Morphological and Transcriptional Characteristics of the Symbiotic Interaction between Pinus massoniana and Suillus bovinus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Materials
2.2. In Vitro Mycorrhizal Formation between P. massoniana and S. bovinus
2.3. Morphological Observations
2.4. RNA Extraction, Sequencing and Analysis
2.5. Determination of Differentially Expressed Genes and Enrichment Analysis
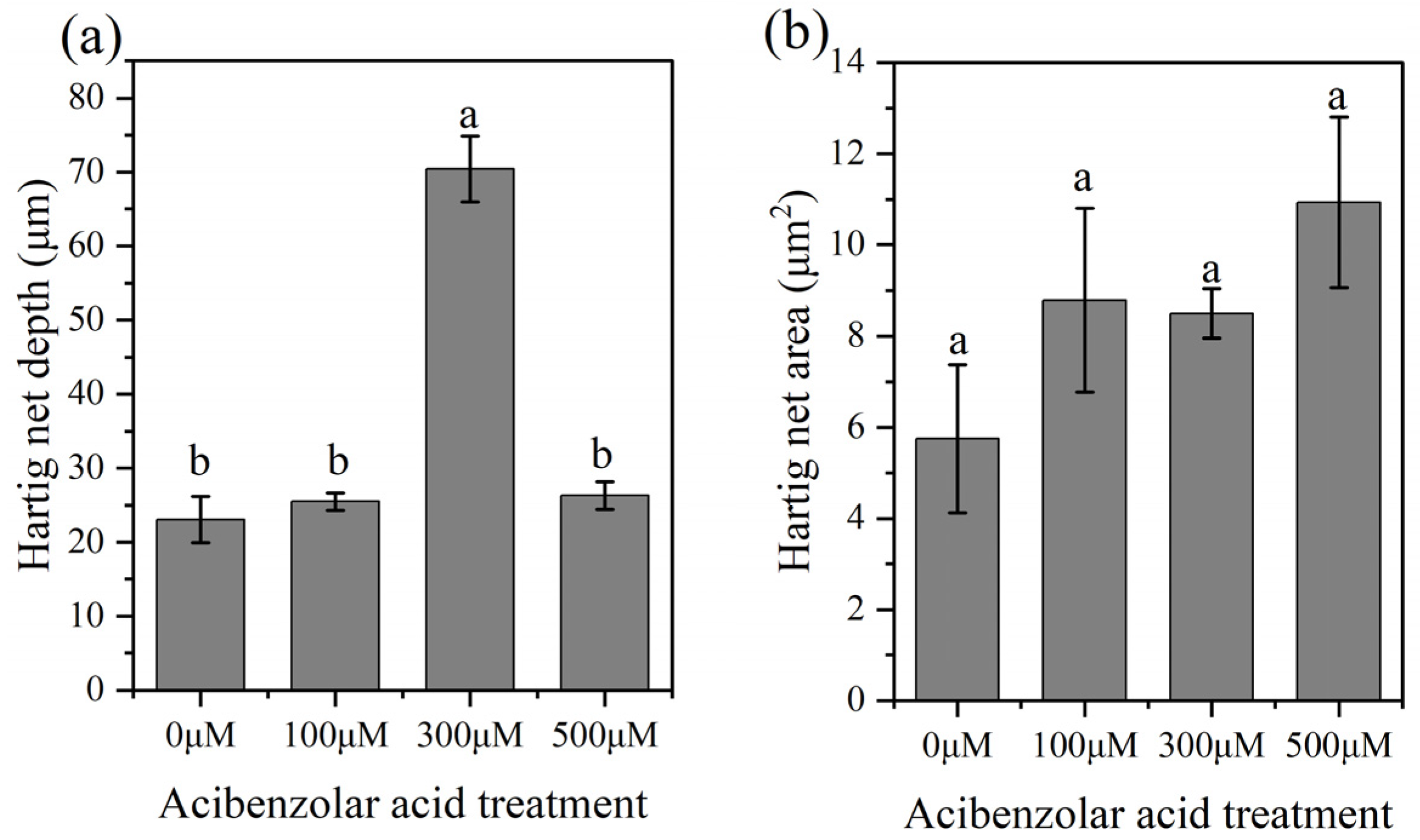
2.6. Effect of Acibenzolar Acid on Mycorrhizal Development
2.7. Statistical Analysis
3. Results
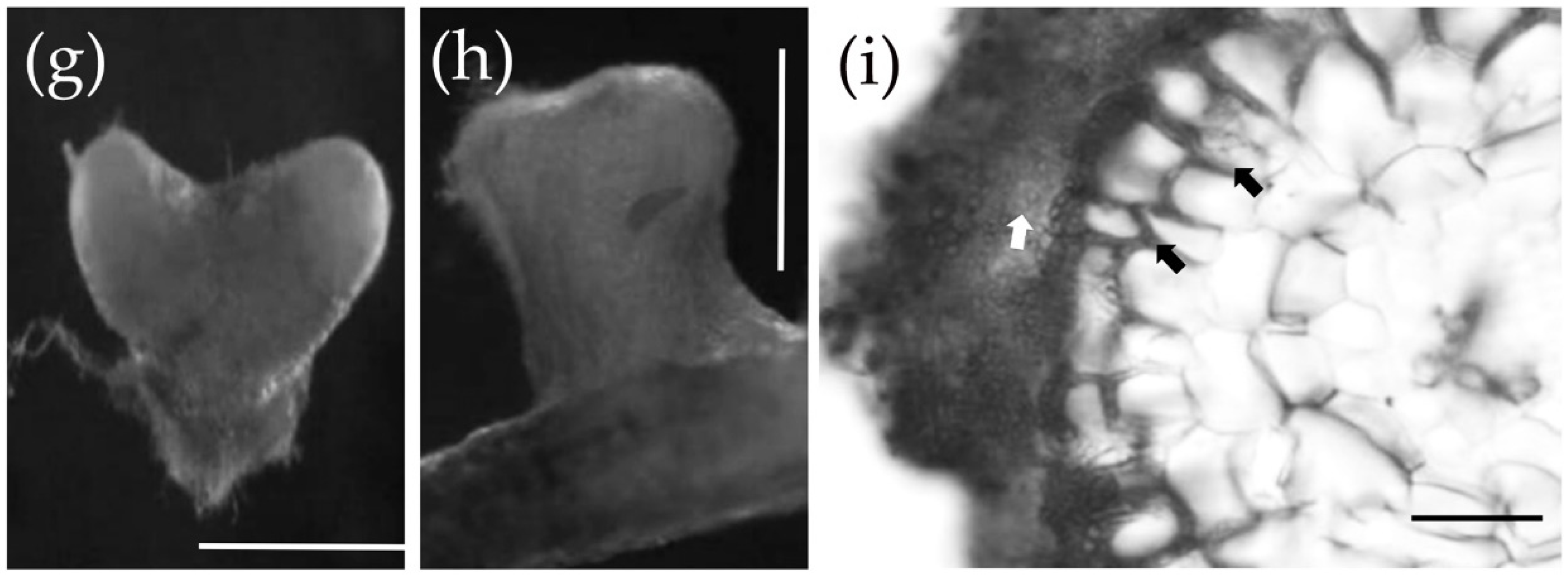
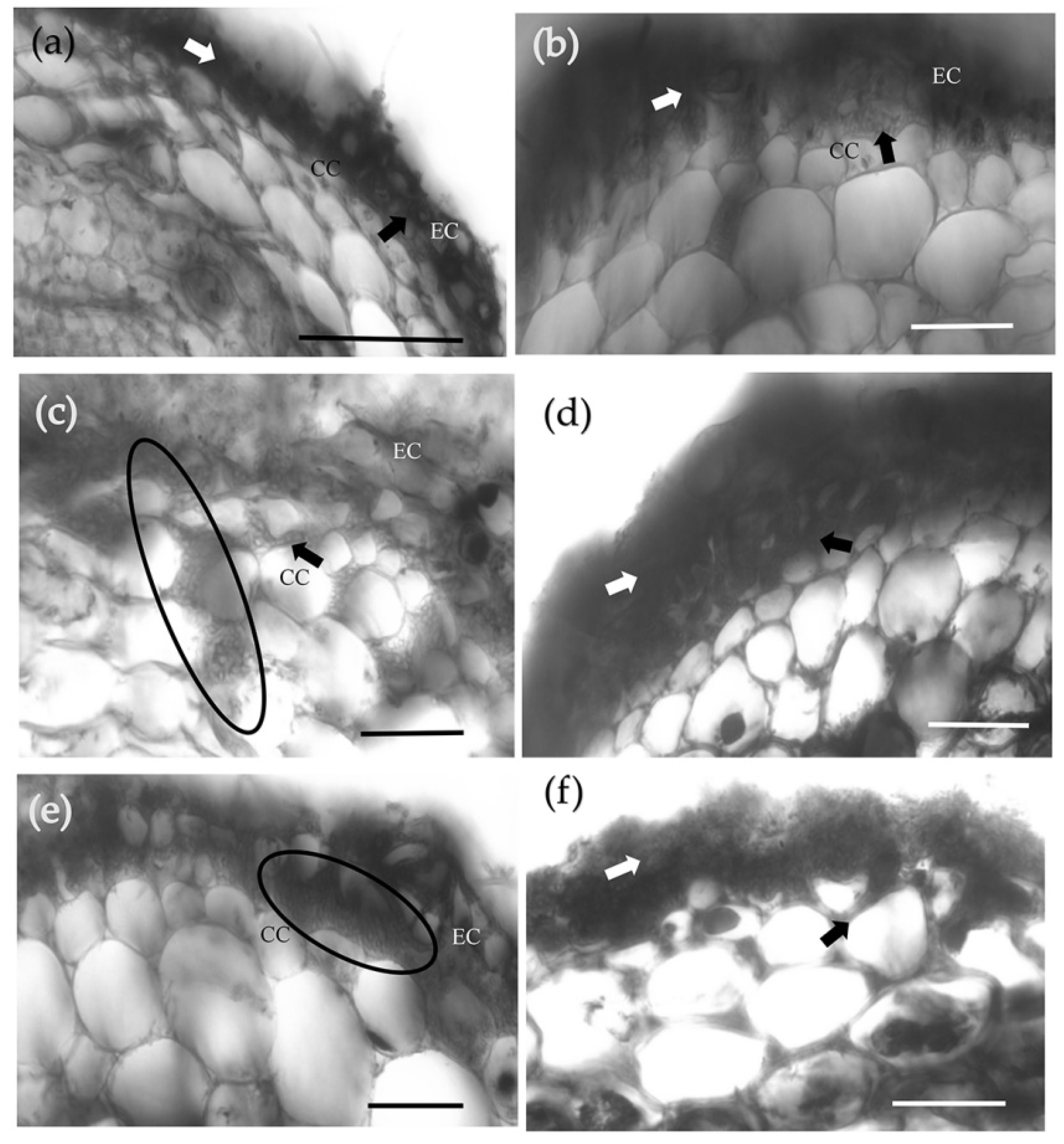
3.1. Pre-Symbiotic Interactions between P. massoniana and S. bovinus
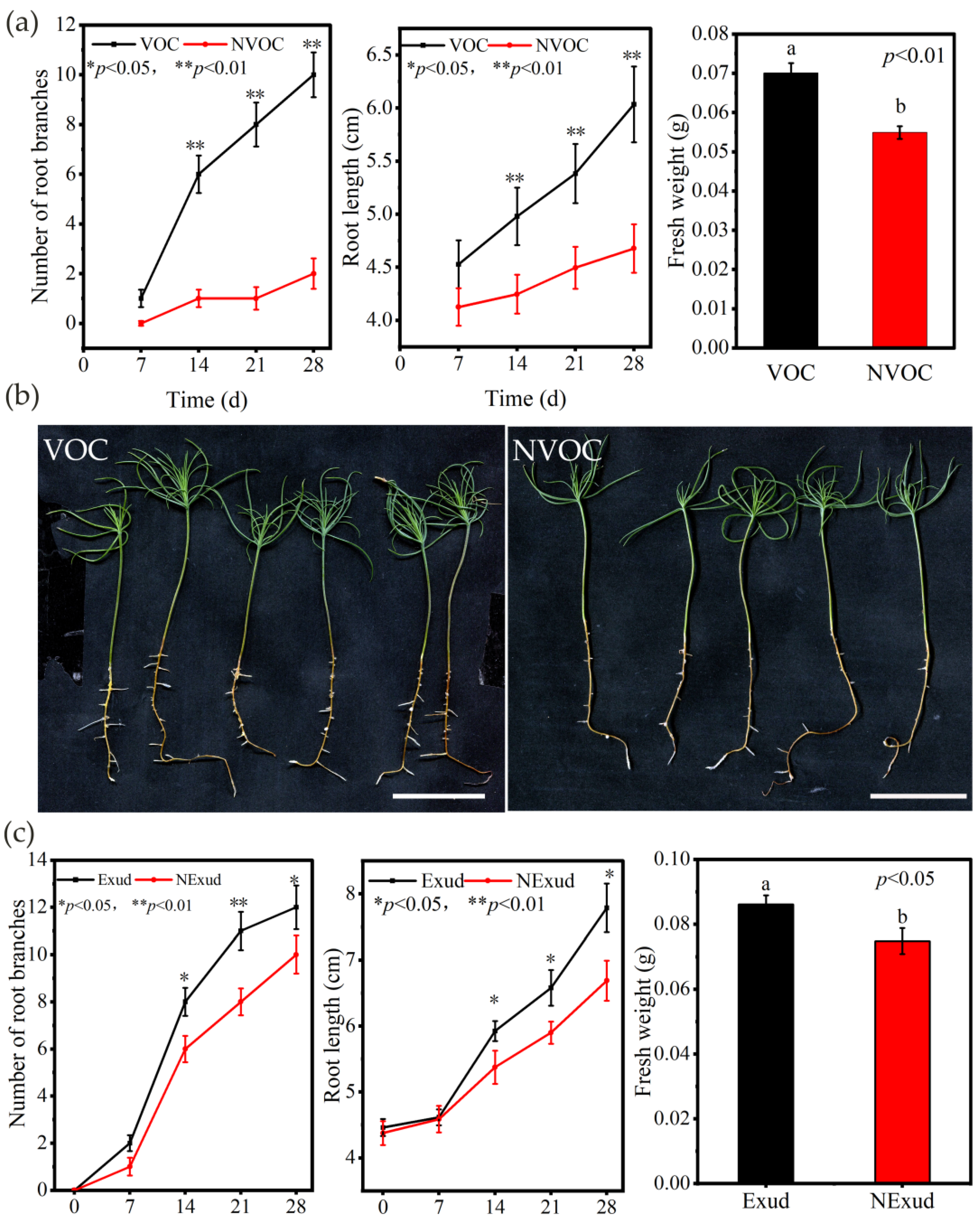
3.2. Symbiotic Interactions between P. massoniana Roots and S. bovinus over Time
3.3. Root Transcriptional Analysis during Symbiotic Interactions between P. massoniana and S. bovinus
3.3.1. Quality Analysis
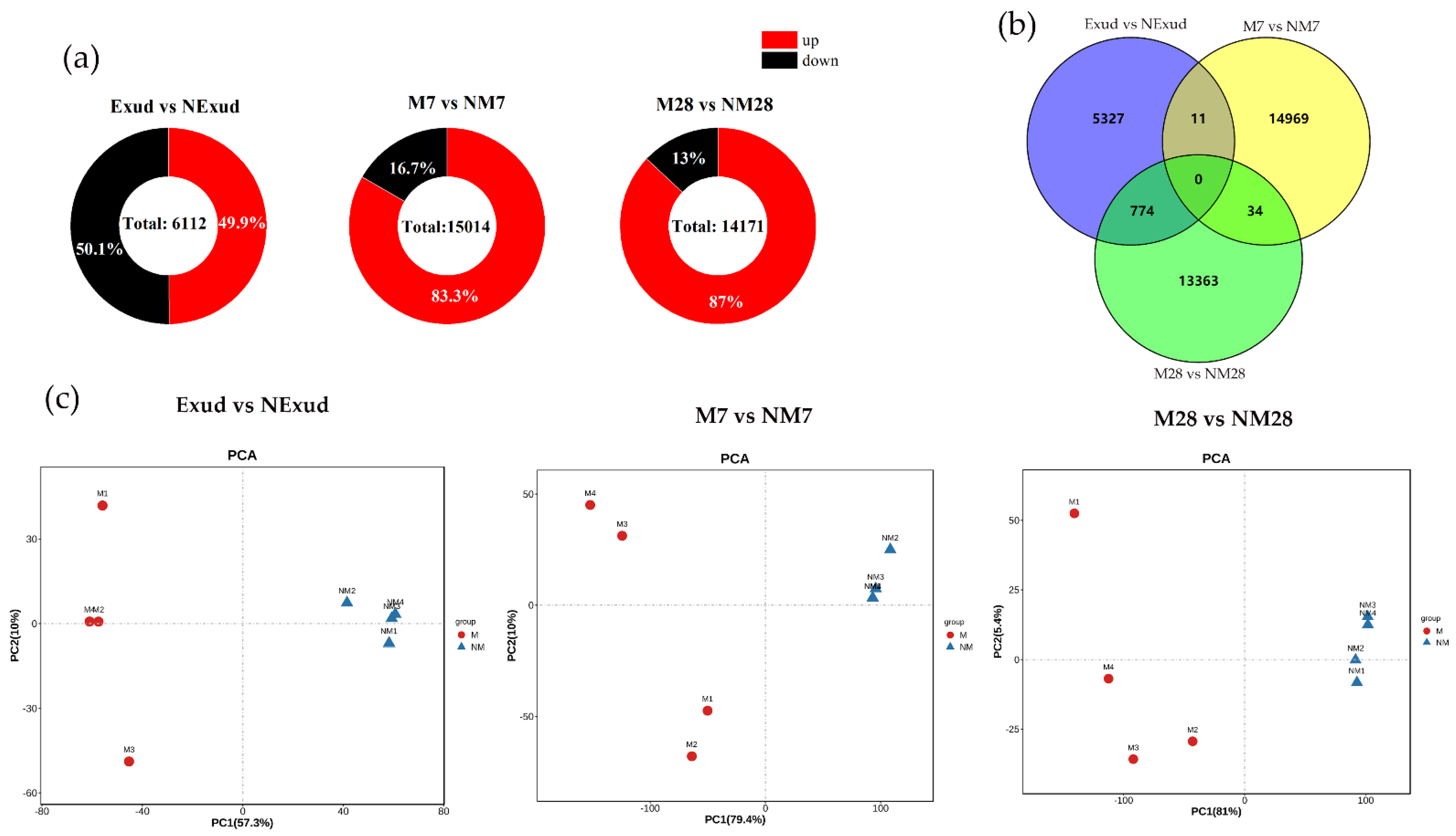
3.3.2. Analysis of DEGs
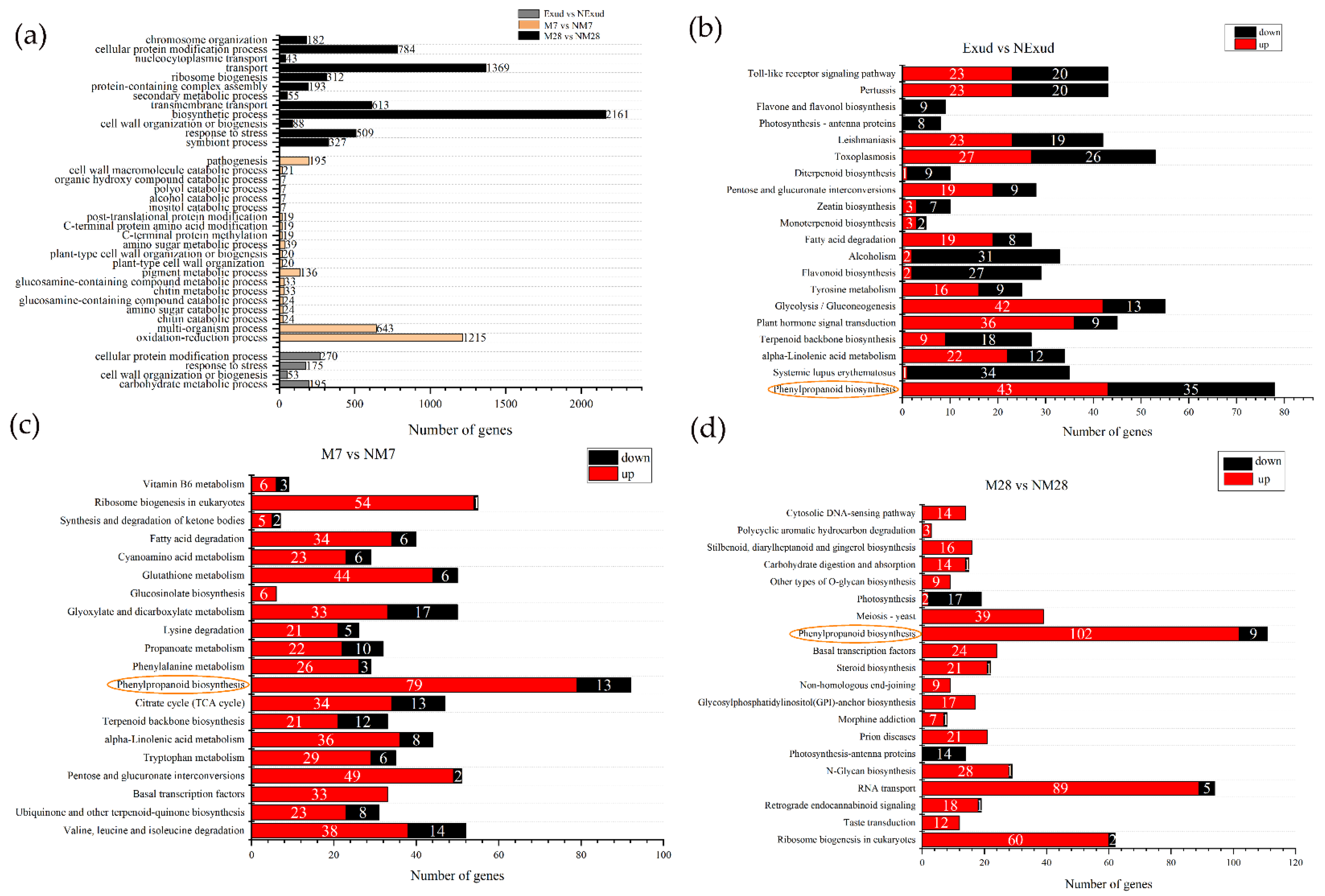
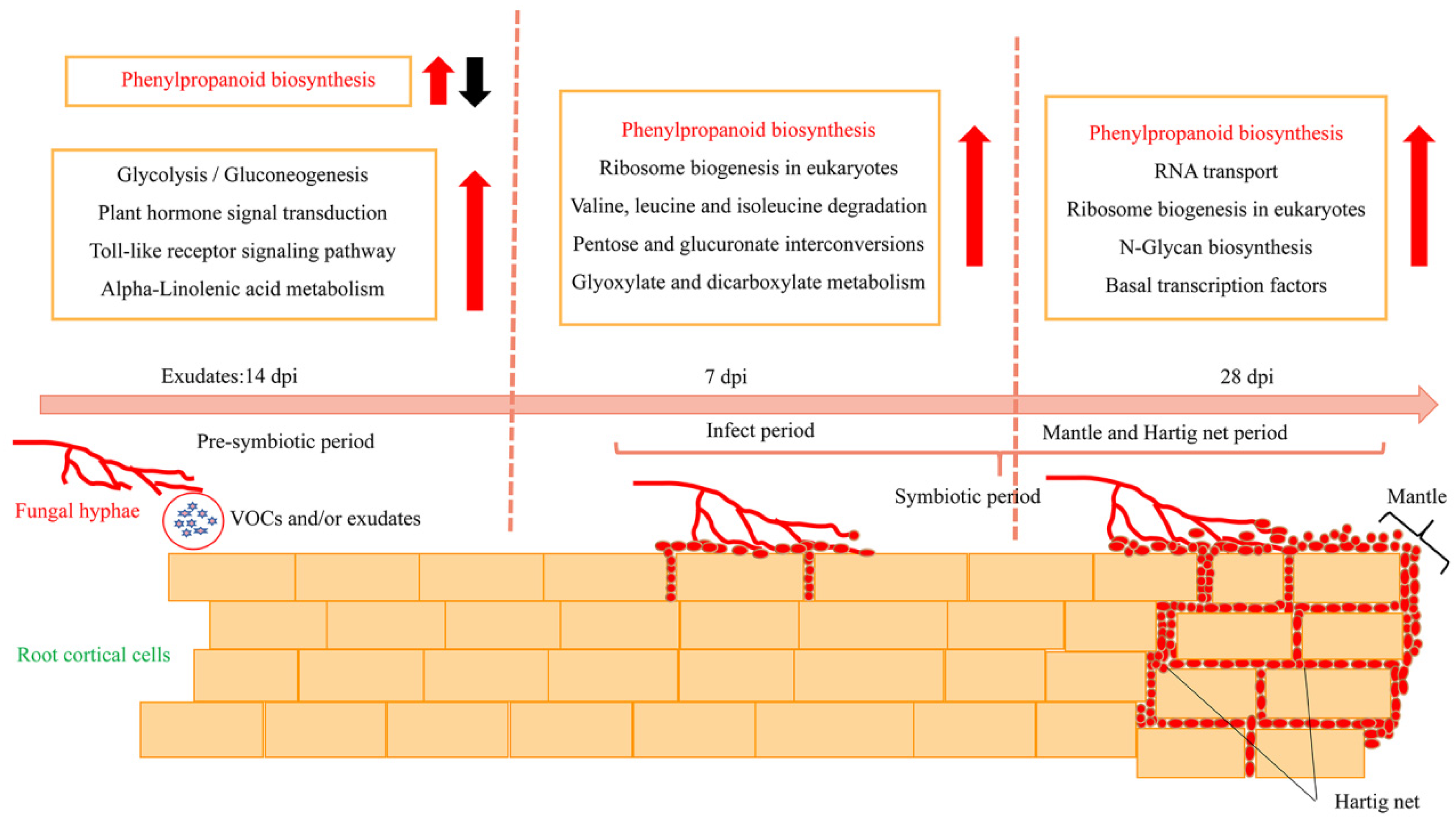
3.3.3. Enrichment Analysis of DEGs
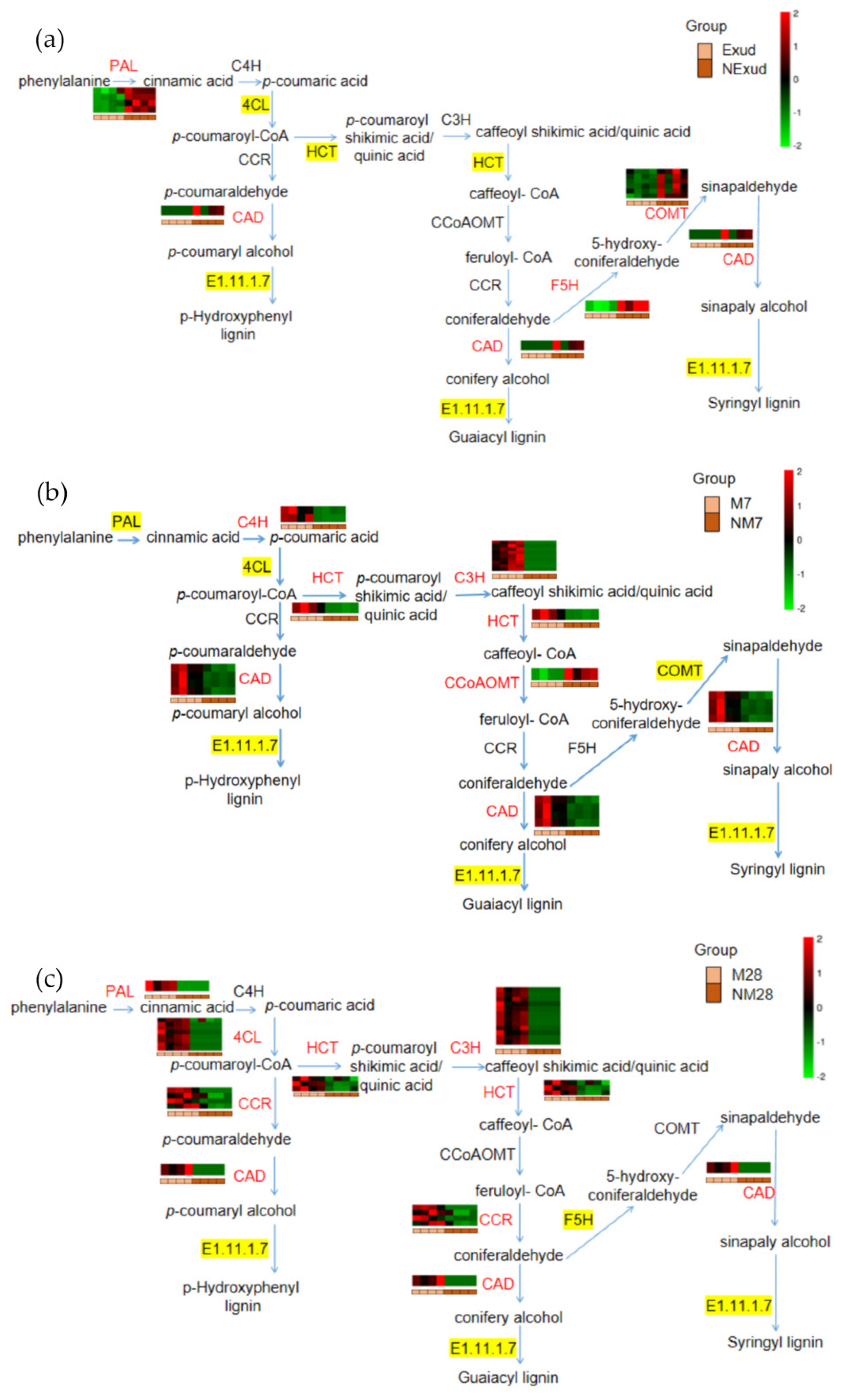
3.3.4. Genes Involved in the Phenylpropanoid Biosynthesis Pathway throughout Colonization
3.4. Effect of Inhibiting HCT Activity on ECM Morphogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; pp. 192–268. [Google Scholar]
- Becquer, A.; Guerrero-Galán, C.; Eibensteiner, J.L.; Houdinet, G.; Bücking, H.; Zimmermann, S.D.; Garcia, K. The ectomycorrhizal contribution to tree nutrition. In Advances in Botanical Research; Cánovas, F.M., Ed.; Academic Press: London, UK, 2019; Volume 89, pp. 77–126. [Google Scholar]
- Nehls, U.; Plassard, C. Nitrogen and phosphate metabolism in ectomycorrhizas. New Phytol. 2018, 220, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zak, D.R.; Pellitier, P.T.; Argiroff, W.; Castillo, B.; James, T.Y.; Nave, L.E.; Averill, C.; Beidler, K.V.; Bhatnagar, J.; Blesh, J.; et al. Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytol. 2019, 223, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehls, U.; Grunze, N.; Willmann, M.; Reich, M.; Kuster, H. Sugar for my honey: Carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry 2007, 68, 82–91. [Google Scholar] [CrossRef]
- Casieri, L.; Ait Lahmidi, N.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A.; Bonneau, L.; Courty, P.E.; Garcia, K.; Charbonnier, M.; Delteil, A.; et al. Biotrophic transportome in mutualistic plant–fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi–potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, F.Q.; Zhang, C.; Liu, C.; Yang, M.; Li, S. Edible ectomycorrhizal fungi and their cultivation in China. In Mushrooms, Humans and Nature in a Changing World; Pérez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F.Q., Eds.; Springer: Cham, Germany, 2020; pp. 31–60. [Google Scholar]
- Zhao, Y.Z.; Sun, X.G.; Feng, J.W.; Guo, Q.Q. Diversity of edible mycorrhizal fungi in Pinus massoniana forests of central Guizhou, southwest China. Mycosystema 2021, 40, 108–123. (In Chinese) [Google Scholar]
- Horan, D.P.; Chilvers, G.A. Chemotropism-the key to ectomycorrhizal formation? New Phytol. 1990, 116, 297–301. [Google Scholar] [CrossRef]
- Lagrange, H.; Jay-Allemand, C.; Lapeyrie, F. Rutin, the phenolglycoside from eucalyptus root exudates, stimulates Pisolithus hyphal growth at picomolar concentrations. New Phytol. 2001, 149, 349–355. [Google Scholar] [CrossRef]
- Kikuchi, K.; Matsushita, N.; Suzuki, K.; Hogetsu, T. Flavonoids induce germination of basidiospores of the ectomycorrhizal fungus Suillus bovinus. Mycorrhiza 2007, 17, 563–570. [Google Scholar] [CrossRef]
- Splivallo, R.; Fischer, U.; Göbel, C.; Feussner, I.; Karlovsky, P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009, 150, 2018–2029. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.; Henke, C.; Asiimwe, T.; Ulbricht, A.; Klemmer, S.; Schachtschabel, D.; Boland, W.; Kothe, E. Biosynthesis and secretion of indole-3-acetic acid and its morphological effects on Tricholoma vaccinum–spruce ectomycorrhiza. App. Environ. Microb. 2015, 81, 7003–7011. [Google Scholar] [CrossRef] [PubMed]
- Ditengou, F.A.; Müller, A.; Rosenkranz, M.; Felten, J.; Lasok, H.; van Doorn, M.M.; Legué, V.; Palme, K.; Schnitzler, J.P.; Polle, A. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat. Commun. 2015, 6, 6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, K.; Delaux, P.M.; Cope, K.R.; Ané, J.M. Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses. New Phytol. 2015, 208, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, Y.; Plett, J.M.; Veneault-Fourrey, C. Signaling pathways driving the development of ectomycorrhizal symbiosis. In Molecular Mycorrhizal Symbiosis; Martin, F., Ed.; John Wiley & Sons, Inc.: Hoboken, UK, 2016; pp. 141–157. [Google Scholar]
- Feng, W.Y.; Sun, X.G.; Ding, G.J. Advance of ectomycorrhizal signal recognition mechanism in pre-symbiosis. Plant Physiol. J. 2021, 57, 749–758. (In Chinese) [Google Scholar]
- Martin, F.; Duplessis, S.; Ditengou, F.; Lagrange, H.; Voiblet, C.; Lapeyrie, F. Developmental cross talking in the ectomycorrhizal symbiosis: Signals and communication genes. New Phytol. 2001, 151, 145–154. [Google Scholar] [CrossRef]
- Plett, J.M.; Tisserant, E.; Brun, A.; Morin, E.; Grigoriev, I.V.; Kuo, A.; Martin, F.; Kohler, A. The mutualist Laccaria bicolor expresses a core gene regulon during the coloniza-tion of diverse host plants and a variable regulon to counteract host-specific defenses. Mol. Plant Microbe Interact. 2015, 28, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, J.; Kemppainen, M.; Delhomme, N.; Shutava, I.; Zhou, J.J.; Takahashi, J.; Pardo, A.G.; Lundberg-Felten, J. Laccaria bicolor pectin methylesterases are involved in ectomycorrhiza development with Populus tremula × Populus tremuloides. New Phytol. 2022, 236, 639–655. [Google Scholar] [CrossRef]
- Voiblet, C.; Duplessis, S.; Encelot, N.; Martin, F. Identification of symbiosis-regulated genes in Eucalyptus globulus–Pisolithus tinctorius ectomycorrhiza by differential hybridization of arrayed cDNAs. Plant J. 2001, 25, 181–191. [Google Scholar] [CrossRef]
- Hogekamp, C.; Küster, H. A roadmap of cell-type specific gene expression during sequential stages of the arbuscular mycorrhiza symbiosis. BMC Genom. 2013, 14, 306. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.E.; Sreedasyam, A.; Trivedi, G.; Desai, S.; Dai, Y.; Cseke, L.J.; Collart, F.R. Multi-omics approach identifies molecular mechanisms of plant-fungus mycorrhizal interaction. Front. Plant Sci. 2016, 6, 1061. [Google Scholar] [CrossRef] [Green Version]
- Johansson, T.; Le Quéré, A.; Ahren, D.; Söderström, B.; Erlandsson, R.; Lundeberg, J.; Uhlén, M.; Tunlid, A. Transcriptional responses of Paxillus involutus and Betula pendula during formation of ectomycorrhizal root tissue. Mol. Plant Microbe. Interact. 2004, 17, 202–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duplessis, S.; Courty, P.E.; Tagu, D.; Martin, F. Transcript patterns associated with ectomycorrhiza development in Eucalyptus globulus and Pisolithus microcarpus. New Phytol. 2005, 165, 599–611. [Google Scholar] [CrossRef]
- Le Quéré, A.; Wright, D.P.; Söderström, B.; Tunlid, A.; Johansson, T. Global patterns of gene regulation associated with the development of ectomycorrhiza between birch (Betula pendula Roth.) and Paxillus involutus (Batsch) Fr. Mol. Plant Microbe. Interact. 2005, 18, 659–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Coelho, I.; de Queiroz, M.V.; Costa, M.D.; Kasuya, M.C.M.; de Araújo, E.F. Identification of differentially expressed genes of the fungus Hydnangium sp. during the pre-symbiotic phase of the ectomycorrhizal association with Eucalyptus grandis. Mycorrhiza 2010, 20, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Flores-Monterroso, A.; Canales, J.; de la Torre, F.; Ávila, C.; Cánovas, F.M. Identification of genes differentially expressed in ectomycorrhizal roots during the Pinus pinaster–Laccaria bicolor interaction. Planta 2013, 237, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Sebastiana, M.; Vieira, B.; Lino-Neto, T.; Monteiro, F.; Figueiredo, A.; Sousa, L.; Salomé Pais, M.; Tavares, R.; Paulo, O.S. Oak root response to ectomycorrhizal symbiosis establishment: RNA-Seq derived transcript identification and expression profiling. PLoS ONE 2014, 9, e98376. [Google Scholar]
- Li, H.; Peng, H.; Wang, L.; Wei, H.; Li, N.; Jing, Q. Identification of fungal genes involved in the preinfection events between ectomycorrhizal association (Pisolithus tinctorius and Pinus massoniana). Mycol. Prog. 2014, 13, 123–130. [Google Scholar] [CrossRef]
- Doré, J.; Perraud, M.; Dieryckx, C.; Kohler, A.; Morin, E.; Henrissat, B.; Lindquist, E.; Zimmermann, S.D.; Girard, V.; Kuo, A.; et al. Comparative genomics, proteomics and transcriptomics give new insight into the exoproteome of the basidiomycete Hebeloma cylindrosporum and its involvement in ectomycorrhizal symbiosis. New Phytol. 2015, 208, 1169–1187. [Google Scholar] [CrossRef]
- Plett, K.L.; Raposo, A.E.; Anderson, I.C.; Piller, S.C.; Plett, J.M. Protein arginine methyltransferase expression affects ectomycorrhizal symbiosis and the regulation of hormone signaling pathways. Mol. Plant Microbe Interact. 2019, 32, 1291–1302. [Google Scholar] [CrossRef]
- Bouffaud, M.L.; Herrmann, S.; Tarkka, M.T.; Bönn, M.; Feldhahn, L.; Buscot, F. Oak displays common local but specific distant gene regulation responses to different mycorrhizal fungi. BMC Genom. 2020, 21, 399. [Google Scholar] [CrossRef]
- Tang, N.; Lebreton, A.; Xu, W.; Dai, Y.; Yu, F.; Martin, F.M. Transcriptome profiling reveals differential gene expression of secreted proteases and highly specific gene repertoires involved in Lactarius–Pinus symbioses. Front. Plant Sci. 2021, 12, 1775. [Google Scholar] [CrossRef] [PubMed]
- Doré, J.; Kohler, A.; Dubost, A.; Hundley, H.; Singan, V.; Peng, Y.; Kuo, A.; Grigoriev, I.V.; Martin, F.; Marmeisse, R.; et al. The ectomycorrhizal basidiomycete Hebeloma cylindrosporum undergoes early waves of transcriptional reprogramming prior to symbiotic structures differentiation. Environ. Microbiol. 2017, 19, 1338–1354. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.L.; Chen, Y.; Vilgalys, R. Metatranscriptomic study of common and host-specific patterns of gene expression between pines and their symbiotic ectomycorrhizal fungi in the genus Suillus. PLoS Genet. 2016, 12, e1006348. [Google Scholar] [CrossRef]
- Heller, G.; Adomas, A.; Li, G.; Osborne, J.; Zyl, L.V.; Sederoff, R.; Finlay, R.D.; Stenlid, J.; Asiegbu, F.O. Transcriptional analysis of Pinus sylvestris roots challenged with the ectomycorrhizal fungus Laccaria bicolor. BMC Plant Biol. 2008, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veneault-Fourrey, C.; Commun, C.; Kohler, A.; Morin, E.; Balestrini, R.; Plett, J.; Danchin, E.; Coutinho, P.; Wiebenga, A.; de Vries, R.P.; et al. Genomic and transcriptomic analysis of Laccaria bicolor CAZome reveals insights into polysaccharides remodelling during symbiosis establishment. Fungal Genet Biol. 2014, 72, 168–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastiana, M.; Figueiredo, A.; Acioli, B.; Sousa, L.; Pessoa, F.; Baldé, A.; Pais, M.S. Identification of plant genes involved on the initial contact between ectomycorrhizal symbionts (Castanea sativa–European chestnut and Pisolithus tinctorius). Eur. J. Soil Biol. 2009, 45, 275–282. [Google Scholar] [CrossRef]
- Plett, J.M.; Kemppainen, M.; Kale, S.D.; Kohler, A.; Legué, V.; Brun, A.; Tyler, B.M.; Pardo, A.G.; Martin, F. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 2011, 21, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Plett, J.M.; Daguerre, Y.; Wittulsky, S.; Vayssieres, A.; Deveau, A.; Melton, S.J.; Brun, A.; Veneault-Fourrey, C.; Martin, F. Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc. Natl. Acad. Sci. USA 2014, 111, 8299–8304. [Google Scholar] [CrossRef] [Green Version]
- Daguerre, Y.; Basso, V.; Hartmann-Wittulski, S.; Schellenberger, R.; Meyer, L.; Bailly, J.; Kohler, A.; Plett, J.M.; Martin, F.; Veneault-Fourrey, C. The mutualism effector MiSSP7 of Laccaria bicolor alters the interactions between the Poplar JAZ6 protein and its associated proteins. Sci. Rep. 2020, 10, 20362. [Google Scholar] [CrossRef]
- Kang, H.; Chen, X.; Kemppainen, M.; Pardo, A.G.; Veneault-Fourrey, C.; Kohler, A.; Kohler, A.; Martin, F.M. The small secreted effector protein MiSSP7.6 of Laccaria bicolor is required for the establishment of ectomycorrhizal symbiosis. Environ. Microbiol. 2020, 22, 1435–1446. [Google Scholar] [CrossRef]
- Pellegrin, C.; Daguerre, Y.; Ruytinx, J.; Guinet, F.; Kemppainen, M.; Frey, N.F.D.; Puech-Pagès, V.; Hecker, A.; Pardo, A.; Martin, F.; et al. Laccaria bicolor MiSSP8 is a small-secreted protein decisive for the establishment of the ectomycorrhizal symbiosis. Environ. Microbiol. 2019, 21, 3765–3779. [Google Scholar] [CrossRef] [PubMed]
- Plett, J.M.; Plett, K.L.; Wong-Bajracharya, J.; Freitas Pereira, M.; Costa, M.D.; Kohler, A.; Martin, F.; Anderson, I.C. Mycorrhizal effector PaMiSSP10b alters polyamine biosynthesis in Eucalyptus root cells and promotes root colonization. New Phytol. 2020, 228, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Kim, S.J.; Podila, G.K. Differential expression of a malate synthase gene during the preinfection stage of symbiosis in the ectomycorrhizal fungus Laccaria bicolor. New Phytol. 2002, 154, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Anasontzis, G.E.; Labourel, A.; Champion, C.; Haon, M.; Kemppainen, M.; Commun, C.; Deveau, A.; Pardo, A.; Veneault-Fourrey, C.; et al. The ectomycorrhizal basidiomycete Laccaria bicolor releases a secreted β-1,4 endoglucanase that plays a key role in symbiosis development. New Phytol. 2018, 220, 1309–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Labourel, A.; Haon, M.; Kemppainen, M.; Machado, E.D.S.; Brouilly, N.; Veneault-Fourrey, C.; Kohler, A.; Rosso, M.N.; Pardo, A.; et al. The ectomycorrhizal basidiomycete Laccaria bicolor releases a GH28 polygalacturonase that plays a key role in symbiosis establishment. New Phytol. 2022, 233, 2534–2547. [Google Scholar] [CrossRef]
- Becquer, A.; Garcia, K.; Amenc, L.; Rivard, C.; Doré, J.; Trives-Segura, C.; Szponarski, W.; Russet, S.; Baeza, Y.; Lassalle-Kaiser, B.; et al. The Hebeloma cylindrosporum HcPT2 Pi transporter plays a key role in ectomycorrhizal symbiosis. New Phytol. 2018, 220, 1185–1199. [Google Scholar] [CrossRef] [Green Version]
- Lucic, E.; Fourrey, C.; Kohler, A.; Martin, F.; Chalot, M.; Brun-Jacob, A. A gene repertoire for nitrogen transporters in Laccaria bicolor. New Phytol. 2008, 180, 343–364. [Google Scholar] [CrossRef]
- Martin, F.; Aerts, A.; Ahrén, D.; Brun, A.; Danchin, E.G.J.; Duchaussoy, F.; Gibon, J.; Kohler, A.; Lindquist, E.; Pereda, V.; et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 2008, 452, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Tranvan, H.; Habricot, Y.; Jeannette, E.; Gay, G.; Sotta, B. Dynamics of symbiotic establishment between an IAA-overproducing mutant of the ectomycorrhizal fungus Hebeloma cylindrosporum and Pinus pinaster. Tree Physiol. 2000, 20, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q. Studies on symbiotic mycorrhiza fungi with Masson pine. For. Res. 1989, 2, 357–362. (In Chinese) [Google Scholar]
- Zhou, Z.X. Chinese Masson Pine; China Forestry Press: Beijing, China, 2001. [Google Scholar]
- Ding, G.; Zhou, Z.; Wang, Z. Cultivation and Utilization of Pulpwood Stand for Pinus massoniana; China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- Wu, X.Q.; Sun, M.Q. Mycorrhizal formation between seven ectomycorrhizal fungi and seedlings of three pines species. Acta Ecol. Sin. 2006, 26, 4186–4191. (In Chinese) [Google Scholar]
- Sun, X.G.; Feng, W.Y.; Li, M.; Shi, J.; Ding, G.J. Phenology and cultivation of Suillus bovinus, an edible mycorrhizal fungus, in a Pinus massoniana plantation. Can. J. For. Res. 2019, 48, 960–968. [Google Scholar] [CrossRef]
- Ruiz-Díez, B.; Rincón, A.M.; De Felipe, M.R.; Fernández-Pascual, M. Molecular characterization and evaluation of mycorrhizal capacity of Suillus isolates from Central Spain for the selection of fungal inoculants. Mycorrhiza 2006, 16, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Yamada, A.; Yokota, S.; Maruyama, T.; Shimokawa, T.; Neda, H. Innate traits of Pinaceae-specific ectomycorrhizal symbiont Suillus luteus that differentially associates with arbuscular mycorrhizal broad-leaved trees in vitro. Mycoscience 2015, 56, 606–611. [Google Scholar] [CrossRef]
- Peay, K.G.; Schubert, M.G.; Nguyen, N.H.; Bruns, T.D. Measuring ectomycorrhizal fungal dispersal: Macroecological patterns driven by microscopic propagules. Mol. Ecol. 2012, 21, 4122–4136. [Google Scholar] [CrossRef]
- Rudawska, M.; Wilgan, R.; Janowski, D.; Iwański, M.; Leski, T. Shifts in taxonomical and functional structure of ectomycor-rhizal fungal community of Scots pine (Pinus sylvestris L.) underpinned by partner tree ageing. Pedobiologia 2018, 71, 20–30. [Google Scholar] [CrossRef]
- Marx, D.H. The influence of ectotrophic fungi on the resistance of pine root to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 1969, 59, 153–163. [Google Scholar]
- Gupta, P.K.; Durzan, D.J. Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Negrel, J.; Klinguer, A.; Adrian, M. In vitro inhibition of shikimate hydroxycinnamoyltransferase by acibenzolar acid, the first metabolite of the plant defence inducer acibenzolar-S-methyl. Plant Physiol. Biochem. 2021, 163, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Felten, J.; Kohler, A.; Morin, E.; Bhalerao, R.P.; Palme, K.; Martin, F.; Ditengou, F.A.; Legué, V. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol. 2009, 151, 1991–2005. [Google Scholar] [CrossRef] [Green Version]
- Felten, J.; Legué, V.; Anicet Ditengou, F. Lateral root stimulation in the early interaction between Arabidopsis thaliana and the ectomycorrhizal fungus Laccaria bicolor: Is fungal auxin the trigger? Plant Signal Behav. 2010, 5, 864–867. [Google Scholar] [CrossRef] [Green Version]
- Sitrit, Y.; Roth-Bejerano, N.; Kagan-Zur, V.; Turgeman, T. Pre-symbiotic interactions between the desert truffle Terfezia boudieri and its host plant Helianthemum sessiliflorum. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 81–92. [Google Scholar]
- Balestrini, R.; Kottke, I. Structure and development of ectomycorrhizal roots. In Molecular Mycorrhizal Symbiosis; Martin, F., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2016; pp. 47–61. [Google Scholar]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Horan, D.P.; Chilvers, G.A.; Lapeyrie, F.F. Time sequence of the infection process eucalypt ectomycorrhizas. New Phytol. 1988, 109, 451–458. [Google Scholar] [CrossRef]
- Burgess, T.; Dell, B.; Malajczuk, N. In vitro synthesis of Pisolithus-Eucalyptus ectomycorrhizae: Synchronization of lateral tip emergence and ectomycorrhizal development. Mycorrhiza 1996, 6, 189–196. [Google Scholar] [CrossRef]
- Plett, J.M.; Khachane, A.; Ouassou, M.; Sundberg, B.; Kohler, A.; Martin, F. Ethylene and jasmonic acid act as negative modulators during mutualistic symbiosis between Laccaria bicolor and Populus roots. New Phytol. 2014, 202, 270–286. [Google Scholar] [CrossRef]
- Hill, R.A.; Wong-Bajracharya, J.; Anwar, S.; Coles, D.; Wang, M.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Martin, F.; Anderson, I.C.; et al. Abscisic acid supports colonization of Eucalyptus grandis roots by the mutualistic ectomycorrhizal fungus Pisolithus microcarpus. New Phytol. 2022, 233, 966–982. [Google Scholar] [CrossRef]
- Yu, J.Y. Infecting Micromechanism of Ectomycorrhizal Fungi to Picea koraiensis. Master’s Thesis, Northeast Forestry University, Harbin, China, 2007. [Google Scholar]
- Nylund, J.; Unestam, T. Structure and physiology of ectomycorrhizae. I. The process of mycorrhiza formation in Norway spruce in vitro. New Phytol. 1982, 91, 63–79. [Google Scholar] [CrossRef]
- Melville, L.H.; Massicotte, H.B.; Peterson, R.L. Ontogeny of early stages of ectomycorrhizae synthesized between Dryas integrifolia and Hebeloma cylindrosporum. Bot. Gaz. 1987, 148, 332–341. [Google Scholar] [CrossRef]
- Brunner, I. Comparative studies on ectomycorrhizae synthesized with various in vitro techniques using Picea abies and two Hebeloma species. Trees 1991, 5, 90–94. [Google Scholar] [CrossRef]
- Fusconi, A. The development of the fungal sheath on Cistus incanus short roots. Can. J. Bot. 1983, 61, 2546–2553. [Google Scholar] [CrossRef]
- Grellier, B.; Letouze, R.; Strullu, D.G. Micropropagation of birch and mycorrhizal formation in vitro. New Phytol. 1984, 97, 591–599. [Google Scholar] [CrossRef]
- Gay, G.; Normand, L.; Marmeisse, R.; Sotta, B.; Debaud, J.C. Auxin overproducer mutants of Hebeloma cylindrosporum Romagnesi have increased mycorrhizal activity. New Phytol. 1994, 128, 645–657. [Google Scholar] [CrossRef]
- Balestrini, R.; Hahn, M.G.; Bonfante, P. Location of cell-wall components in ectomycorrhizae of Corylus avellana and Tuber magnatum. Protoplasma 1996, 191, 55–69. [Google Scholar] [CrossRef]
- Martin, F.; Ramstedt, M.; Söderhäll, K.; Canet, D. Carbohydrate and amino acid metabolism in the ectomycorrhizal ascomycete Sphaerosporella brunnea during glucose utilization: A 13C NMR study. Plant Physiol. 1988, 86, 935–940. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.; Mikolajewski, S.; Peipp, H.; Schmitt, U.; Schmidt, J.; Wray, V.; Strack, D. Tissue-specific and development-dependent accumulation of phenylpropanoids in larch mycorrhizas. Plant Physiol. 1997, 114, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Plett, K.L.; Buckley, S.; Plett, J.M.; Anderson, I.C.; Lundberg-Felten, J.; Jämtgård, S. Novel microdialysis technique reveals a dramatic shift in metabolite secretion during the early stages of the interaction between the ectomycorrhizal fungus Pisolithus microcarpus and its host Eucalyptus grandis. Microorganisms 2021, 9, 1817. [Google Scholar] [CrossRef]
- Weiss, M.; Schmidt, J.; Neumann, D.; Wray, V.; Christ, R.; Strack, D. Phenylpropanoids in mycorrhizas of the Pinaceae. Planta 1999, 208, 491–502. [Google Scholar] [CrossRef]
- Feugey, L.; Strullu, D.G.; Poupard, P.; Simoneau, P. Induced defence responses limit Hartig net formation in ectomycorrhizal birch roots. New Phytol. 1999, 144, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Baldacci-Cresp, F.; Kohler, A.; Morreel, K.; Goeminne, G.; Van Acker, R.; Veneault-Fourrey, C.; Mol, A.; Pilate, G.; Boerjan, W.; et al. Alterations in the phenylpropanoid pathway affect poplar ability for ectomycorrhizal colonisation and susceptibility to root-knot nematodes. Mycorrhiza 2020, 30, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Besseau, S.; Geoffroy, P.; Ritzenthaler, C.; Meyer, D.; Lapierre, C.; Pollet, B.; Legrand, M. Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell 2004, 16, 1446–1465. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Sun, X.; Ding, G. Morphological and Transcriptional Characteristics of the Symbiotic Interaction between Pinus massoniana and Suillus bovinus. J. Fungi 2022, 8, 1162. https://doi.org/10.3390/jof8111162
Feng W, Sun X, Ding G. Morphological and Transcriptional Characteristics of the Symbiotic Interaction between Pinus massoniana and Suillus bovinus. Journal of Fungi. 2022; 8(11):1162. https://doi.org/10.3390/jof8111162
Chicago/Turabian StyleFeng, Wanyan, Xueguang Sun, and Guijie Ding. 2022. "Morphological and Transcriptional Characteristics of the Symbiotic Interaction between Pinus massoniana and Suillus bovinus" Journal of Fungi 8, no. 11: 1162. https://doi.org/10.3390/jof8111162
APA StyleFeng, W., Sun, X., & Ding, G. (2022). Morphological and Transcriptional Characteristics of the Symbiotic Interaction between Pinus massoniana and Suillus bovinus. Journal of Fungi, 8(11), 1162. https://doi.org/10.3390/jof8111162





