Impact of the Disk Diffusion Test on Fluconazole De-Escalation in Patients with Candidemia
Abstract
1. Introduction
2. Materials and Methods
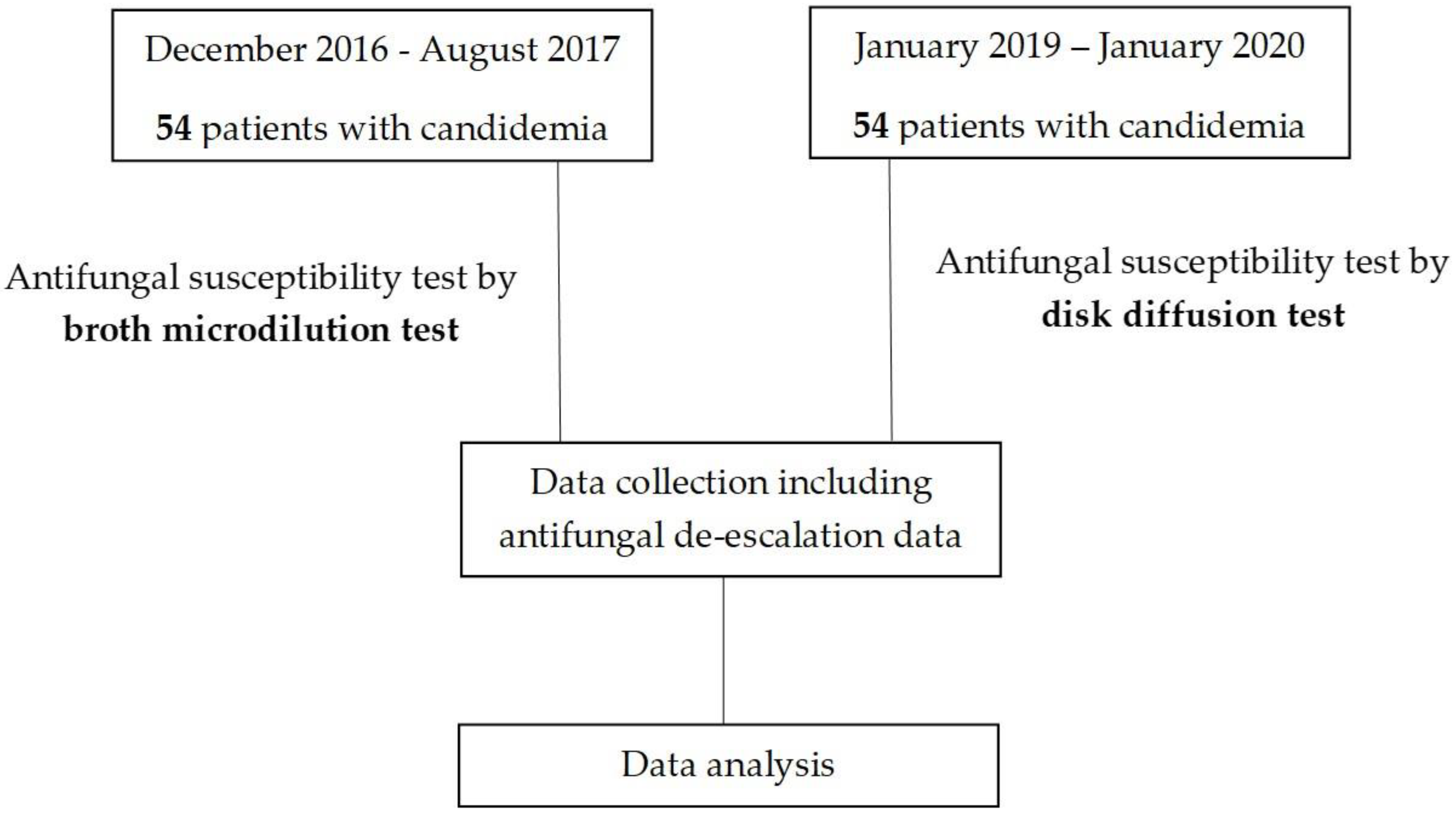
2.1. Study Design
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Patient Data
3.2. Antifungal De-Escalation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Gudlaugsson, O.; Gillespie, S.; Lee, K.; Vande Berg, J.; Hu, J.; Messer, S.; Herwaldt, L.; Pfaller, M.; Diekema, D. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 2003, 37, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Candida bloodstream infections: Comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob. Agents Chemother. 2011, 55, 561–566. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 2011, 49, 396–399. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance Group. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Capoor, M.R.; Nair, D.; Deb, M.; Verma, P.K.; Srivastava, L.; Aggarwal, P. Emergence of non-albicans Candida species and antifungal resistance in a tertiary care hospital. Jpn. J. Infect. Dis. 2005, 58, 344–348. [Google Scholar] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 2012, 50, 2846–2856. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Fourth Informational Supplement M27-S4; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017. [Google Scholar]
- Pfaller, M.A.; Hazen, K.C.; Messer, S.A.; Boyken, L.; Tendolkar, S.; Hollis, R.J.; Diekema, D.J. Comparison of results of fluconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS global antifungal surveillance program. J. Clin. Microbiol. 2004, 42, 3607–3612. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Zone Diameter Interpretive Standards, Corresponding Minimal Inhibitory Concentration (MIC) Interpretive Breakpoints, and Quality Control Limits for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Third International Supplement CLSI Document-M444-S3; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2009. [Google Scholar]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Bates, D.W.; Su, L.; Yu, D.T.; Chertow, G.M.; Seger, D.L.; Gomes, D.R.; Dasbach, E.J.; Platt, R. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin. Infect. Dis. 2001, 32, 686–693. [Google Scholar] [CrossRef]
- Chaiwarith, R.; Ounbang, P.; Khamwan, C.; Nuntachit, N.; Sirisanthana, T.; Supparatpinyo, K. Epidemiology of adult candidemia at Chiang Mai University Hospital. Southeast Asian J. Trop. Med. Public Health 2011, 42, 1505–1514. [Google Scholar] [PubMed]
- Boonyasiri, A.; Jearanaisilavong, J.; Assanasen, S. Candidemia in Siriraj Hospital: Epidemiology and factors associated with mortality. J. Med. Assoc. Thai 2013, 96 (Suppl. S2), S91–S97. [Google Scholar]
- Tan, T.Y.; Hsu, L.Y.; Alejandria, M.M.; Chaiwarith, R.; Chinniah, T.; Chayakulkeeree, M.; Choudhury, S.; Chen, Y.H.; Shin, J.H.; Kiratisin, P.; et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med. Mycol. 2016, 54, 471–477. [Google Scholar] [CrossRef]
- Ngamchokwathana, C.; Chongtrakool, P.; Waesamaae, A.; Chayakulkeeree, M. Risk factors and outcomes of non-albicans Candida bloodstream infection in patients with candidemia at Siriraj Hospital-Thailand’s largest national tertiary referral hospital. J. Fungi 2021, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Jaffal, K.; Poissy, J.; Rouze, A.; Preau, S.; Sendid, B.; Cornu, M.; Nseir, S. De-escalation of antifungal treatment in critically ill patients with suspected invasive Candida infection: Incidence, associated factors, and safety. Ann. Intensive Care 2018, 8, 49. [Google Scholar] [CrossRef]
- Moreno-García, E.; Puerta-Alcalde, P.; Gariup, G.; Fernández-Ruiz, M.; Cortés, L.E.L.; Cuervo, G.; Salavert, M.; Merino, P.; Machado, M.; Guinea, J.; et al. Early stepdown from echinocandin to fluconazole treatment in candidemia: A post hoc analysis of three cohort studies. Open Forum Infect. Dis. 2021, 8, ofab250. [Google Scholar] [CrossRef] [PubMed]
- Van Engen, A.; Casamayor, M.; Kim, S.; Watt, M.; Odeyemi, I. “De-escalation” strategy using micafungin for the treatment of systemic Candida infections: Budget impact in France and Germany. Clin. Outcomes Res. 2017, 9, 763–774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017. [Google Scholar]
- Jaruvongvanich, V.; Worasilchai, N.; Kongnatthasate, K.; Jaruvongvanich, S.; Damkerngsuntorn, W.; Atikarnbodee, D.; Thammahong, A.; Lerdlitruangsin, S.; Chindamporn, A. Correlation between broth microdilution, E-test and disk diffusion methods for testing antifungal susceptibility of Candida species isolated from Thai blood samples. Asian Biomed. 2016, 10, 75–80. [Google Scholar]
- Israel, S.; Perlman, A.; Moran-Gilad, J.; Korem, M. Direct fluconazole disk susceptibility testing for Candida glabrata-positive blood cultures. J. Clin. Microbiol. 2021, 59, e0031121. [Google Scholar] [CrossRef]
- Nunnally, N.S.; Damm, T.; Lockhart, S.R.; Berkow, E.L. Categorizing susceptibility of clinical isolates of Candida auris to amphotericin B, caspofungin, and fluconazole by use of the CLSI M44-A2 disk diffusion method. J. Clin. Microbiol. 2021, 59, e02355-20. [Google Scholar] [CrossRef]
- Jeon, S.; Shin, J.H.; Lim, H.J.; Choi, M.J.; Byun, S.A.; Lee, D.; Lee, S.Y.; Won, E.J.; Kim, S.H.; Shin, M.G. Disk diffusion susceptibility testing for the rapid detection of fluconazole resistance in Candida Isolates. Ann. Lab. Med. 2021, 41, 559–567. [Google Scholar] [CrossRef] [PubMed]
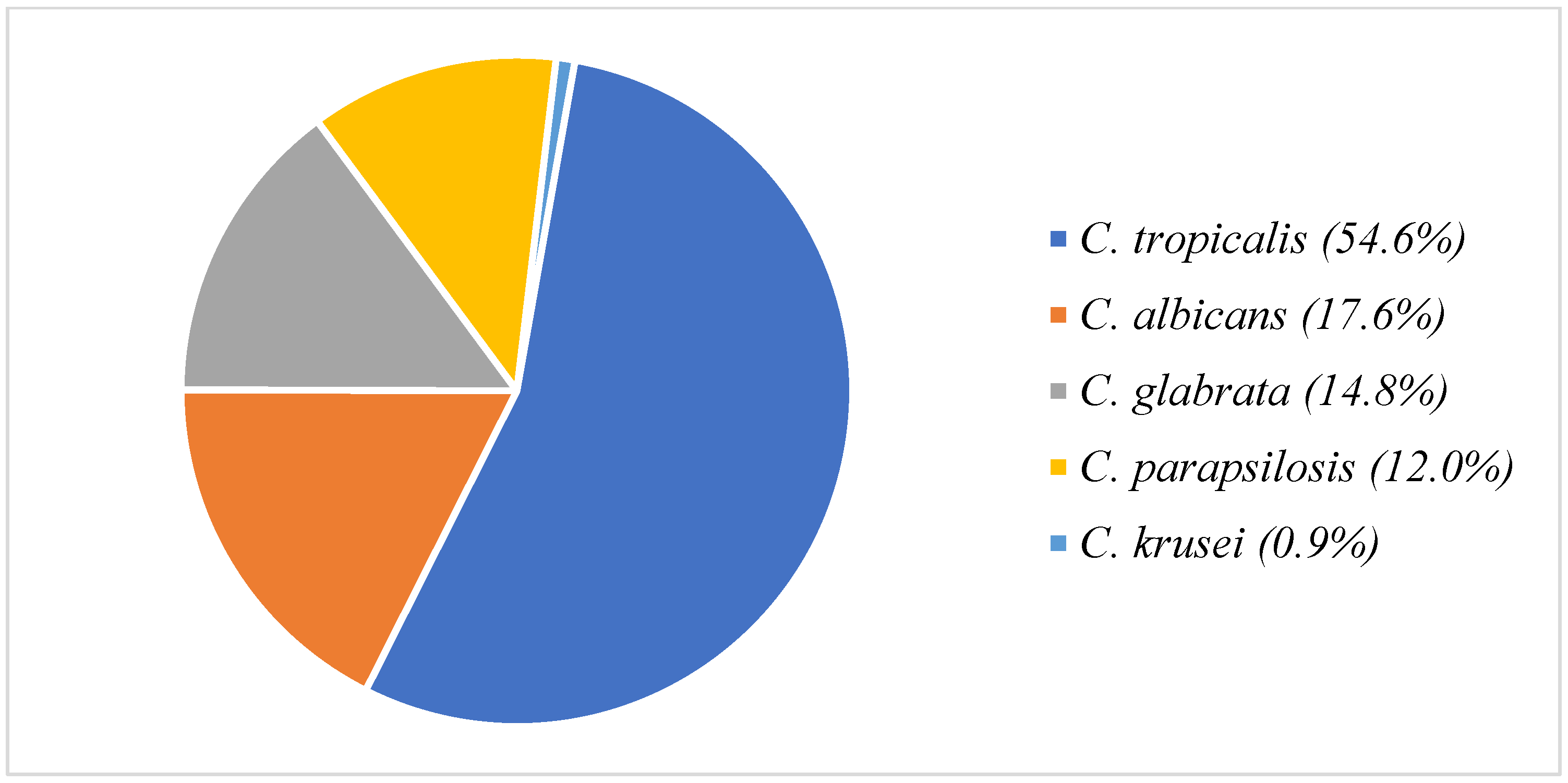
| Clinical Characteristics | DD Group (N = 54) | BMD Group (N = 54) | p-Value |
|---|---|---|---|
| Age (years), mean (SD) | 61.18 (2.4) | 61.89 (2.3) | 0.236 |
| Sex, Male | 30 (55.6%) | 36 (66.7%) | 0.724 |
| Clinical data at diagnosis of candidemia, N (%) | |||
| Central venous catheter | 38 (70.4%) | 38 (70.4%) | 1.000 |
| Mechanical ventilator | 33 (61.1%) | 31 (57.4%) | 0.695 |
| Parenteral nutrition | 25 (46.6%) | 18 (33.3%) | 0.169 |
| Hemodialysis | 17 (31.5%) | 21 (38.9%) | 0.420 |
| Peritoneal dialysis | 0 | 1 (1.9%) | 1.000 |
| Recent abdominal surgery | 8 (14.8%) | 9 (16.7%) | 0.792 |
| ICU admission | 25 (46.3%) | 27 (50.0%) | 0.700 |
| Urinary catheter | 39 (72.2%) | 40 (74.1%) | 0.823 |
| Presence of prosthesis | 4 (7.4%) | 12 (22.2%) | 0.030 |
| Receive carbapenem >7 days | 26 (48.1%) | 28 (51.9%) | 0.700 |
| Receive cephalosporin >7 days | 9 (16.7%) | 5 (9.3%) | 0.252 |
| Receive fluoroquinolone >7 days | 5 (9.3%) | 11 (20.4%) | 0.104 |
| Receive at least two ATB >10 days | 13 (24.1%) | 8 (14.8%) | 0.224 |
| Receive at least three ATB >10 days | 3 (5.6%) | 9 (16.7%) | 0.066 |
| Receive at least four ATB >10 days | 8 (18.4%) | 3 (5.6%) | 0.112 |
| Receive corticosteroids * | 5 (9.3%) | 10 (18.5%) | 0.164 |
| ANC < 500 cells/mm3 | 12 (22.2%) | 10 (18.5%) | 0.633 |
| Presence of yeast in urine | 25 (53.2%) | 31 (67.4%) | 0.162 |
| Presence of yeast in sputum | 21 (46.7%) | 24 (52.2%) | 0.599 |
| Presence of yeast in feces | 3 (14.3%) | 5 (31.2%) | 0.254 |
| Prior antifungal exposure ** | 8 (14.8%) | 12 (22.2%) | 0.322 |
| Prior azoles exposure ** | 7 (13.0%) | 10 (18.5%) | 0.428 |
| Co-morbidities, N (%) | |||
| Chronic cardiac disease | 10 (18.5%) | 11 (20.4%) | 0.808 |
| Chronic lung disease | 3 (5.6%) | 7 (13.0%) | 0.184 |
| Chronic kidney disease | 14 (25.9%) | 21 (38.9%) | 0.150 |
| Chronic liver disease | 3 (5.6%) | 6 (11.1%) | 0.489 |
| Diabetes mellitus | 16 (29.6%) | 17 (31.5%) | 0.835 |
| HIV infection | 0 | 1 (1.9%) | 1.000 |
| Autoimmune disease | 4 (7.4%) | 4 (7.4%) | 1.000 |
| Hematological malignancies | 11 (20.4%) | 11 (20.4%) | 1.000 |
| Hematopoietic stem cell transplantation | 2 (3.7%) | 2 (3.7%) | 1.000 |
| Solid organ transplantation | 1 (1.19%) | 3 (5.6%) | 0.618 |
| Laboratory at diagnosis of candidemia | |||
| AST (U/L) | 30 | 37 | 0.353 |
| ALT (U/L) | 24 | 20 | 0.805 |
| Creatinine (mg/dL) | 1.69 | 1.25 | 0.783 |
| DD Group (N = 54) | BMD Group (N = 54) | p-Value | |
|---|---|---|---|
| Primary outcomes | |||
| Proportion of antifungal de-escalation within 72 h, N (%) | 14 (25.9%) | 5 (9.3%) | 0.023 |
| Median time to antifungal de-escalation, days (IQR) | 3 (2.0–5.0) | 6 (3.0–9.0) | 0.037 |
| Secondary outcomes | |||
| Fourteen-day mortality, N (%) | 12 (23.5%) | 20 (37.0%) | 0.133 |
| Thirty-day mortality, N (%) | 20 (41.7%) | 28 (53.8%) | 0.223 |
| Median length of stay, days (IQR) | 17 (10.0–37.5) | 15 (8.0–28.5) | 0.239 |
| Median antifungal cost, Baht (IQR) | 33,575 (13,581–67,113) | 48,470 (97,750–134,333) | 0.621 |
| Treatment-related complications, N (%) | |||
| -Acute kidney injury | 9 (29.0%) | 10 (33.3%) | 0.717 |
| -Hypokalemia | 23 (62.2%) | 15 (45.5%) | 0.161 |
| -Hepatitis | 6 (11.1%) | 7 (13.0%) | 0.767 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantasuwan, S.; Chongtrakool, P.; Waesamaae, A.; Chayakulkeeree, M. Impact of the Disk Diffusion Test on Fluconazole De-Escalation in Patients with Candidemia. J. Fungi 2022, 8, 1185. https://doi.org/10.3390/jof8111185
Tantasuwan S, Chongtrakool P, Waesamaae A, Chayakulkeeree M. Impact of the Disk Diffusion Test on Fluconazole De-Escalation in Patients with Candidemia. Journal of Fungi. 2022; 8(11):1185. https://doi.org/10.3390/jof8111185
Chicago/Turabian StyleTantasuwan, Suchavadee, Piriyaporn Chongtrakool, Amiroh Waesamaae, and Methee Chayakulkeeree. 2022. "Impact of the Disk Diffusion Test on Fluconazole De-Escalation in Patients with Candidemia" Journal of Fungi 8, no. 11: 1185. https://doi.org/10.3390/jof8111185
APA StyleTantasuwan, S., Chongtrakool, P., Waesamaae, A., & Chayakulkeeree, M. (2022). Impact of the Disk Diffusion Test on Fluconazole De-Escalation in Patients with Candidemia. Journal of Fungi, 8(11), 1185. https://doi.org/10.3390/jof8111185






