The Transcriptional Regulation of Genes Involved in the Immune Innate Response of Keratinocytes Co-Cultured with Trichophyton rubrum Reveals Important Roles of Cytokine GM-CSF
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Keratinocytes and Growth Conditions
2.3. Co-Culture Assay and Conditions
2.3.1. Conidial Suspension Conditions
2.4. Heat-Inactivated T. rubrum Conidia in the Germinative and Conidial Phase
2.5. Human Keratinocyte and T. rubrum Co-Culture Assay with Well Inserts
2.6. Lipopolysaccharide-Keratinocyte Challenge Assay
2.7. Lactate Dehydrogenase Assay
2.8. Cytokine Secretion during Co-Culture with T. rubrum Fungal Elements and LPS-HaCat Challenge Assay
2.9. RNA Extraction, cDNA Synthesis and RT-qPCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Release of Lactate Dehydrogenase by HaCat Keratinocytes during Co-Culture with T. rubrum
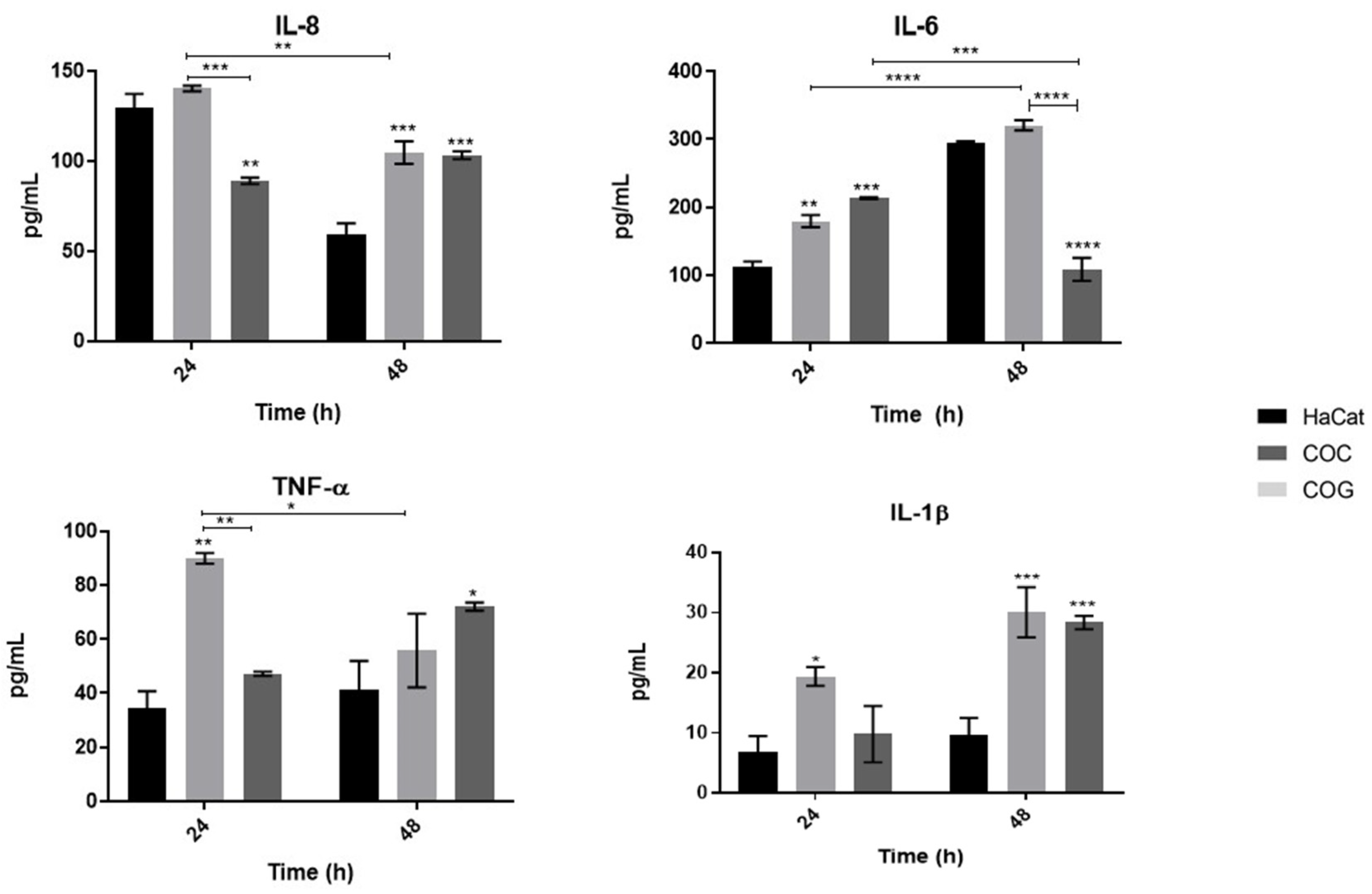
3.2. Fungal Elements of T. rubrum in Different Developmental Stages Promote Distinct Proinflammatory Cytokine Secretion Profiles of Human Keratinocytes
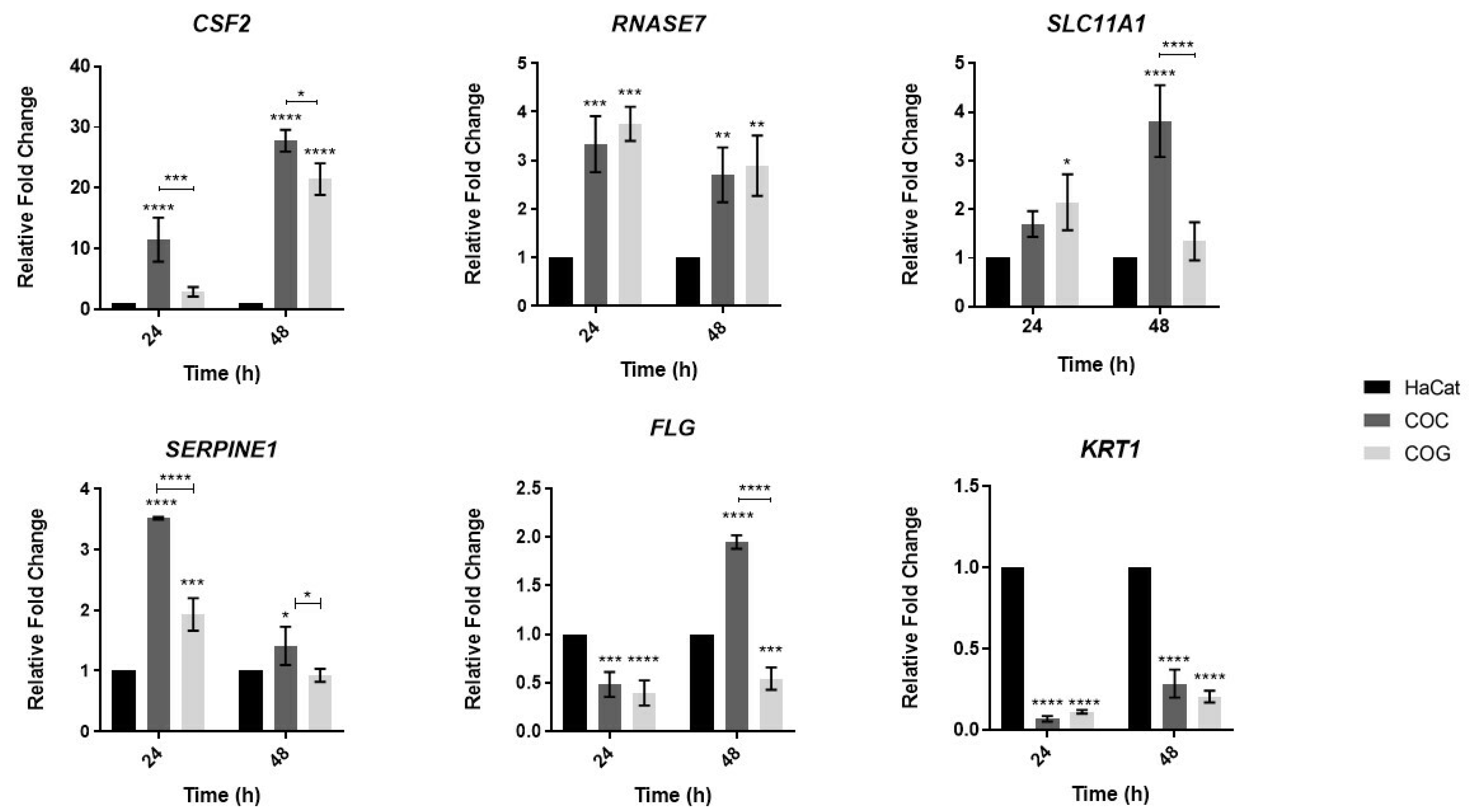
3.3. Analysis of Expression of Human Genes Involved in the Innate Immune Response and Epithelial Barrier Integrity during Co-Culture with T. rubrum
3.4. Analysis of Expression of Genes Involved in the Innate Immune Response of Keratinocytes Co-Cultured with Heat-Inactivated Fungal Elements of T. rubrum
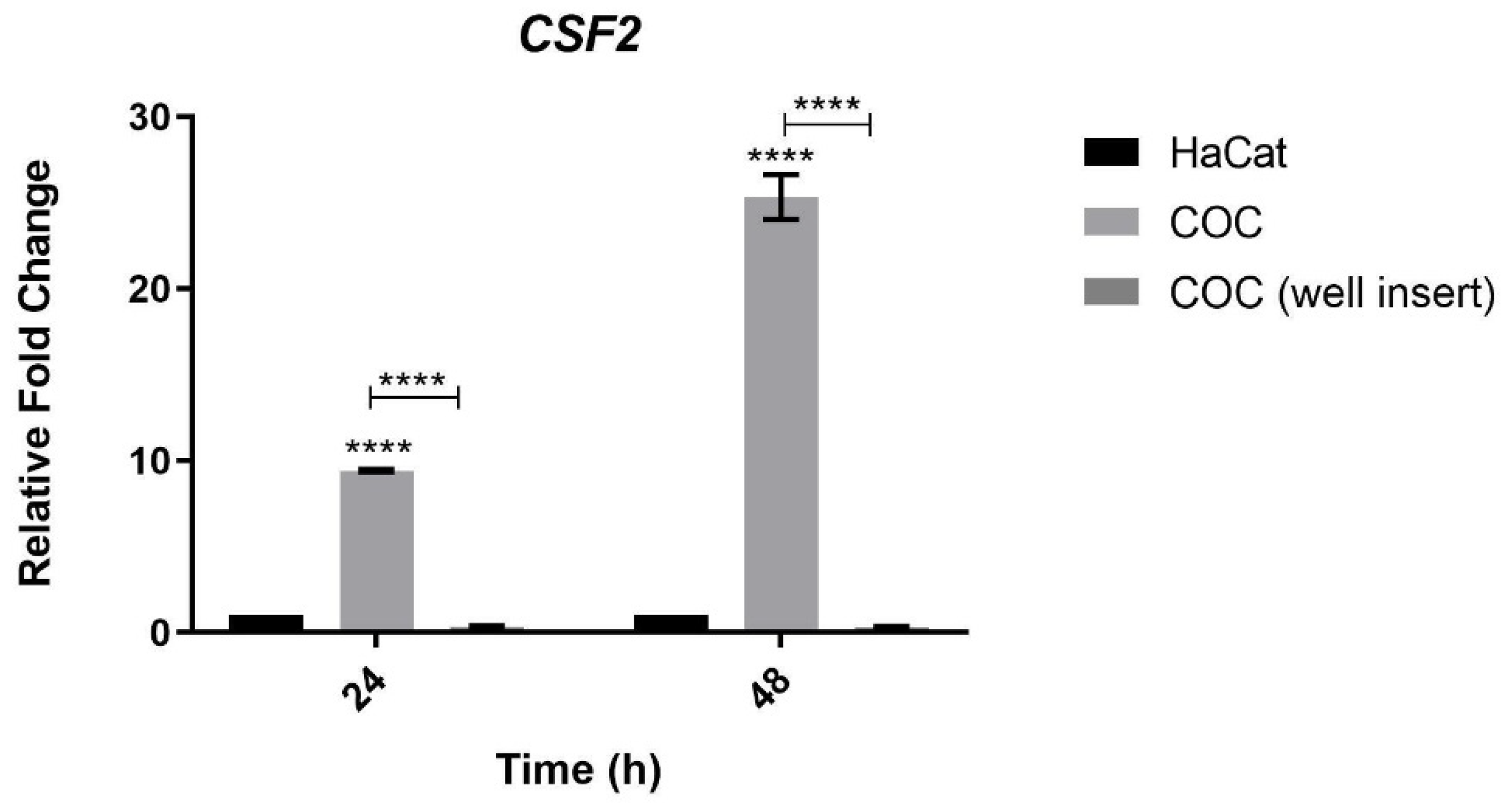
3.5. Analysis of CSF2 Expression during Co-Culture of Keratinocytes and T. rubrum Separated by Permeable Inserts
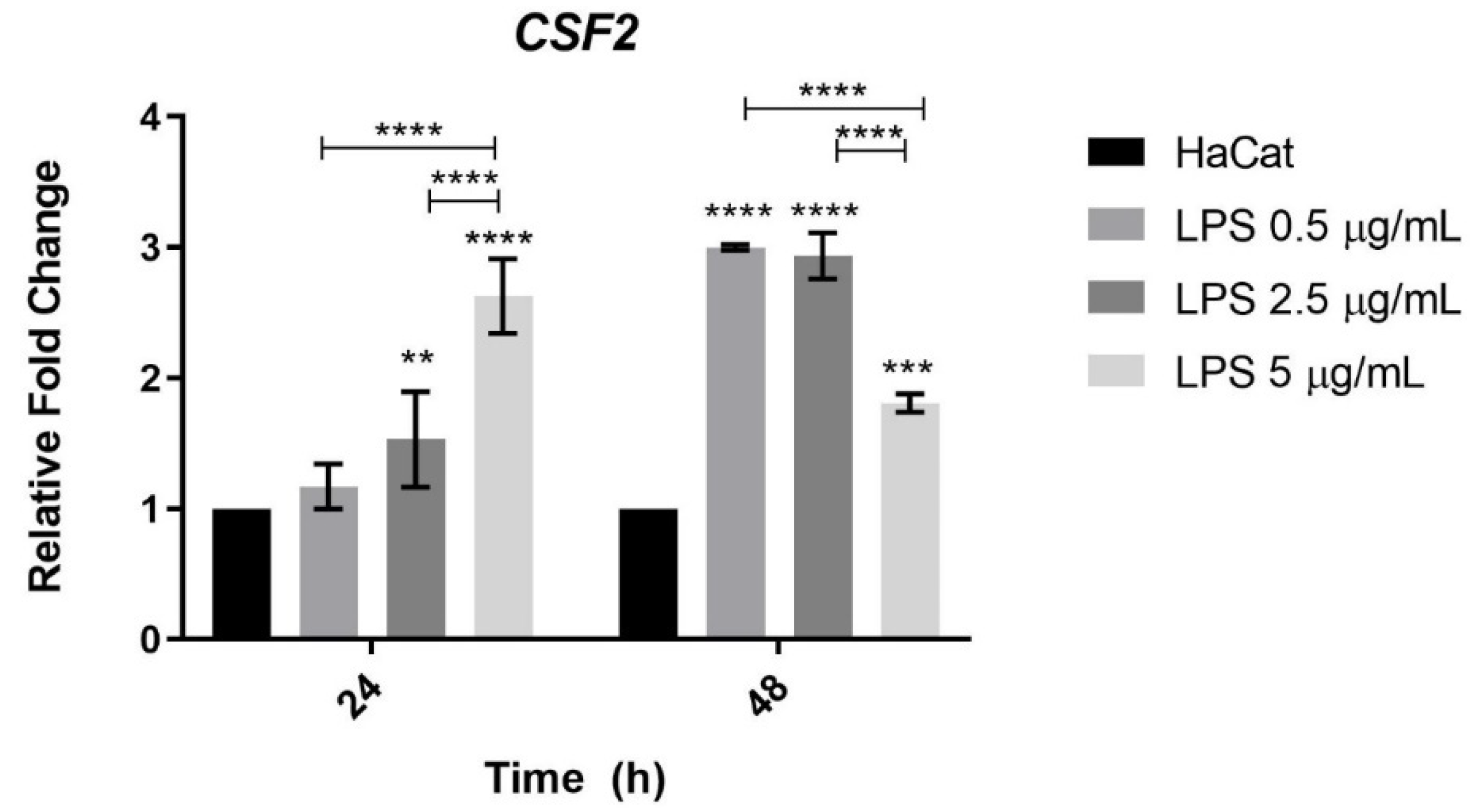
3.6. CSF2 Gene Expression in Keratinocytes Challenged with Bacterial LPS
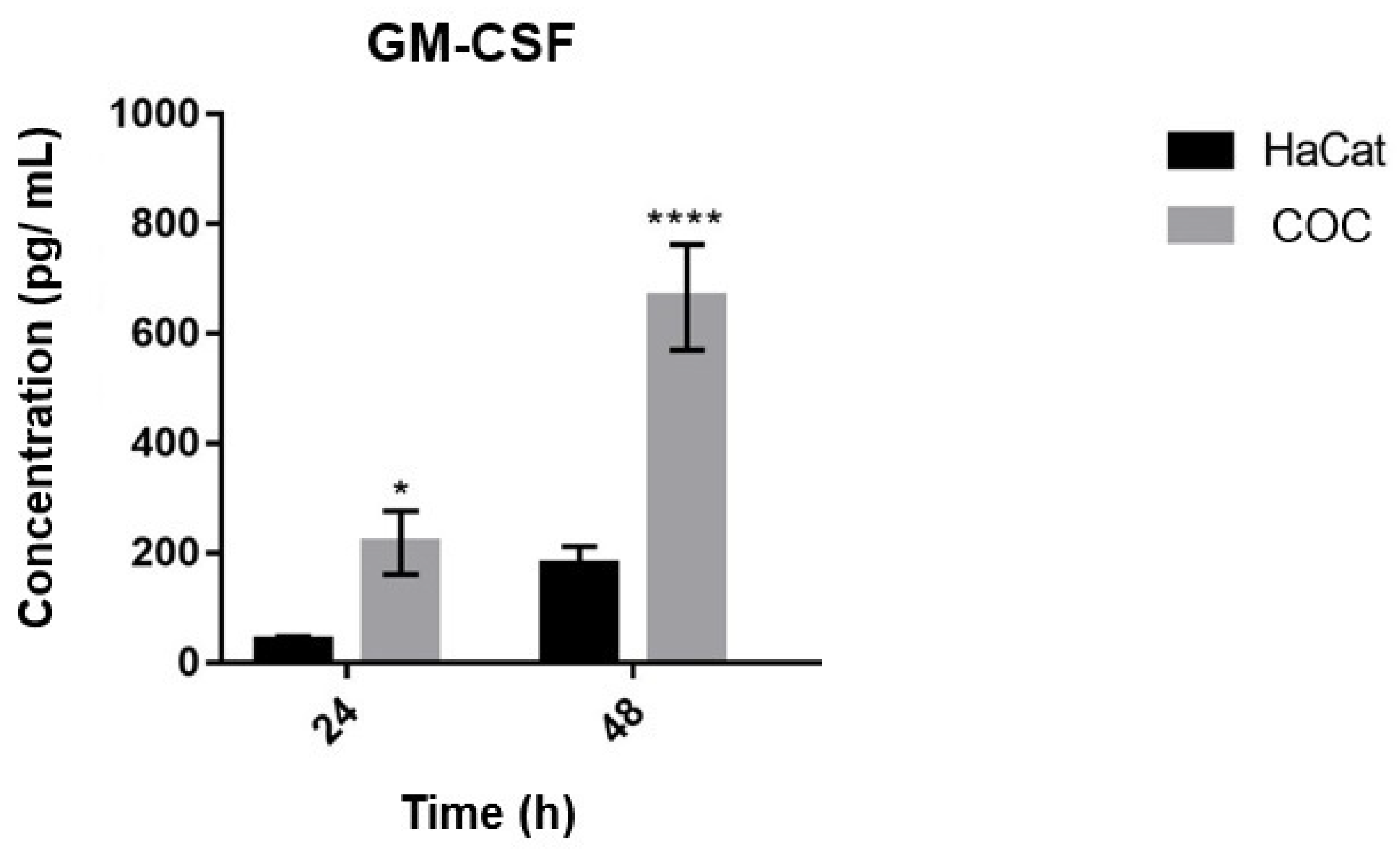
3.7. Secretion of GM-CSF by Keratinocytes Co-Cultured with T. rubrum and Challenged with Bacterial LPS
4. Discussion
4.1. The Levels of Proinflammatory Cytokine Secretion by Human Keratinocytes May Be Related to the Stage of Fungal Development
4.2. The CSF2 Gene May Play an Important Role in the Immune Response of Keratinocytes
4.3. The CSF2 Gene of Keratinocytes Is Less Expressed during Co-Culture with Heat-Inactivated T. rubrum Fungal Elements
4.4. The Expression of CSF2 Is Dependent on the Contact between Human Keratinocytes and T. rubrum Fungal Elements
4.5. Expression of the CSF2 Gene Was Lower When HaCat Keratinocytes Were Challenged with Bacterial LPS
4.6. The Secretion of GM-CSF by Human Keratinocytes Was Greater at 48 h during Co-Culture with T. rubrum, and Was Not Detected during Co-Culture with LPS
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnat, S.; Nowakiewicz, A.; Łagowski, D.; Zięba, P. Host-and Pathogen-Dependent Susceptibility and Predisposition to Dermatophytosis. J. Med. Microbiol. 2019, 68, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, M.F.; de Abreu, M.H.; Cantelli, B.A.M.; Segura, G.G.; Nishimura, F.G.; Bitencourt, T.A.; Marins, M.; Fachin, A.L. Epidemiology and Diagnostic Perspectives of Dermatophytoses. J. Fungi 2020, 6, 310. [Google Scholar] [CrossRef] [PubMed]
- Faway, É.; Lambert de Rouvroit, C.; Poumay, Y. In Vitro Models of Dermatophyte Infection to Investigate Epidermal Barrier Alterations. Exp. Dermatol. 2018, 27, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Blechert, O.; Mei, H.; Ge, L.; Liu, J.; Tao, Y.; Li, D.; de Hoog, G.S.; Liu, W. Whole-Genome Resequencing of Trichophyton Rubrum Provides Insights into Population Differentiation and Drug Resistance. Mycopathologia 2019, 185, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J. Pathogenesis of Tinea. JDDG J. Dtsch. Dermatol. Ges. 2010, 8, 780–786. [Google Scholar] [CrossRef]
- Firat, Y.H.; Simanski, M.; Rademacher, F.; Schroder, L.; Brasch, J.; Harder, J. Infection of Keratinocytes with Trichophytum Rubrum Induces Epidermal Growth Factor-Dependent RNase 7 and Human Beta-Defensin-3 Expression. PLoS ONE 2014, 9, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Mörig, A.; Neumann, B.; Proksch, E. Expression of Antimicrobial Peptides and Toll-like Receptors Is Increased in Tinea and Pityriasis Versicolor. Mycoses 2014, 57, 147–152. [Google Scholar] [CrossRef]
- Blutfield, M.S.; Lohre, J.M.; Pawich, D.A.; Vlahovic, T.C. The Immunologic Response to Trichophyton Rubrum in Lower Extremity Fungal Infections. J. Fungi 2015, 1, 130–137. [Google Scholar] [CrossRef]
- Sardana, K.; Gupta, A.; Mathachan, S. Immunopathogenesis of Dermatophytoses and Factors Leading to Recalcitrant Infections. Indian Dermatol. Online J. 2021, 12, 389–399. [Google Scholar] [CrossRef]
- Petrucelli, M.F.; Peronni, K.; Sanches, P.R.; Komoto, T.T.; Matsuda, J.B.; da Silva Junior, W.A.; Beleboni, R.O.; Martinez-Rossi, N.M.; Marins, M.; Fachin, A.L. Dual RNA-Seq Analysis of Trichophyton Rubrum and HaCat Keratinocyte Co-Culture Highlights Important Genes for Fungal-Host Interaction. Genes 2018, 9, 362. [Google Scholar] [CrossRef]
- Lee, K.M.C.; Achuthan, A.A.; Hamilton, J.A. GM-CSF: A Promising Target in Inflammation and Autoimmunity. Immunotargets Ther. 2020, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, Y.; Ishibashi, Y.; Hiruma, M.; Nishikawa, A.; Ikeda, S. Cytokine Secretion Profiles of Human Keratinocytes during Trichophyton Tonsurans and Arthroderma Benhamiae Infections. J. Med. Microbiol. 2006, 55, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Budnick, I.; Singh, M.; Thiruppathi, M.; Alharshawi, K.; Elshabrawy, H.; Holterman, M.J.; Prabhakar, B.S. Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy. J. Interferon Cytokine Res. 2015, 35, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Petrina, M.; Martin, J.; Basta, S. Granulocyte Macrophage Colony-Stimulating Factor Has Come of Age: From a Vaccine Adjuvant to Antiviral Immunotherapy. Cytokine Growth Factor Rev. 2021, 59, 101. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Wang, T.S.; Lee, E.; Cremer, P.C.; Carey, B.; Rajendram, P.; Hudock, K.; Korbee, L.; van Tassell, B.W.; et al. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front. Immunol. 2020, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Dardoufas, K.; Markoulatos, P.; Sotiropoulou-Lontou, A.; Kyprianou, K.; Kolitsi, G.; Pissakas, G.; Skarleas, C.; Kouloulias, V.; Papanicolaou, V.; et al. Oral Pseudomembranous Candidiasis, Herpes Simplex Virus-1 Infection, and Oral Mucositis in Head and Neck Cancer Patients Receiving Radiotherapy and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Mouthwash. J. Oral Pathol. Med. 2001, 30, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gavino, C.; Cotter, A.; Lichtenstein, D.; Lejtenyi, D.; Fortin, C.; Legault, C.; Alirezaie, N.; Majewski, J.; Sheppard, D.C.; Behr, M.A.; et al. CARD9 Deficiency and Spontaneous Central Nervous System Candidiasis: Complete Clinical Remission With GM-CSF Therapy. Clin. Infect. Dis. 2014, 59, 81–84. [Google Scholar] [CrossRef]
- Drummond, R.A.; Zahra, F.T.; Natarajan, M.; Swamydas, M.; Hsu, A.P.; Wheat, L.J.; Gavino, C.; Vinh, D.C.; Holland, S.M.; Mikelis, C.M.; et al. GM-CSF Therapy in Human Caspase Recruitment Domain–Containing Protein 9 Deficiency. J. Allergy Clin. Immunol. 2018, 142, 1334–1338.e5. [Google Scholar] [CrossRef]
- Komoto, T.T.; Bitencourt, A.; Silva, G.; Oliveira Beleboni, R.; Marins, M.; Fachin, A.L. Gene Expression Response of Trichophyton Rubrum during Coculture on Keratinocytes Exposed to Antifungal Agents. Evid.-Based Complement. Altern. Med. 2015, 2015, 180535. [Google Scholar] [CrossRef]
- Chai, L.Y.A.; Netea, M.G.; Sugui, J.; Vonk, A.G.; van de Sande, W.W.J.; Warris, A.; Kwon-Chung, K.J.; Jan Kullberg, B. Aspergillus Fumigatus Conidial Melanin Modulates Host Cytokine Response. Immunobiology 2010, 215, 915–920. [Google Scholar] [CrossRef]
- Li, L.; Dongari-Bagtzoglou, A. Epithelial GM-CSF Induction by Candida Glabrata. J. Dent. Res. 2009, 88, 746–751. [Google Scholar] [CrossRef] [PubMed]
- de Camargo Pereira, G.; Guimarães, G.N.; Planello, A.C.; Santamaria, M.P.; de Souza, A.P.; Line, S.R.; Marques, M.R. Porphyromonas Gingivalis LPS Stimulation Downregulates DNMT1, DNMT3a, and JMJD3 Gene Expression Levels in Human HaCaT Keratinocytes. Clin. Oral Investig. 2013, 17, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.; Evje, L.; Syversen, T. Silver Nanoparticle-Induced Cytotoxicity in Rat Brain Endothelial Cell Culture. Toxicol. Vitro 2013, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Segura, G.G.; Cantelli, B.A.; Peronni, K.; Sanches, P.R.; Komoto, T.T.; Rizzi, E.; Beleboni, R.O.; da Silva Junior, W.A.; Martinez-Rossi, N.M.; Marins, M.; et al. Cellular and Molecular Response of Macrophages Thp-1 during Co-Culture with Inactive Trichophyton Rubrum Conidia. J. Fungi 2020, 6, 363. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, D.; Hu, P.-C.; Li, Q.; Lin, C.-Y. HOXB7-S3 Inhibits the Proliferation and Invasion of MCF-7 Human Breast Cancer Cells. Mol. Med. Rep. 2015, 12, 4901–4908. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095497. [Google Scholar] [CrossRef]
- Weitzman, I.; Summerbell, R.C. The Dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Yang, J.; Chen, L.; Liu, B.; Liu, T.; Jin, Q. Integrated MicroRNA and MRNA Analysis in the Pathogenic Filamentous Fungus Trichophyton rubrum. BMC Genom. 2018, 19, 933. [Google Scholar] [CrossRef]
- Petrucelli, M.F.; Matsuda, J.B.; Peroni, K.; Sanches, P.R.; Silva, W.A.; Beleboni, R.O.; Martinez-Rossi, N.M.; Marins, M.; Fachin, A.L. The Transcriptional Profile of Trichophyton rubrum Co-Cultured with Human Keratinocytes Shows New Insights about Gene Modulation by Terbinafine. Pathogens 2019, 8, 274. [Google Scholar] [CrossRef]
- Celestrino, G.A.; Verrinder Veasey, J.; Benard, G.; Sousa, M.G.T. Host Immune Responses in Dermatophytes Infection. Mycoses 2021, 64, 477–483. [Google Scholar] [CrossRef]
- Hau, C.S.; Tada, Y.; Kanda, N.; Watanabe, S. Immunoresponses in Dermatomycoses. J. Dermatol. 2015, 42, 236–244. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kano, R.; Hasegawa, A.; Watanabe, S. Interleukin-8 and Tumor Necrosis Factor Alpha Production in Human Epidermal Keratinocytes Induced by Trichophyton Mentagrophytes. Clin. Diagn. Lab. Immunol. 2002, 9, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Adachi, M.; Nakamura, Y.; Kano, R.; Makimura, K.; Hasegawa, A.; Kanda, N.; Watanabe, S. The Effect of Dermatophytes on Cytokine Production by Human Keratinocytes. Arch. Dermatol. Res. 2007, 299, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.V.A.; Rademacher, F.; Hinrichs, H.; Beck-Jendroschek, V.; Harder, M.; Brasch, J.; Gläser, R.; Harder, J. Expression of Epidermal Antimicrobial Peptides Is Increased in Tinea Pedis. Mycoses 2021, 64, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Fritz, P.; Beck-Jendroschek, V.; Brasch, J. Inhibition of Dermatophytes by the Antimicrobial Peptides Human β-Defensin-2, Ribonuclease 7 and Psoriasin. Med. Mycol. 2012, 50, 579–584. [Google Scholar] [CrossRef]
- Nairz, M.; Fritsche, G.; Crouch, M.L.V.; Barton, H.C.; Fang, F.C.; Weiss, G. Slc11a1 Limits Intracellular Growth of Salmonella Enterica Sv. Typhimurium by Promoting Macrophage Immune Effector Functions and Impairing Bacterial Iron Acquisition. Cell Microbiol. 2009, 11, 1365–1381. [Google Scholar] [CrossRef]
- Kidane, Y.H.; Lawrence, C.; Murali, T.M. Computational Approaches for Discovery of Common Immunomodulators in Fungal Infections: Towards Broad-Spectrum Immunotherapeutic Interventions. BMC Microbiol. 2013, 13, 224. [Google Scholar] [CrossRef]
- Harslund, J.; Frees, D.; Leifsson, P.S.; Offenberg, H.; Rømer, M.U.; Brünner, N.; Olsen, J.E. The Role of Serpine-1 and Tissue Inhibitor of Metalloproteinase Type-1 in Early Host Responses to Staphylococcus Aureus Intracutaneous Infection of Mice. Pathog. Dis. 2013, 68, 96–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jensen, J.-M.; Pfeiffer, S.; Akaki, T.; Schröder, J.-M.; Kleine, M.; Neumann, C.; Proksch, E.; Brasch, J. Barrier Function, Epidermal Differentiation, and Human β-Defensin 2 Expression in Tinea Corporis. J. Investig. Dermatol. 2007, 127, 1720–1727. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Xu Landén, N. Molecular Sciences The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Lasbury, M.E.; Merali, S.; Durant, P.J.; Tschang, D.; Ray, C.A.; Lee, C.H. Polyamine-Mediated Apoptosis of Alveolar Macrophages during Pneumocystis Pneumonia. J. Biol. Chem. 2007, 282, 11009–11020. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M.; Rudd, C.E.; Morrison, T.E. Th1/Th2 Paradigm Extended: Macrophage Polarization as an Unappreciated Pathogen-Driven Escape Mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar] [CrossRef]
- Damiani, G.; McCormick, T.S.; Leal, L.O.; Ghannoum, M.A. Recombinant Human Granulocyte Macrophage-Colony Stimulating Factor Expressed in Yeast (Sargramostim): A Potential Ally to Combat Serious Infections. Clin. Immunol. 2020, 210, 108292. [Google Scholar] [CrossRef]
- das Gupta, M.; Fliesser, M.; Springer, J.; Breitschopf, T.; Schlossnagel, H.; Schmitt, A.-L.; Kurzai, O.; Hünniger, K.; Einsele, H.; Löffler, J. Aspergillus Fumigatus Induces MicroRNA-132 in Human Monocytes and Dendritic Cells. Int. J. Med. Microbiol. 2014, 304, 592–596. [Google Scholar] [CrossRef]
- Chen, S.; Yan, H.; Zhang, L.; Kong, W.; Sun, Y.; Zhang, W.; Chen, Y.; Deng, A. Cryptococcus Neoformans Infection and Immune Cell Regulation in Human Monocytes. Cell. Physiol. Biochem. 2015, 37, 537–547. [Google Scholar] [CrossRef]
- Huang, X.; Yi, J.; Yin, S.; Chen, R.; Li, M.; Gong, Z.; Lai, W.; Chen, J. Exposure to Heat-Inactivated Trichophyton Rubrum Resulting in a Limited Immune Response of Human Keratinocytes. Chin. Med. J. 2013, 126, 215–219. [Google Scholar]
- Cochet, F.; Peri, F. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-like Receptor 4 (TLR4) Signalling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef]
- Pivarcsi, A.; Bodai, L.; Réthi, B.; Kenderessy-Szabó, A.; Koreck, A.; Széll, M.; Beer, Z.; Bata-Csörgo, Z.; Magócsi, M.; Rajnavölgyi, E.; et al. Expression and Function of Toll-like Receptors 2 and 4 in Human Keratinocytes. Int. Immunol. 2003, 15, 721–730. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Perspective Article: Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Siobhan, N.; Agarwal, N.; Parker Chris, C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Zeng, R.Y.; Tan, H.S.W.; et al. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and T-Cell Responses: What We Do and Don’t Know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Yew, W.S.; Seneviratne, C.J.; Chang, M.W.; Chai, L.Y.A. Immunomodulation as Therapy for Fungal Infection: Are We Closer? Front. Microbiol. 2018, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Goldman, C.; Akiyama, M.J.; Torres, J.; Louie, E.; Meehan, S.A. Scedosporium Apiospermum Infections and the Role of Combination Antifungal Therapy and GM-CSF: A Case Report and Review of the Literature. Med. Mycol. Case Rep. 2016, 11, 40–43. [Google Scholar] [CrossRef]
- Dongari-Bagtzoglou, A.; Kashleva, H. Granulocyte-Macrophage Colony-Stimulating Factor Responses of Oral Epithelial Cells to Candida Albicans. Oral Microbiol. Immunol. 2003, 18, 165–170. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrucelli, M.F.; Cantelli, B.A.M.; Marins, M.; Fachin, A.L. The Transcriptional Regulation of Genes Involved in the Immune Innate Response of Keratinocytes Co-Cultured with Trichophyton rubrum Reveals Important Roles of Cytokine GM-CSF. J. Fungi 2022, 8, 1151. https://doi.org/10.3390/jof8111151
Petrucelli MF, Cantelli BAM, Marins M, Fachin AL. The Transcriptional Regulation of Genes Involved in the Immune Innate Response of Keratinocytes Co-Cultured with Trichophyton rubrum Reveals Important Roles of Cytokine GM-CSF. Journal of Fungi. 2022; 8(11):1151. https://doi.org/10.3390/jof8111151
Chicago/Turabian StylePetrucelli, Monise Fazolin, Bruna Aline M. Cantelli, Mozart Marins, and Ana Lúcia Fachin. 2022. "The Transcriptional Regulation of Genes Involved in the Immune Innate Response of Keratinocytes Co-Cultured with Trichophyton rubrum Reveals Important Roles of Cytokine GM-CSF" Journal of Fungi 8, no. 11: 1151. https://doi.org/10.3390/jof8111151
APA StylePetrucelli, M. F., Cantelli, B. A. M., Marins, M., & Fachin, A. L. (2022). The Transcriptional Regulation of Genes Involved in the Immune Innate Response of Keratinocytes Co-Cultured with Trichophyton rubrum Reveals Important Roles of Cytokine GM-CSF. Journal of Fungi, 8(11), 1151. https://doi.org/10.3390/jof8111151





