Metaprofiling of the Bacterial Community in Colonized Compost Extracts by Agaricus subrufescens
Abstract
1. Introduction
2. Materials and Methods
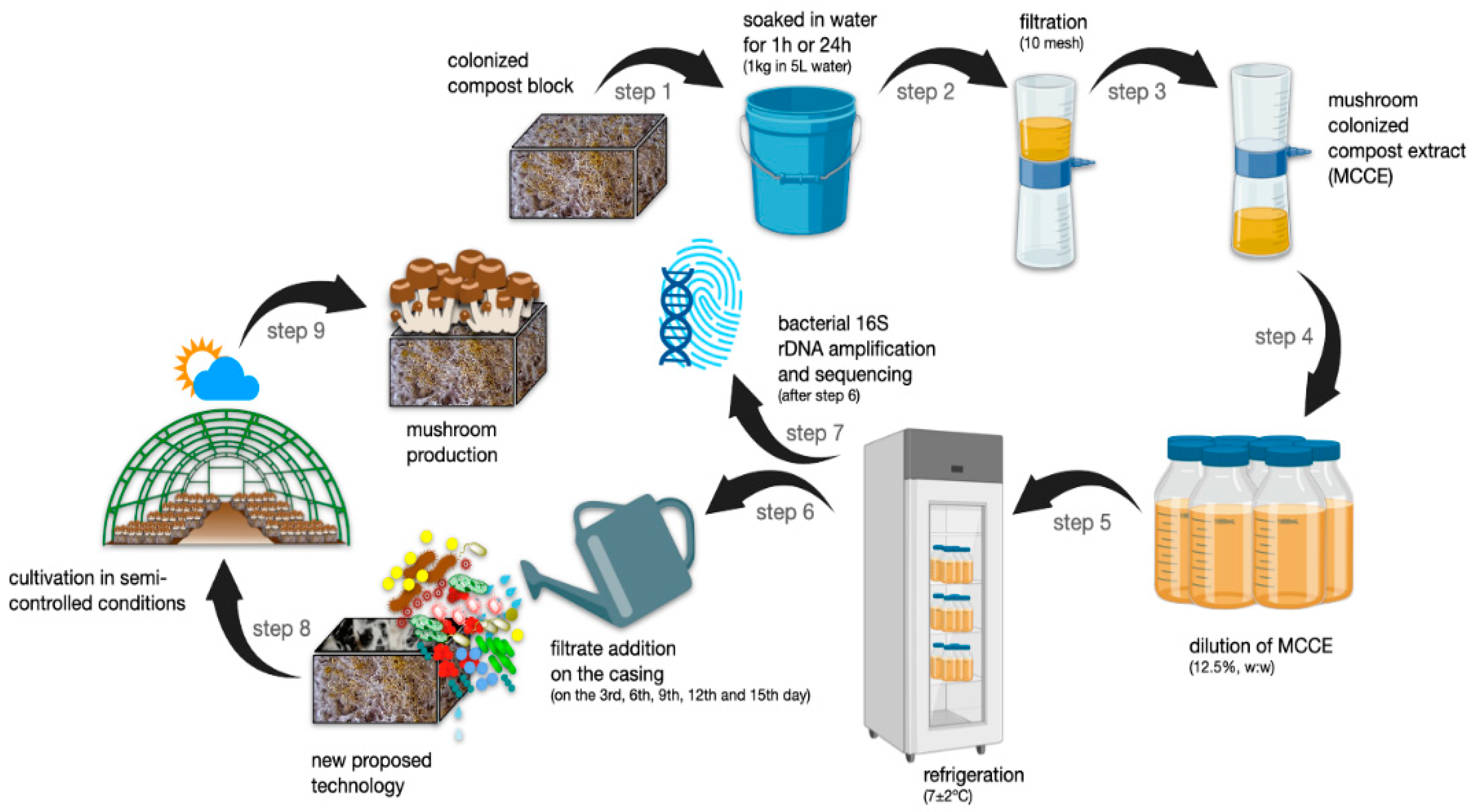
2.1. Experimental Design
2.2. Compost Preparation
2.3. Spawn Production
2.4. Casing Layer and Fruiting Induction
2.5. Mushroom-Colonized Compost Extract (MCCE) Preparation and Addition to the Casing Layer
2.6. Harvest Phase
2.7. Agronomic Trait Evaluation
2.8. Statiscal Analysis
2.9. Estimation of Bacterial Diversity by 16S rRNA Gene Sequencing
2.10. Bioinformatic Analysis
3. Results
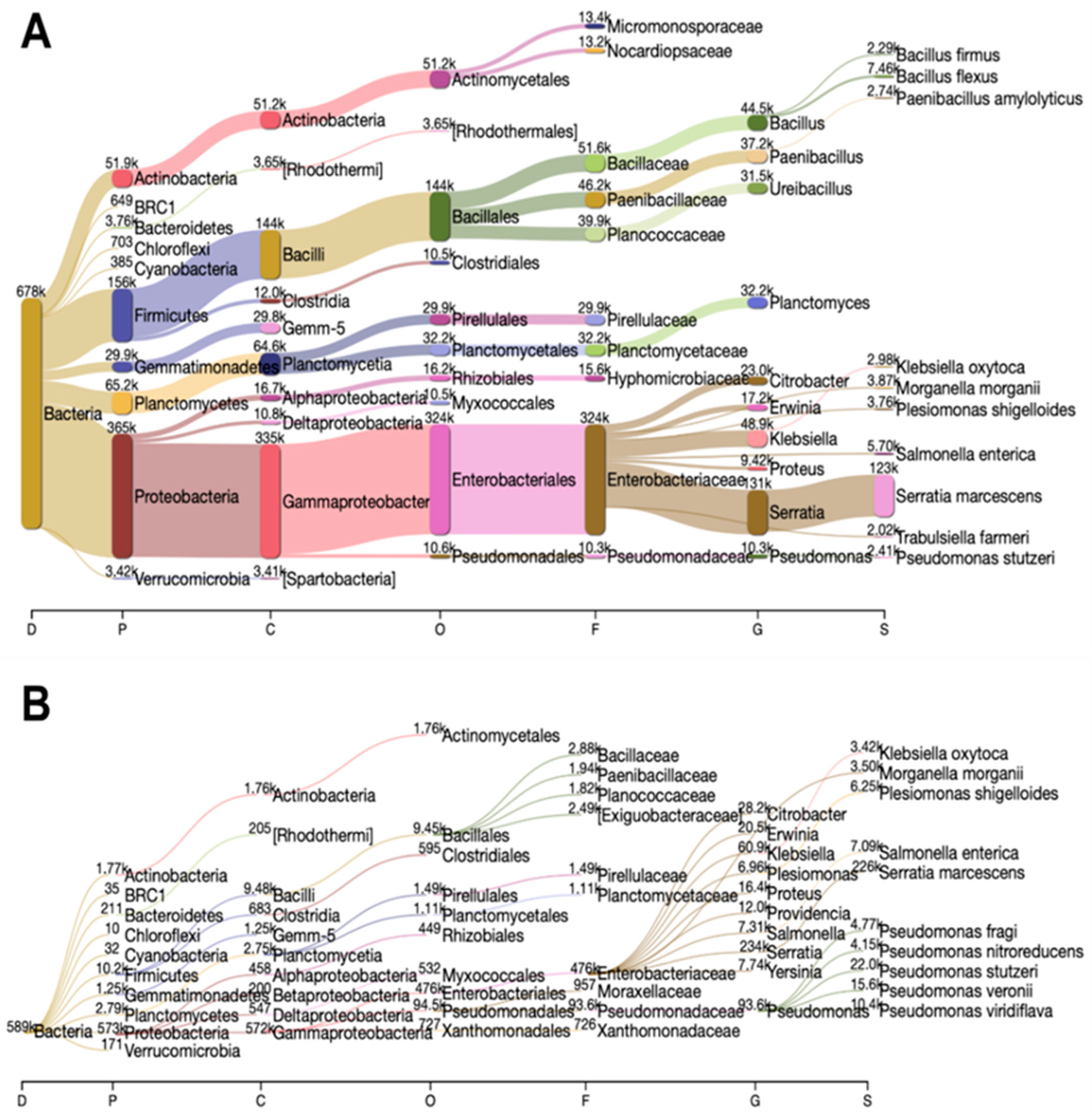
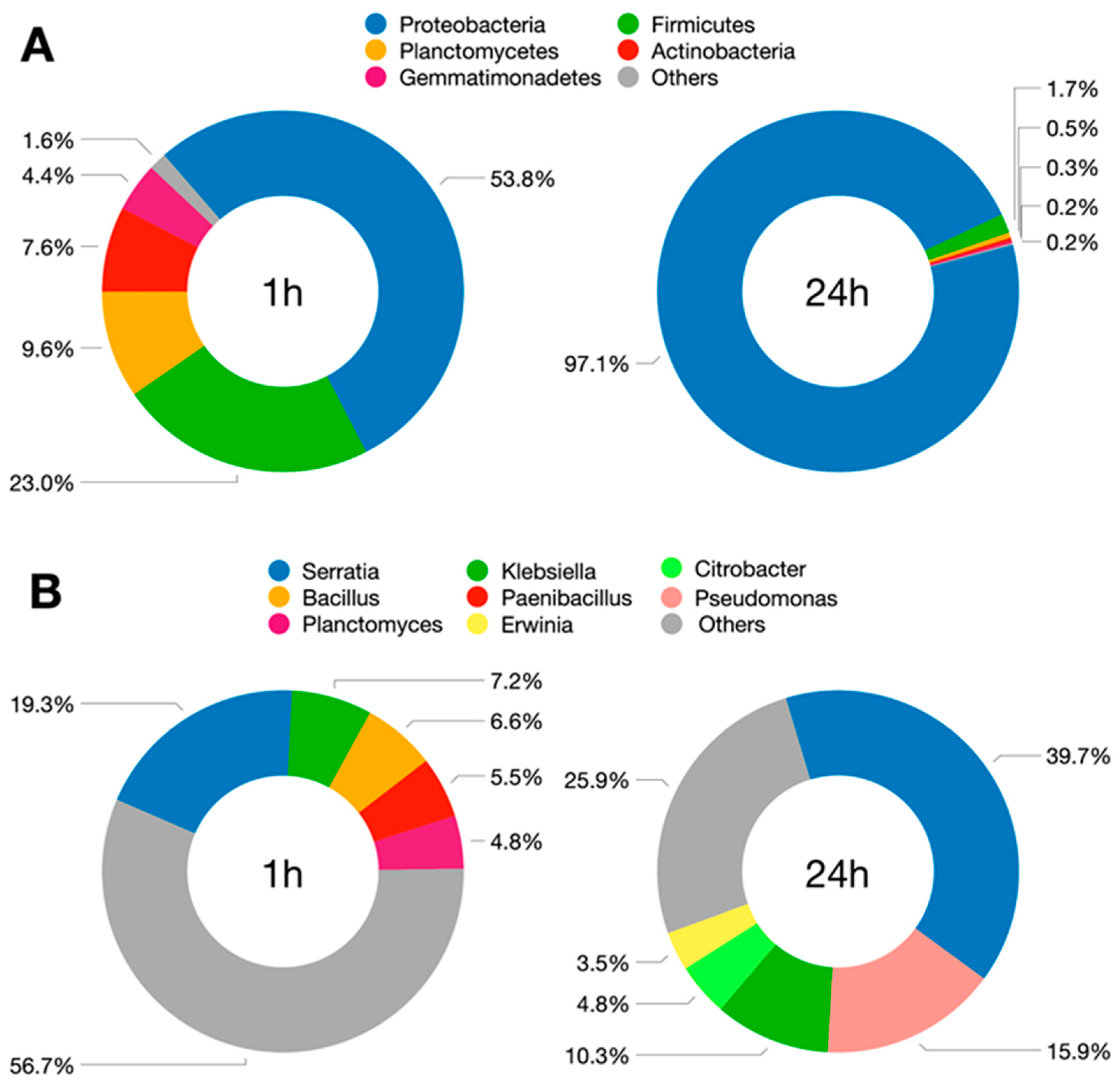
3.1. Composition and Dynamics of MCCE Microbiome
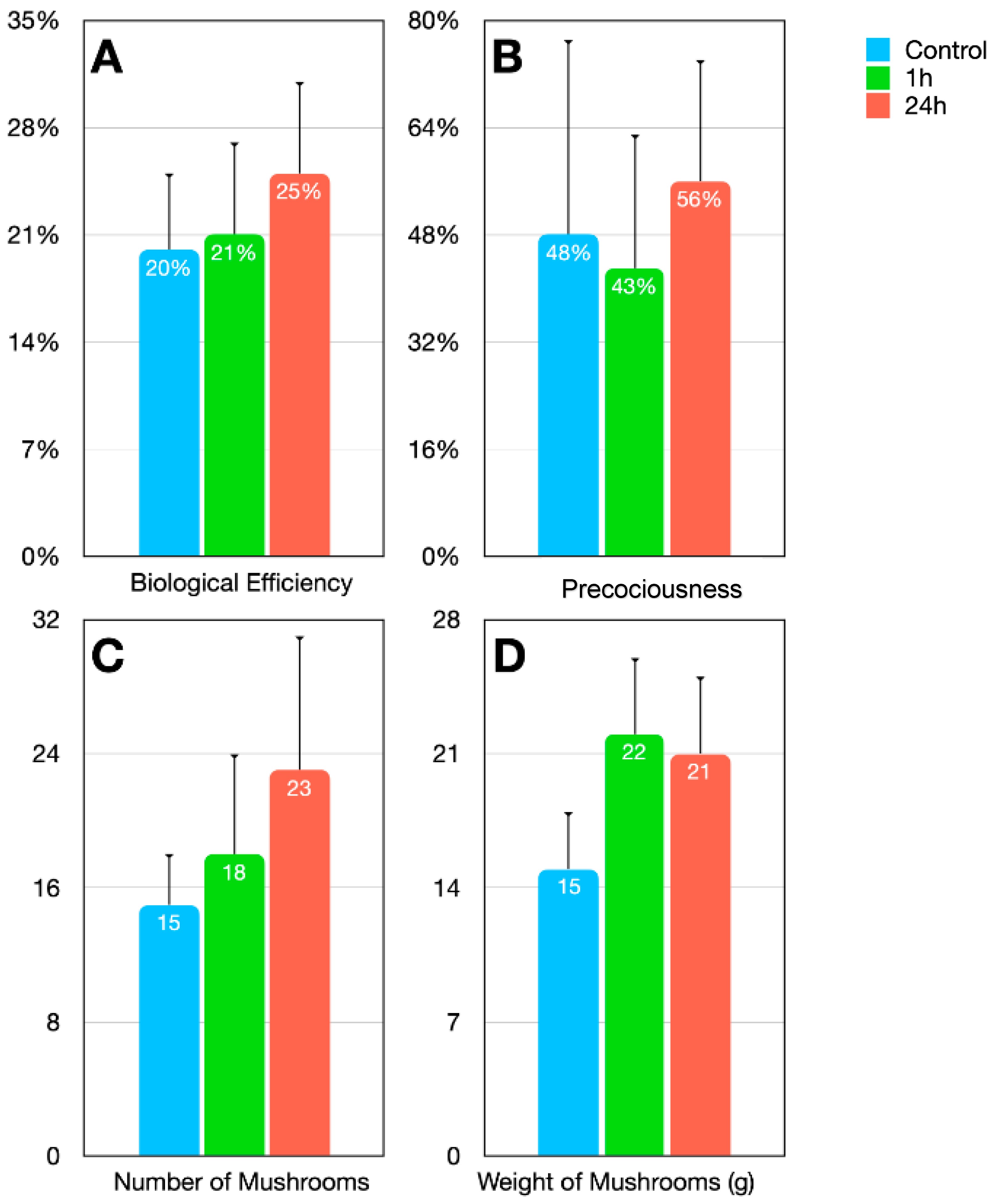
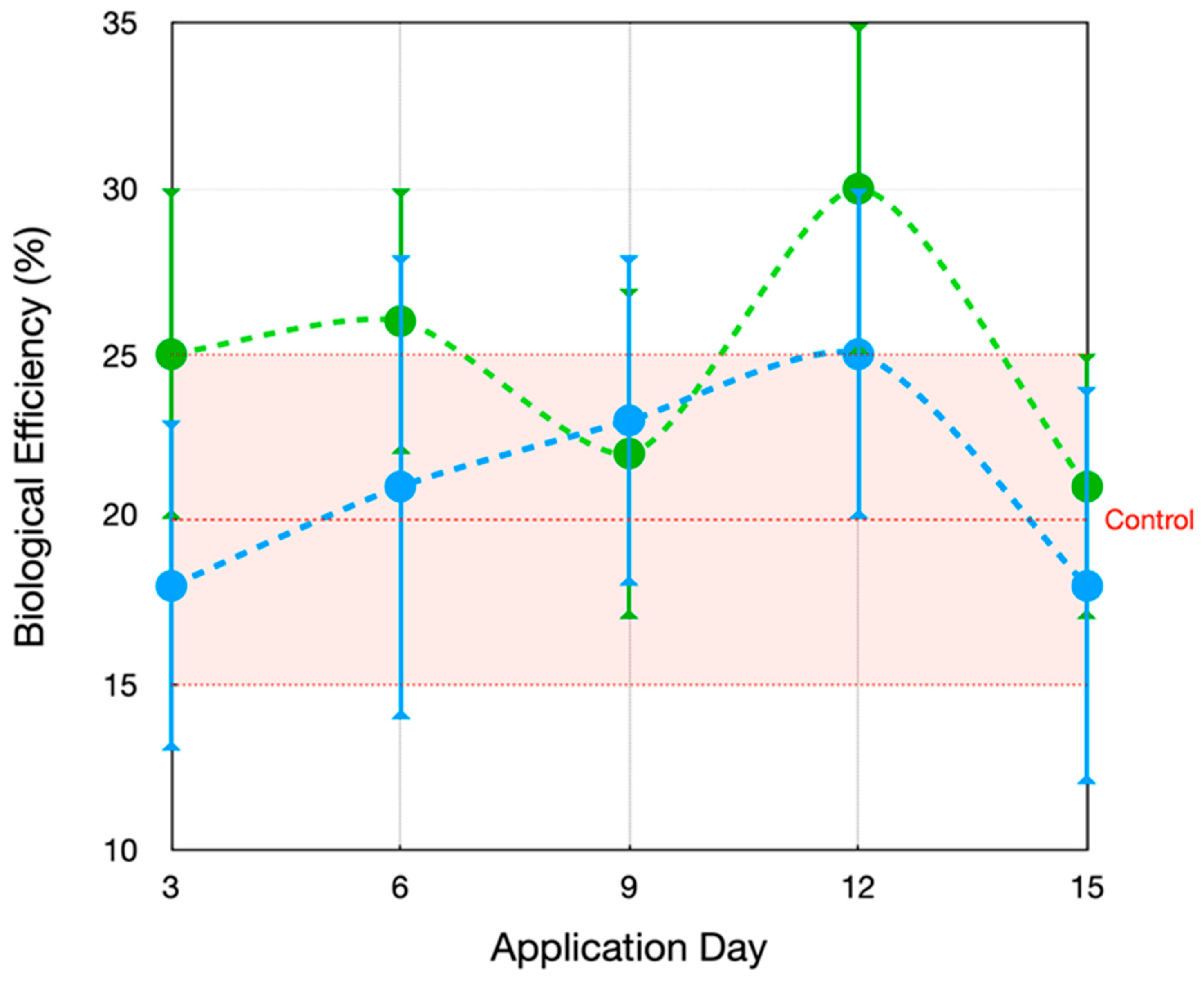
3.2. Evaluation of the Application of MCCE on the Agronomic Traits of Mushrooms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Largeteau, M.L.; Llarena-Hernández, R.C.; Regnault-Roger, C.; Savoie, J.M. The medicinal Agaricus mushroom cultivated in Brazil: Biology, cultivation and non-medicinal valorisation. Appl. Microbiol. Biotechnol. 2011, 92, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.M.; Dias, E.S.; Assis, L.J.D.; Gomide, P.H.O.; Santos, J.B.D. Genetic variability of mushroom isolates Agaricus blazei using markers RAPD. Ciência E Agrotecnologia 2007, 31, 1242–1249. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Karunarathna, S.C.; Thongklang, N.; Zhao, R.; Callac, P.; Moukha, S.; Férandon, C.; Chukeatirote, E.; Hyde, K.D. Agaricus subrufescens: A review. Saudi J. Biol. Sci. 2012, 19, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Colauto, N.B.; Linde, G.A. Avances sobre el cultivo del “cogumelo do sol” en Brasil. In El Cultivo de los Hongos Comestibles en Iberoamérica; Sánchez, J.E., Mata, G., Eds.; Colegio de la Frontera Sur: Tapachula, Mexico, 2012; pp. 103–128. [Google Scholar]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef]
- Ghasemi, K.; Emadi, M.; Bagheri, A.; Mohammadi, M. Casing material and thickness effects on the yield and nutrient concentration of Agaricus bisporus. Sarhad. J. Agric. 2020, 36, 958–965. [Google Scholar] [CrossRef]
- Murmu, R.; Maurya, A.K.; John, V. Mycoflora of certain casing materials used in the production of white button mushroom (Agaricus bisporus (Lange) Imbach). Int. J. Chem. Stud. 2020, 8, 2863–2868. [Google Scholar] [CrossRef]
- Levanon, D.; Danai, O.; Raz, D. Method of Indoor Mushroom Cultivation. European Patent Application EP 3106025A1, 2016. [Google Scholar]
- Navarro, M.J.; Gea, F.J.; Pardo-Giménez, A.; Martínez, A.; Raz, D.; Levanon, D.; Danay, O. Agronomical valuation of a drip irrigation system in a commercial mushroom farm. Sci. Hort. 2020, 265, 109234. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Adelodun, B.; Bedeković, D.; Kos, I.; Širić, I.; Silva, L.F. Sustainable Use of Sewage Sludge as a Casing Material for Button Mushroom (Agaricus bisporus) Cultivation: Experimental and Prediction Modeling Studies for Uptake of Metal Elements. J. Fungi 2022, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.; Goodchild, M.; Jenkins, D.; Burton, K.; Walker, D.; Moorhouse, E. Precision Irrigation of Mushrooms Using a Novel Dielectric Tensiometer. Mushroom News 2017, 65, 8–17. [Google Scholar]
- Zied, D.C.; Caitano, C.E.C.; Pardo-Giménez, A.; Dias, E.; Zeraik, M.L.; Pardo, J.E. Using of appropriated strains in the practice of compost supplementation for Agaricus subrufescens production. Front. Sustain. Food Syst. 2018, 2, 26. [Google Scholar] [CrossRef]
- Gea, F.J.; Carrasco, J.; Diánez, F.; Santos, M.; Navarro, M.J. Control of dry bubble disease (Lecanicillium fungicola) in button mushroom (Agaricus bisporus) by spent mushroom substrate tea. Eur. J. Plant Pathol. 2014, 138, 711–720. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Pecchia, J.A. Bacterial community patterns in the Agaricus bisporus cultivation system, from compost raw materials to mushroom caps. Microb. Ecol. 2021, 84, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, S.; Muto-Fujita, A.; Maruyama, F. Evaluation of PCR conditions for characterizing bacterial communities with full-length 16S rRNA genes using a portable nanopore sequencer. Sci. Rep. 2020, 10, 12580. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, L.; Rodríguez-Pérez, H.; Flores, C. Nanopore sequencing and its application to the study of microbial communities. Comput. Struct. Biotechnol. J. 2021, 19, 1497–1511. [Google Scholar] [CrossRef]
- Matsuo, Y.; Komiya, S.; Yasumizu, Y.; Yasuoka, Y.; Mizushima, K.; Takagi, T.; Kryukov, K.; Fukuda, A.; Morimoto, Y.; Naito, Y.; et al. Full-length 16S rRNA gene amplicon analysis of human gut microbiota using MinION™ nanopore sequencing confers species-level resolution. BMC Microbiol. 2021, 21, 35. [Google Scholar] [CrossRef]
- Andrade, M.C.N.; Kopytowski Filho, J.; Minhoni, M.T.D.; Coutinho, L.N.; Figueiredo, M.B. Productivity, biological efficiency, and number of Agaricus blazei mushrooms grown in compost in the presence of Trichoderma sp. and Chaetomium olivacearum contaminants. Braz. J. Microbiol. 2007, 38, 243–247. [Google Scholar] [CrossRef]
- Aguiar, L.V.B.D.; Sales-Campos, C.; Gouvêa, P.R.D.S.; Vianez, B.F.; Dias, E.S.; Chevreuil, L.R. Substrate disinfection methods on the production and nutritional composition of a wild oyster mushroom from the Amazon. Ciência E Agrotecnologia 2021, 45, e010321. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo, J.E.; Dias, E.S.; Rinker, D.L.; Caitano, C.E.C.; Zied, D.C. Optimization of cultivation techniques improves the agronomic behavior of Agaricus subrufescens. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Stoknes, K.; Scholwin, F.; Jasinska, A.; Wojciechowska, E.; Mleczek, M.; Hanc, A.; Niedzielski, P. Cadmium mobility in a circular food-to-waste-to-food system and the use of a cultivated mushroom (Agaricus subrufescens) as a remediation agent. J. Environ. Manag. 2019, 245, 48–54. [Google Scholar] [CrossRef]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci. Hort. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1991; pp. 115–148. [Google Scholar]
- Boratyn, G.M.; Schäffer, A.A.; Agarwala, R.; Altschul, S.F.; Lipman, D.J.; Madden, T.L. Domain enhanced lookup time accelerated BLAST. Biol. Direct. 2012, 17, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Coster, W.D.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Broeckhoven, C.V. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome. Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 15, 3094–3100. [Google Scholar] [CrossRef]
- Breitwieser, F.P.; Salzberg, S.L. Pavian: Interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics 2020, 36, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi. J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Mcgee, C.F.; Byrne, H.; Irvine, A.; Wilson, J. Diversity and dynamics of the DNA and cDNA-derived bacterial compost communities throughout the Agaricus bisporus mushroom cropping process. Ann. Microbiol. 2017, 67, 751–761. [Google Scholar] [CrossRef]
- An, Q.; Wu, X.J.; Dai, Y.C. Comparative genomics of 40 edible and medicinal mushrooms provide an insight into the evolution of lignocellulose decomposition mechanisms. 3 Biotech 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wei, J.K.; Wang, Q.H.; Yang, R.; Gao, X.J.; Sang, Y.X.; Cai, P.P.; Zhang, G.Q.; Chen, Q.J. Lignocellulose utilization and bacterial communities of millet straw based mushroom (Agaricus bisporus) production. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Carrasco, J.; Tello, M.L.; Toro, M.; Tkacz, A.; Poole, P.; Pérez-Clavijo, M.; Preston, G. Casing microbiome dynamics during button mushroom cultivation: Implications for dry and wet bubble diseases. Microbiology 2019, 165, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; García-Delgado, C.; Lavega, R.; Tello, M.L.; Toro, M.; Barba-Vicente, V.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Pérez, M.; Preston, G. Holistic assessment of the microbiome dynamics in the substrates used for commercial champignon (Agaricus bisporus) cultivation. Microb. Biotechnol. 2020, 13, 1933–1974. [Google Scholar] [CrossRef]
- Sun, S.; Li, F.; Xu, X.; Liu, Y.; Kong, X.; Chen, J.; Liu, T.; Chen, L. Study on the community structure and function of symbiotic bacteria from different growth and developmental stages of Hypsizygus marmoreus. BMC Microbiol. 2020, 20, 311. [Google Scholar] [CrossRef] [PubMed]
- Pecchia, J.; Cortese, R.; Albert, I. Investigation into the microbial community changes that occur in the casing layer during cropping of the white button mushroom, Agaricus bisporus. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products, New Delhi, India, 19–22 November 2014; ICAR-Directorate of Mushroom Research: Himachal Pradesh, India, 2014; pp. 309–313. [Google Scholar]
- Vos, A.M.; Heijboer, A.; Boschker, H.T.; Bonnet, B.; Lugones, L.G.; Wösten, H.A. Microbial biomass in compost during colonization of Agaricus bisporus. AMB Express 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sheng, J.P.; Chen, L.; Zheng, Y.Y.; David, Y.W.L.; Yang, Y.; Xu, M.S.; Shen, L. Biocontrol activity of Bacillus subtilis isolated from Agaricus bisporus mushroom compost against pathogenic fungi. J. Agric. Food Chem. 2015, 63, 6009–6018. [Google Scholar] [CrossRef]
- Pandin, C.; Védie, R.; Rousseau, T.; Le Coq, D.; Aymerich, S.; Briandet, R. Dynamics of compost microbiota during the cultivation of Agaricus bisporus in the presence of Bacillus velezensis QST713 as biocontrol agent against Trichoderma aggressivum. Biol. Control 2018, 127, 39–54. [Google Scholar] [CrossRef]
- Ralph, N.; Andreja, D.P.; Philip, J.H.; Jemma, P.; Alison, R. Volatile C 8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia 2009, 101, 583–591. [Google Scholar] [CrossRef]
- Colauto, N.B.; Fermor, T.R.; Eira, A.F.; Linde, G.A. Pseudomonas putida stimulates primordia on Agaricus bitorquis. Curr. Microbiol. 2016, 72, 482–488. [Google Scholar] [CrossRef]
- Jarial, R.S.; Shandilya, T.R.; Kumad, J. Casing in Mushroom Beds—A review. Agric. Rev. 2005, 26, 261–271. [Google Scholar]
- Kim, M.K.; Math, R.K.; Cho, K.M.; Shin, K.J.; Kim, J.O.; Ryu, J.S.; Lee, Y.H.; Yun, H.D. Effect of Pseudomonas sp. P7014 on the growth of edible mushroom Pleurotus eryngii in bottle culture for commercial production. Bioresour Technol. 2008, 99, 3306–3308. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, J.S.; Crowley, D.E.; Cho, B.G. Growth promotion of the edible fungus Pleurotus ostreatus by fluorescent pseudomonads. FEMS Microbiol. Lett. 2003, 218, 271–276. [Google Scholar] [CrossRef]
- Brurberg, M.B.; Eijsink, V.G.; Haandrikman, A.J.; Venema, G.; Nes, I.F. Chitinase B from Serratia marcescens BJL200 is exported to the periplasm without processing. Microbiology 1995, 141, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kaomek, M.; Mizuno, K.; Fujinira, T.; Sriyotha, P.; Cairna, J.R.K. Cloning, expression and characterization of an antifungal chitinase from Leucaena leucocephla De Wit. Biosci. Biotechnol. Biochem. 2003, 67, 667–676. [Google Scholar] [CrossRef]
- Van Houdt, R.; Givskov, M.; Michiels, C.W. Quorum sensing in Serratia. FEMS Microbiol. Rev. 2007, 31, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, F.P.; Passarelli-Araujo, H.; Regis, J.A.R.; da Rocha, L.O.; de Souza, E.M.; Aravind, L.; Olivares, F.L.; Venancio, T.M. Genome sequencing and assessment of plant growth-promoting properties of a Serratia marcescens strain isolated from vermicompost. BMC Genom. 2018, 39, 750. [Google Scholar] [CrossRef]
- Das, M. Chitinase produced by Serratia marcescens SMG isolated from decomposed Volvariella volvacea. Afr. J. Microbiol. Res. 2011, 5, 3220–3222. [Google Scholar] [CrossRef]
- Sun, S.J.; Liu, Y.C.; Weng, C.H.; Sun, S.W.; Li, F.; Li, H.; Zhu, H. Cyclic dipeptides mediating quorum sensing and their biological effects in Hypsizygus marmoreus. Biomolecules 2020, 10, 298. [Google Scholar] [CrossRef]
- Schmidt, R.; Jager, V.D.; Zühlke, D.; Wolff, C.; Bernhardt, J.; Cankar, K.; Garbeva, P. Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo-González, J.E.; Zied, D.C. Casing materials and techniques in Agaricus bisporus cultivation. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; Wiley Blackwell: West Sussex, UK, 2017; pp. 149–174. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iossi, M.R.; Palú, I.A.; Soares, D.M.; Vieira, W.G., Jr.; Alves, L.S.; Stevani, C.V.; Caitano, C.E.C.; Atum, S.V.F.; Freire, R.S.; Dias, E.S.; et al. Metaprofiling of the Bacterial Community in Colonized Compost Extracts by Agaricus subrufescens. J. Fungi 2022, 8, 995. https://doi.org/10.3390/jof8100995
Iossi MR, Palú IA, Soares DM, Vieira WG Jr., Alves LS, Stevani CV, Caitano CEC, Atum SVF, Freire RS, Dias ES, et al. Metaprofiling of the Bacterial Community in Colonized Compost Extracts by Agaricus subrufescens. Journal of Fungi. 2022; 8(10):995. https://doi.org/10.3390/jof8100995
Chicago/Turabian StyleIossi, Matheus Rodrigo, Isabela Arruda Palú, Douglas Moraes Soares, Wagner G. Vieira, Jr., Lucas Silva Alves, Cassius V. Stevani, Cinthia E. C. Caitano, Samir V. F. Atum, Renato S. Freire, Eustáquio S. Dias, and et al. 2022. "Metaprofiling of the Bacterial Community in Colonized Compost Extracts by Agaricus subrufescens" Journal of Fungi 8, no. 10: 995. https://doi.org/10.3390/jof8100995
APA StyleIossi, M. R., Palú, I. A., Soares, D. M., Vieira, W. G., Jr., Alves, L. S., Stevani, C. V., Caitano, C. E. C., Atum, S. V. F., Freire, R. S., Dias, E. S., & Zied, D. C. (2022). Metaprofiling of the Bacterial Community in Colonized Compost Extracts by Agaricus subrufescens. Journal of Fungi, 8(10), 995. https://doi.org/10.3390/jof8100995





