM | 20.00 ± 0.91 a | 10.00 ± 0.71 ab | 3.70 ± 0.14 b | 1.11 ± 0.06 b | 15.50 ± 0.65 a | 3.93 ± 0.25 ab |
| 400 μL BAM | 21.00 ± 1.08 a | 11.00 ± 0.41a | 3.90 ± 0.08 a | 1.25 ± 0.13 a | 16.75 ± 0.48 a | 4.13 ± 0.38 a |
| Inoculated with CA | 6.25 ± 0.25 d | 4.75 ± 0.63 c | 1.33 ± 0.23 c | 0.56 ± 0.07 c | 7.75 ± 0.48 d | 1.48 ± 0.18 c |
| 200 μL BAM + Inoculated with CA | 11.75 ± 0.48 bc | 8.75 ± 0.25 b | 1.81 ± 0.20 c | 0.73 ± 0.11 bc | 12.50 ± 0.29 c | 2.73 ± 0.20 bc |
| 400 μL BAM + Inoculated with CA | 14.00 ± 0.71 b | 9.50 ± 0.29 ab | 1.98 ± 0.13 c | 0.68 ± 0.03 bc | 13.75 ± 0.25 bc | 3.12 ± 0.37 c |
| Treatment | Photosynthesis Rate (μmol CO2 m−2 s−1) | Transpiration Rate (mmol H2O m2 s−1) | Stomatal Conductance (mol H2O m−2 s−1) | Sub-Stomatal CO2 Concentration (μmol CO2 mol−1) |
|---|---|---|---|---|
| CF (Ck) | 147.25 ± 2.43 ab | 149.25 ± 3.35 abc | 209.25 ± 4.85 a | 202.58 ± 0.55 a |
| 200 μL BAM | 159.50 ± 2.96 a | 158.13 ± 5.14 ab | 169.75 ± 3.12 b | 202.75 ± 1.44 a |
| 400 μL BAM | 163.78 ± 1.99 ab | 168.00 ± 5.45 a | 218.25 ± 7.09 a | 202.43 ± 0.78 a |
| Inoculated with CA | 120.03 ± 3.76 c | 116.50 ± 6.60 d | 138.00 ± 2.48 c | 202.78 ± 0.50 a |
| 200 μL BAM + Inoculated with CA | 145.50 ± 4.84 ab | 135.00 ± 3.14 cd | 162.40 ± 3.86 b | 203.03 ± 0.26 a |
| 400 μL BAM + Inoculated with CA | 149.25 ± 4.82 b | 143.00 ± 3.37 bc | 172.50 ± 6.44 b | 202.33 ± 0.72 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, T.; Zang, C.; Yu, S.; Pei, X.; Xie, J.; Lin, Y.; Liu, X.; Liang, C. Screening, and Optimization of Fermentation Medium to Produce Secondary Metabolites from Bacillus amyloliquefaciens, for the Biocontrol of Early Leaf Spot Disease, and Growth Promoting Effects on Peanut (Arachis hypogaea L.). J. Fungi 2022, 8, 1223. https://doi.org/10.3390/jof8111223
Ahsan T, Zang C, Yu S, Pei X, Xie J, Lin Y, Liu X, Liang C. Screening, and Optimization of Fermentation Medium to Produce Secondary Metabolites from Bacillus amyloliquefaciens, for the Biocontrol of Early Leaf Spot Disease, and Growth Promoting Effects on Peanut (Arachis hypogaea L.). Journal of Fungi. 2022; 8(11):1223. https://doi.org/10.3390/jof8111223
Chicago/Turabian StyleAhsan, Taswar, Chaoqun Zang, Shuyi Yu, Xue Pei, Jinhui Xie, Ying Lin, Xiaozhou Liu, and Chunhao Liang. 2022. "Screening, and Optimization of Fermentation Medium to Produce Secondary Metabolites from Bacillus amyloliquefaciens, for the Biocontrol of Early Leaf Spot Disease, and Growth Promoting Effects on Peanut (Arachis hypogaea L.)" Journal of Fungi 8, no. 11: 1223. https://doi.org/10.3390/jof8111223
APA StyleAhsan, T., Zang, C., Yu, S., Pei, X., Xie, J., Lin, Y., Liu, X., & Liang, C. (2022). Screening, and Optimization of Fermentation Medium to Produce Secondary Metabolites from Bacillus amyloliquefaciens, for the Biocontrol of Early Leaf Spot Disease, and Growth Promoting Effects on Peanut (Arachis hypogaea L.). Journal of Fungi, 8(11), 1223. https://doi.org/10.3390/jof8111223







