The Effect of Dekkera bruxellensis Concentration and Inoculation Time on Biochemical Changes and Cellulose Biosynthesis by Komagataeibacter intermedius
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Media and Culture Preparation
2.2.2. BC Production Set Up
2.2.3. K. intermedius and D. bruxellensis Cell Enumeration
2.2.4. Biochemical Analysis
2.2.5. BC Yield Measurement
2.2.6. Statistical Analysis
3. Results and Discussion
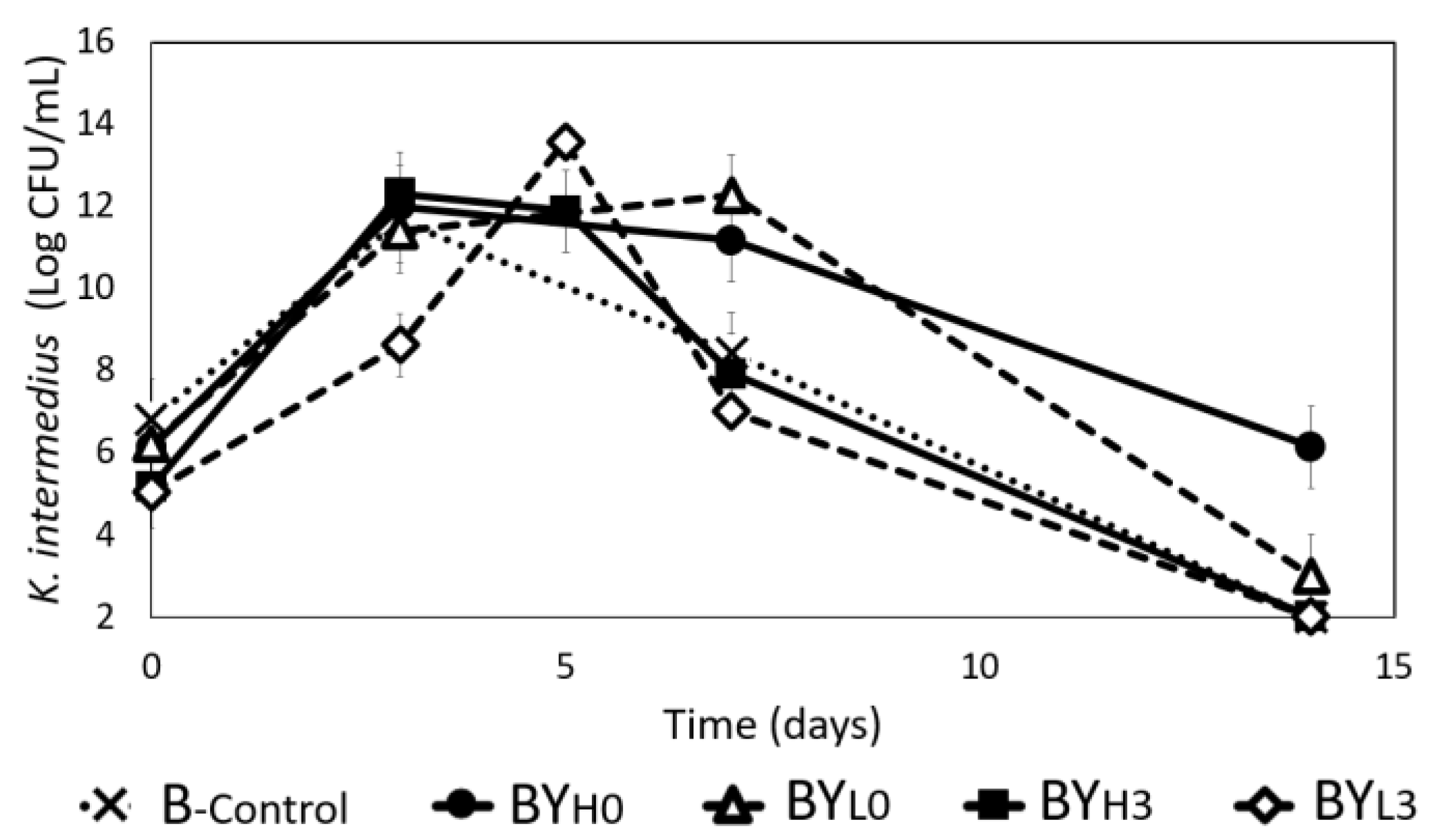
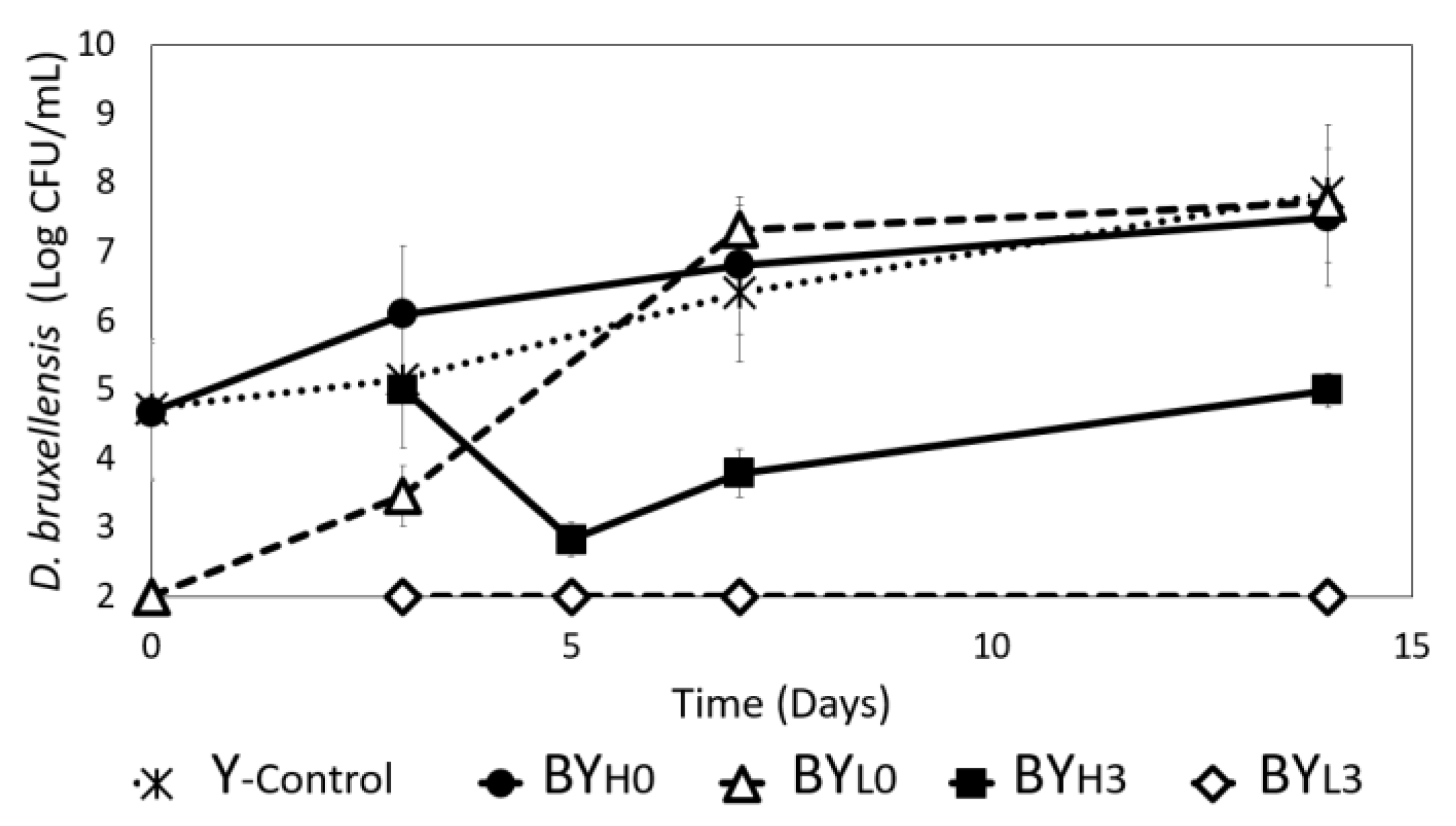
3.1. Effect of D. bruxellensis Concentration and Inoculation Time on Bacteria-Yeast Interactions
3.2. Effect of D. bruxellensis Concentration and Inoculation Time on pH Changes
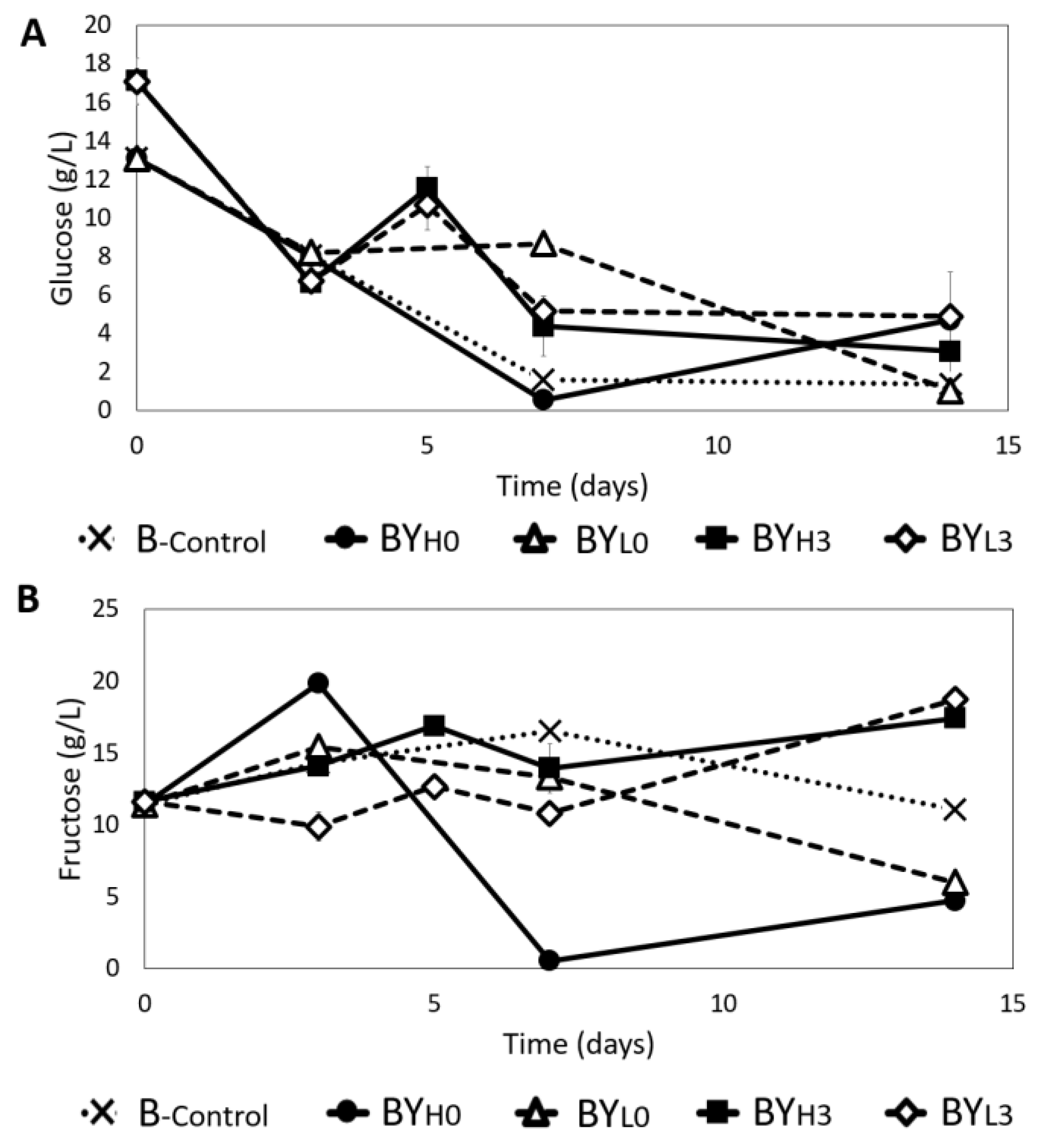
3.3. Effect of D. bruxellensis Concentration and Inoculation Time on Sugar Concentration during Co-Culture with K. intermedius
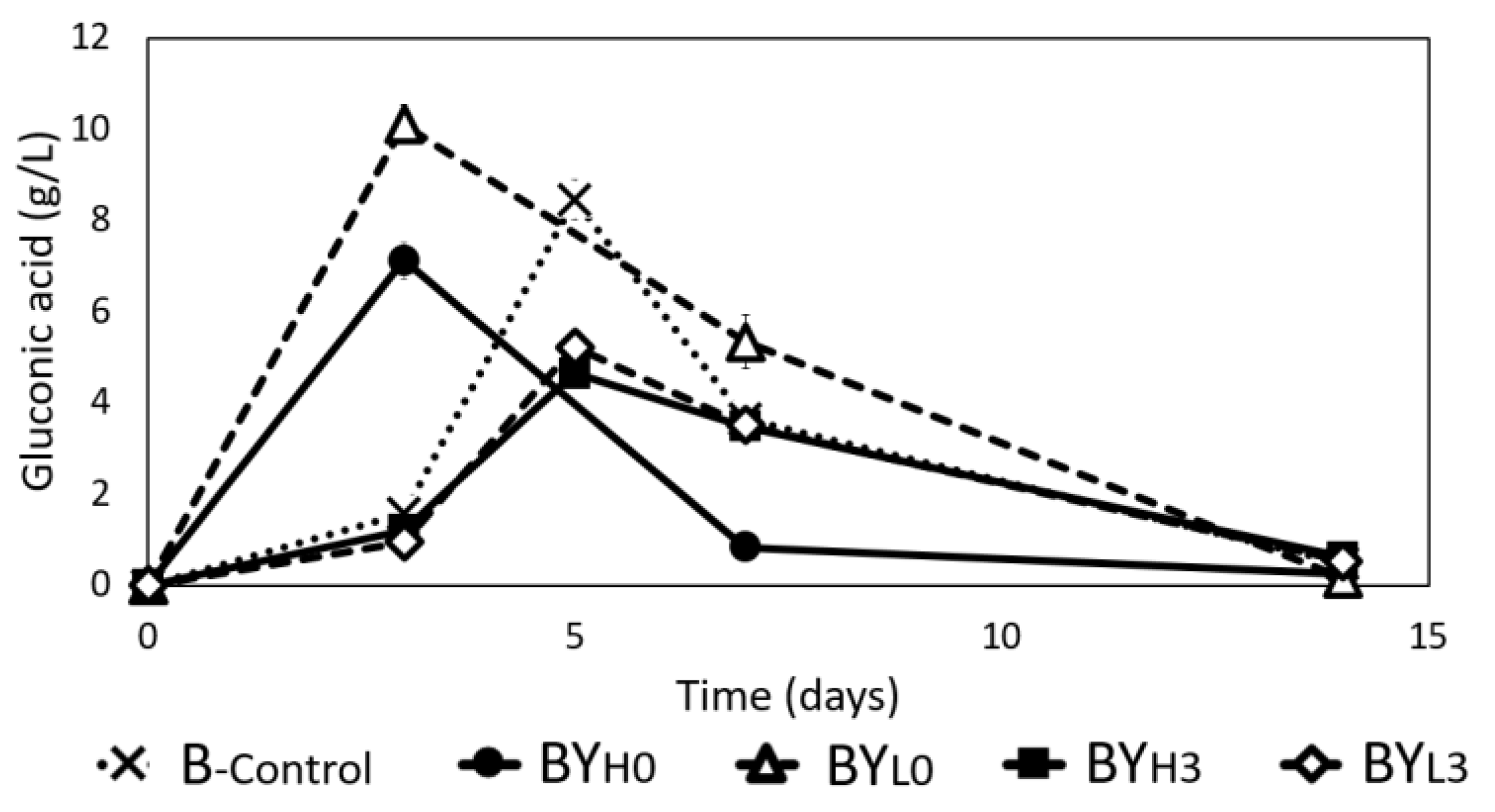
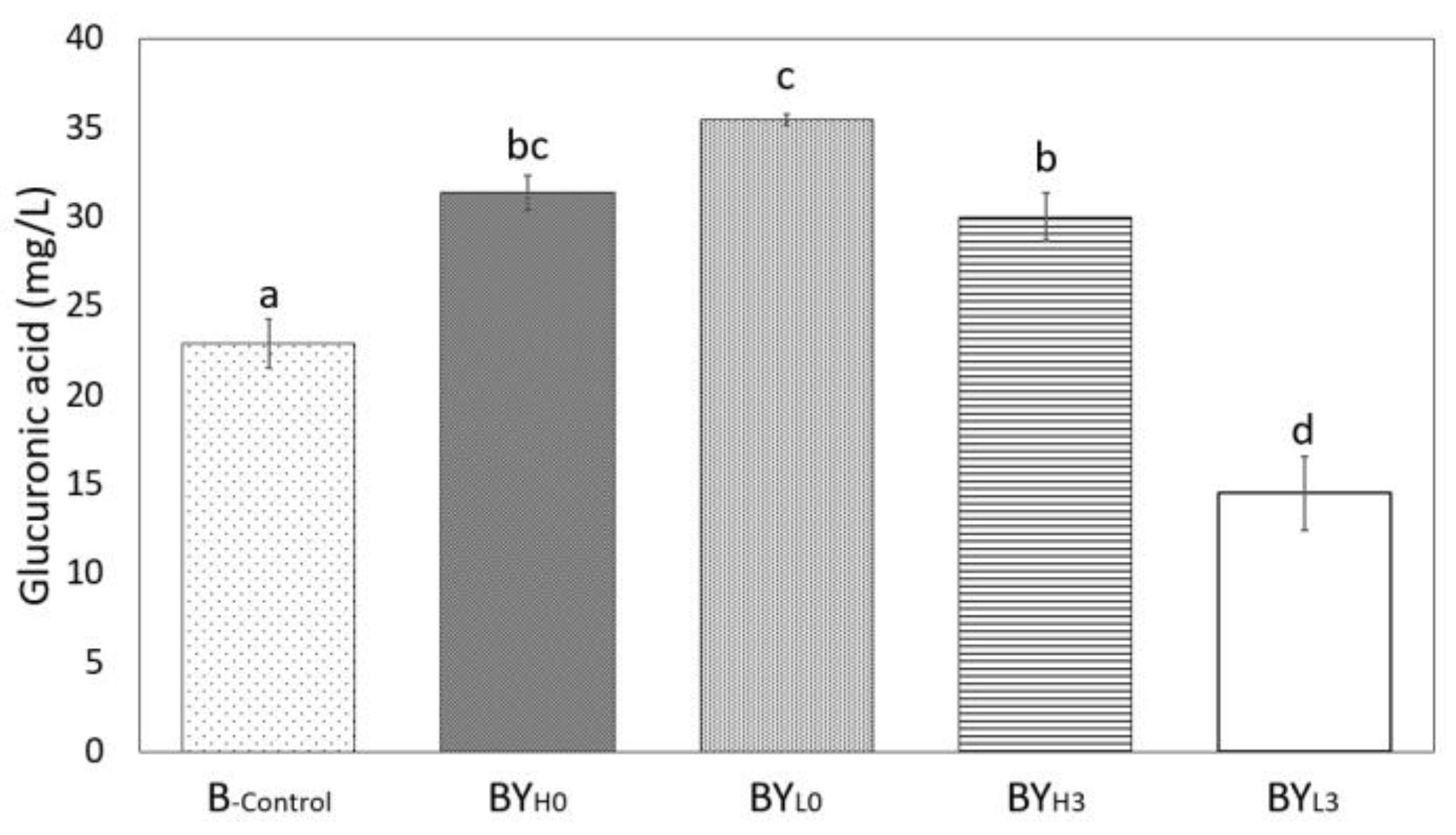
3.4. Effect of D. bruxellensis Concentration and Inoculation Time on Gluconic and Glucuronic Acid Production by K. intermedius
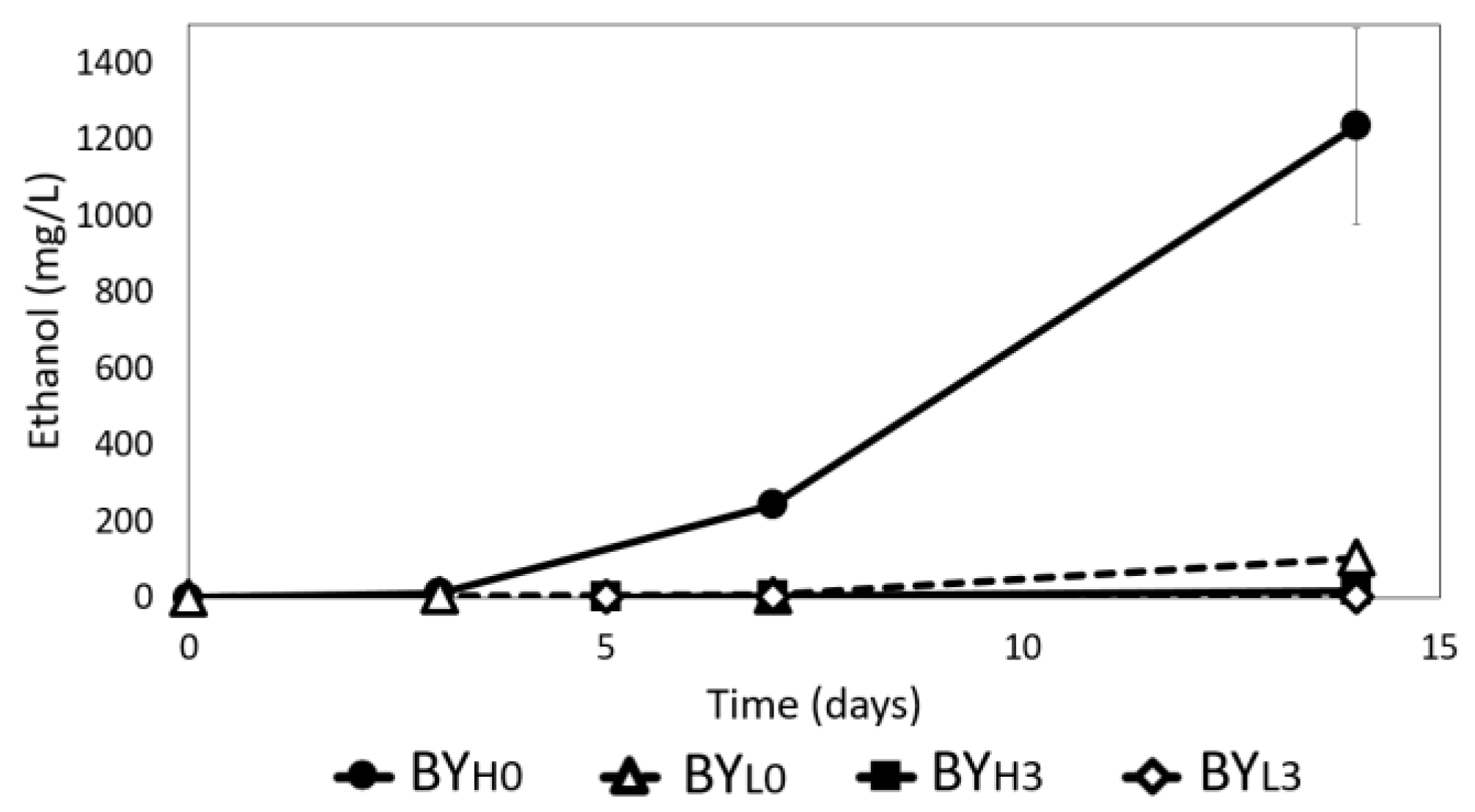
3.5. Effect of D. bruxellensis Concentration and Inoculation Time on Ethanol Production
3.6. Effect of D. bruxellensis Concentration and Inoculation Time on Free Amino Nitrogen (FAN) Concentration
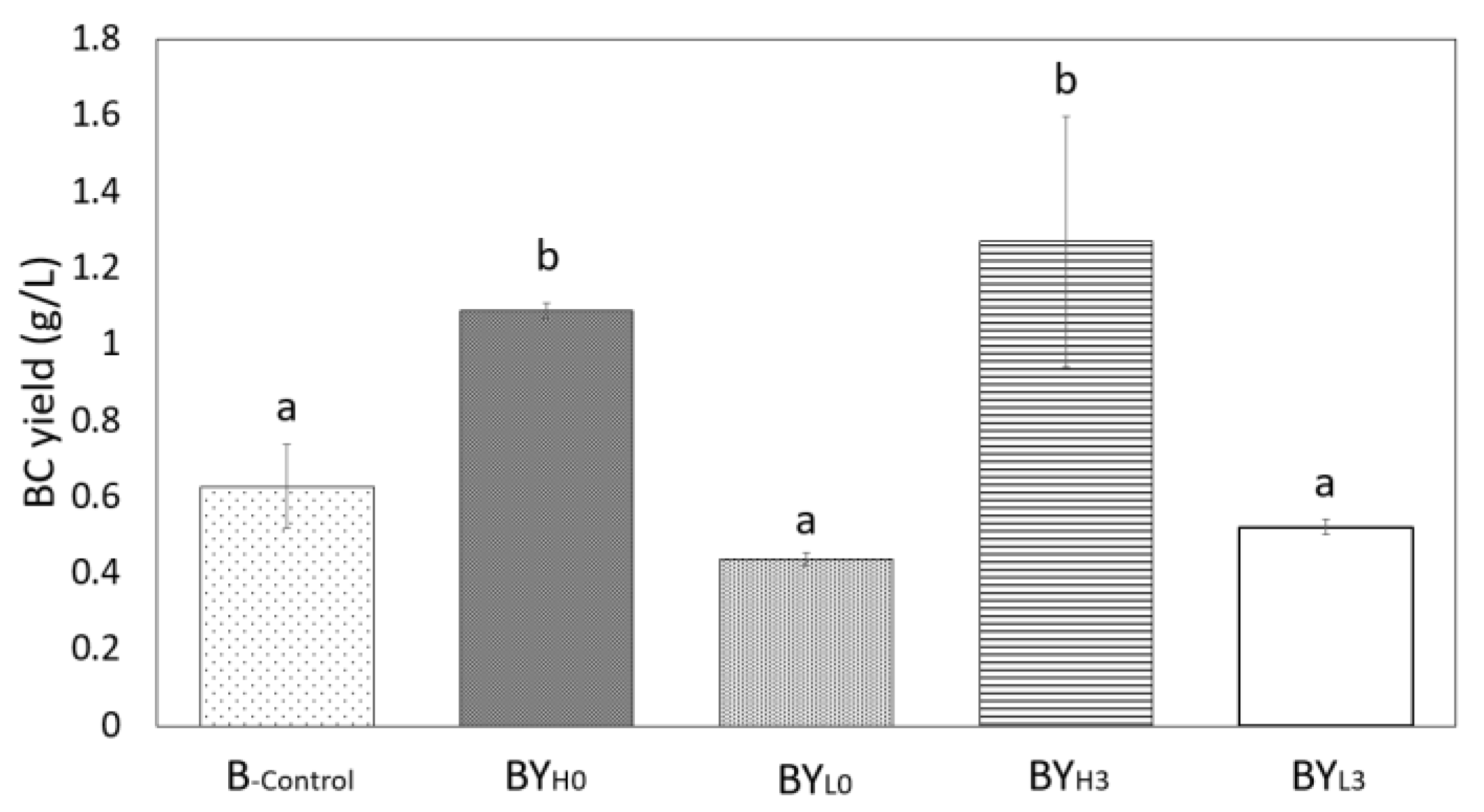
3.7. Effect of D. bruxellensis Concentration and Inoculation Time on BC Production by K. intermedius
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Naomi, R.; Idrus, R.B.H.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef] [PubMed]
- La China, S.; De Vero, L.; Anguluri, K.; Brugnoli, M.; Mamlouk, D.; Gullo, M. Kombucha Tea as a Reservoir of Cellulose Producing Bacteria: Assessing Diversity among Komagataeibacter Isolates. Appl. Sci. 2021, 11, 1595. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktipis, A. Kombucha: A Novel Model System for Cooperation and Conflict in a Complex Multi-Species Microbial Ecosystem. PeerJ 2019, 7, e7565. [Google Scholar] [CrossRef]
- Landis, E.A.; Fogarty, E.; Edwards, J.C.; Popa, O.; Eren, A.M.; Wolfe, B.E. Microbial Diversity and Interaction Specificity in Kombucha Tea Fermentations. mSystems 2022, 7, e00157-22. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Enciso, C.; Torres-Taborda, M.; Zuluaga, R.; Gañán, P.; Rojas, O.J.; Castro, C. Effects of Alternative Energy Sources on Bacterial Cellulose Characteristics Produced by Komagataeibacter medellinensis. Int. J. Biol. Macromol. 2018, 117, 735–741. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Kho, K.; Nurdiansyah, R.; Briot, A.; Taherzadeh, M.J.; Aslanzadeh, S. Do Kombucha Symbiotic Cultures of Bacteria and Yeast Affect Bacterial Cellulose Yield in Molasses? J. Fungi 2021, 7, 705. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, Y.-H.; Hsu, K.-D.; Lai, Y.-J.; Chen, Y.-K.; Cheng, K.-C. Isolation and Identification of Cellulose-Producing Strain Komagataeibacter intermedius from Fermented Fruit Juice. Carbohydr. Polym. 2016, 151, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Morena, A.G.; Valenzuela, S.V.; Pastor, F.I.J.; Díaz, P.; Martínez, J. Microbial Cellulose from a Komagataeibacter intermedius Strain Isolated from Commercial Wine Vinegar. J. Polym. Environ. 2019, 27, 956–967. [Google Scholar] [CrossRef]
- Angela, C.; Young, J.; Kordayanti, S.; Virgina Partha Devanthi, P.; Kho, K. Isolation and Screening of Microbial Isolates from Kombucha Culture for Bacterial Cellulose Production in Sugarcane Molasses Medium. KnE Life Sci. 2020, 5, 111–127. [Google Scholar] [CrossRef]
- Tyagi, N.; Suresh, S. Production of Cellulose from Sugarcane Molasses Using Gluconacetobacter Intermedius SNT-1: Optimization & Characterization. J. Clean. Prod. 2016, 112, 71–80. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Nguyen, P.B.; Nguyen, H.T.; Le, P.H. Screening the Optimal Ratio of Symbiosis between Isolated Yeast and Acetic Acid Bacteria Strain from Traditional Kombucha for High-Level Production of Glucuronic Acid. LWT-Food Sci. Technol. 2015, 64, 1149–1155. [Google Scholar] [CrossRef]
- Li, R.; Xu, Y.; Chen, J.; Wang, F.; Zou, C.; Yin, J. Enhancing the Proportion of Gluconic Acid with a Microbial Community Reconstruction Method to Improve the Taste Quality of Kombucha. LWT 2022, 155, 112937. [Google Scholar] [CrossRef]
- Ciosek, A.; Rusiecka, I.; Poreda, A. Sour Beer Production: Impact of Pitching Sequence of Yeast and Lactic Acid Bacteria. J. Inst. Brew. 2020, 126, 53–58. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Linforth, R.; Onyeaka, H.; Gkatzionis, K. Effects of Co-Inoculation and Sequential Inoculation of Tetragenococcus halophilus and Zygosaccharomyces rouxii on Soy Sauce Fermentation. Food Chem. 2018, 240, 1–8. [Google Scholar] [CrossRef]
- Pratama, F.; Devanthi, P.V.P.; Kho, K. Development of Selective Media for Komagataeibacter intermedius and Dekkera Bruxellensis from a Mixed Culture. Nusant. Sci. Technol. Proc. 2021, 32–38. [Google Scholar] [CrossRef]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2013; ISBN 978-0-12-220851-5. [Google Scholar]
- Serna Saldívar, S.R.O. Cereal Grains: Laboratory Reference and Procedures Manual; Food preservation technology series; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-5565-2. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic Activity and Symbiotic Interactions of Lactic Acid Bacteria and Yeasts Isolated from Water Kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Hu, S.; Li, Z.; Qu, J.; Wu, Z.; Chen, S. Comparison of the Effects of Acetic Acid Bacteria and Lactic Acid Bacteria on the Microbial Diversity of and the Functional Pathways in Dough as Revealed by High-Throughput Metagenomics Sequencing. Int. J. Food Microbiol. 2021, 346, 109168. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.-H.; Cao, H.; Cai, P.; Sørensen, S.J. The Initial Inoculation Ratio Regulates Bacterial Coculture Interactions and Metabolic Capacity. ISME J. 2021, 15, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Parera Olm, I.; Sousa, D.Z. Synthetic Co-Cultures: Novel Avenues for Bio-Based Processes. Curr. Opin. Biotechnol. 2021, 67, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mellefont, L.A.; McMeekin, T.A.; Ross, T. Effect of Relative Inoculum Concentration on Listeria Monocytogenes Growth in Co-Culture. Int. J. Food Microbiol. 2008, 121, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Tanamool, V.; Chantarangsee, M.; Soemphol, W. Simultaneous Vinegar Fermentation from a Pineapple By-Product Using the Co-Inoculation of Yeast and Thermotolerant Acetic Acid Bacteria and Their Physiochemical Properties. 3 Biotech 2020, 10, 115. [Google Scholar] [CrossRef]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces Yeasts—From Spoilage Organisms to Valuable Contributors to Industrial Fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent Advances in Bacterial Cellulose: A Low-Cost Effective Production Media, Optimization Strategies and Applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.-Q.; Cao, X.; Li, L.; Zhu, J.; Yu, H. Effect of PH Buffer and Carbon Metabolism on the Yield and Mechanical Properties of Bacterial Cellulose Produced by Komagataeibacter hansenii ATCC 53582. J. Microbiol. Biotechnol. 2021, 31, 429–438. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, J.-H.; Liou, B.-K.; Lee, C.-K. Utilization of Acetate Buffer to Improve Bacterial Cellulose Production by Gluconacetobacter xylinus. Food Hydrocoll. 2016, 53, 98–103. [Google Scholar] [CrossRef]
- Thongwai, N.; Futui, W.; Ladpala, N.; Sirichai, B.; Weechan, A.; Kanklai, J.; Rungsirivanich, P. Characterization of Bacterial Cellulose Produced by Komagataeibacter maltaceti P285 Isolated from Contaminated Honey Wine. Microorganisms 2022, 10, 528. [Google Scholar] [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Escalante-Aburto, A. Recent Evidence of the Beneficial Effects Associated with Glucuronic Acid Contained in Kombucha Beverages. Curr. Nutr. Rep. 2020, 9, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-J.; Kim, H.-G.; Kim, K.-K.; Kim, H.-S.; Kim, Y.-G.; Lee, S.-J. Increased Production of Bacterial Cellulose by Acetobacter Sp. V6 in Synthetic Media under Shaking Culture Conditions. Bioresour. Technol. 2003, 86, 215–219. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.-P.; Taillandier, P.; Beaufort, S. Metabolome-Microbiome Signatures in the Fermented Beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; de Faria-Tischer, P.C.S.; Tischer, C.A.; Constantino, L.V.; de Rosa, M.F.; Chideroli, R.T.; de Pereira, U.P.; Spinosa, W.A. Komagataeibacter intermedius V-05: An Acetic Acid Bacterium Isolated from Vinegar Industry, with High Capacity for Bacterial Cellulose Production in Soybean Molasses Medium. Food Technol. Biotechnol. 2021, 59, 432–442. [Google Scholar] [CrossRef]
- Wang, S.-S.; Han, Y.-H.; Chen, J.-L.; Zhang, D.-C.; Shi, X.-X.; Ye, Y.-X.; Chen, D.-L.; Li, M. Insights into Bacterial Cellulose Biosynthesis from Different Carbon Sources and the Associated Biochemical Transformation Pathways in Komagataeibacter Sp. W1. Polymers 2018, 10, 963. [Google Scholar] [CrossRef]
- Tantipaibulvut, S.; Pinisakul, A.; Rattanachaisit, P.; Klatin, K.; Onsriprai, B.; Boonyaratsiri, K. Ethanol Production from Desizing Wastewater Using Co-Culture of Bacillus Subtilis and Saccharomyces Cerevisiae. Energy Procedia 2015, 79, 1001–1007. [Google Scholar] [CrossRef]
- Avcioglu, N.H.; Birben, M.; Seyis Bilkay, I. Optimization and Physicochemical Characterization of Enhanced Microbial Cellulose Production with a New Kombucha Consortium. Process Biochem. 2021, 108, 60–68. [Google Scholar] [CrossRef]
- Premjet, S.; Premjet, D.; Ohtani, Y. The Effect of Ingredients of Sugar Cane Molasses on Bacterial Cellulose Production by Acetobacter xylinum ATCC 10245. Seni Gakkaishi 2007, 63, 193–199. [Google Scholar] [CrossRef]
- Lo, S.-C.; Yang, C.-Y.; Mathew, D.C.; Huang, C.-C. Growth and Autolysis of the Kefir Yeast Kluyveromyces marxianus in Lactate Culture. Sci. Rep. 2021, 11, 14552. [Google Scholar] [CrossRef]
- Friedman, M. Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef]
- Koller, H.; Perkins, L.B. Brewing and the Chemical Composition of Amine-Containing Compounds in Beer: A Review. Foods 2022, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Román-Camacho, J.J.; Mauricio, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; García-García, I. Functional Metaproteomic Analysis of Alcohol Vinegar Microbiota during an Acetification Process: A Quantitative Proteomic Approach. Food Microbiol. 2021, 98, 103799. [Google Scholar] [CrossRef]
- Rani, M.U.; Appaiah, K.A.A. Production of Bacterial Cellulose by Gluconacetobacter hansenii UAC09 Using Coffee Cherry Husk. J. Food Sci. Technol. 2013, 50, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.A.R.N.A.; Zakaria, J.; Mohamad, S.; Fathiyah Sy Mohamad, S.; Rahim, M.H.A. Effect of Different Treatment Methods on the Purification of Bacterial Cellulose Produced from OPF Juice by Acetobacter xylinum. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012058. [Google Scholar] [CrossRef]
- Gilbert, C.; Tang, T.-C.; Ott, W.; Dorr, B.A.; Shaw, W.M.; Sun, G.L.; Lu, T.K.; Ellis, T. Living Materials with Programmable Functionalities Grown from Engineered Microbial Co-Cultures. Nat. Mater. 2021, 20, 691–700. [Google Scholar] [CrossRef]
- Pinto, L.; Malfeito-Ferreira, M.; Quintieri, L.; Silva, A.C.; Baruzzi, F. Growth and Metabolite Production of a Grape Sour Rot Yeast-Bacterium Consortium on Different Carbon Sources. Int. J. Food Microbiol. 2019, 296, 65–74. [Google Scholar] [CrossRef]


| KERRYPNX | Cell Concentration (log CFU/mL) | ||
|---|---|---|---|
| Sample | K. intermedius | D. bruxellensis | Day of Yeast Addition |
| B-control | 6 | n.a. | n.a. |
| Y-control | n.a. | 6 | n.a. |
| BYH0 | 6 | 6 | 0 |
| BYL0 | 6 | 3 | 0 |
| BYH3 | 6 | 6 | 3 |
| BYL3 | 6 | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devanthi, P.V.P.; Pratama, F.; Kho, K.; Taherzadeh, M.J.; Aslanzadeh, S. The Effect of Dekkera bruxellensis Concentration and Inoculation Time on Biochemical Changes and Cellulose Biosynthesis by Komagataeibacter intermedius. J. Fungi 2022, 8, 1206. https://doi.org/10.3390/jof8111206
Devanthi PVP, Pratama F, Kho K, Taherzadeh MJ, Aslanzadeh S. The Effect of Dekkera bruxellensis Concentration and Inoculation Time on Biochemical Changes and Cellulose Biosynthesis by Komagataeibacter intermedius. Journal of Fungi. 2022; 8(11):1206. https://doi.org/10.3390/jof8111206
Chicago/Turabian StyleDevanthi, Putu Virgina Partha, Ferren Pratama, Katherine Kho, Mohammad J. Taherzadeh, and Solmaz Aslanzadeh. 2022. "The Effect of Dekkera bruxellensis Concentration and Inoculation Time on Biochemical Changes and Cellulose Biosynthesis by Komagataeibacter intermedius" Journal of Fungi 8, no. 11: 1206. https://doi.org/10.3390/jof8111206
APA StyleDevanthi, P. V. P., Pratama, F., Kho, K., Taherzadeh, M. J., & Aslanzadeh, S. (2022). The Effect of Dekkera bruxellensis Concentration and Inoculation Time on Biochemical Changes and Cellulose Biosynthesis by Komagataeibacter intermedius. Journal of Fungi, 8(11), 1206. https://doi.org/10.3390/jof8111206







