Abstract
Fusarium head blight (FHB) caused by Fusarium graminearum is a significant disease among cereal crops. In F. graminearum, biosynthesis of leucine, which is a branched chain amino acid, is achieved by converting α-isopropylmalate to β-isopropylmalate catalyzed by isopropylmalate isomerase encoded by LEU1. Considering the potential for targeting this pathway by fungicides, we characterized the gene FgLEU1 (FGSG-09589) in the Fusarium graminearum genome using bioinformatics methods. For functional characterization, we constructed a deletion mutant of FgLEU1 (ΔLEU1) through homologous recombination. Compared with the wild-type strain PH-1, ΔLEU1 showed slower colony growth and fewer aerial mycelia. Leucine addition was needed to ensure proper mutant growth. Further, ΔLEU1 showed decreased conidial production and germination rates, and could not produce ascospores. Moreover, ΔLEU1 showed complete loss of pathogenicity and reduced ability to produce deoxynivalenol (DON) and aurofusarin. Upstream and downstream genes of FgLEU1 were significantly upregulated in ΔLEU1. Contrary to previous reports, the deletion mutant was more resistant to osmotic stress and cell wall-damaging agents than the wild-type. Taken together, FgLEU1 plays a crucial role in leucine synthesis, aerial mycelial growth, sexual and asexual reproduction, pathogenicity, virulence, and pigmentation in Fusarium graminearum, indicating its potential as a target for novel antifungal agents.
1. Introduction
Fusarium head blight (FHB) is a serious disease of wheat, barley, and other small grains, usually resulting in lower grain yields, lower quality, and mycotoxin contamination [1]. Fusarium graminearum is recognized as the pathogen of FHB worldwide [1,2]. Ascospores of F. graminearum overwinter on crop residues such as maize, wheat, and barley, then infect the plants upon germination in the following spring and are spread by conidia during flowering [2]. FHB reduces grain quality through the destruction of starch and protein and by the accumulation of mycotoxins such as the trichothecene derivative, deoxynivalenol (DON); these changes can occur within a few weeks [3], and toxin pollution is inevitable once the seeds are infected [2]. DON is a threat to both human and animal health [4,5,6], and its synthesis in F. graminearum involves the Tri gene cluster [7]. Recently, some microorganisms have been found to degrade DON [8,9], but their use in agriculture is limited. The current control of FHB relies on some fungicides [2] and pesticides, which can cause great harm to plants and human health. Control of FHB and mycotoxin contamination thus remains challenging due to the lack of resistant wheat cultivars to FHB and limited effective fungicides against F. graminearum [1]. Considering this situation, the development of effective new fungicides that are less toxic and effective is an urgent need.
Three branch-chain amino acids (BCAAs), including isoleucine, leucine, and valine, cannot be synthesized by humans and other mammals, but can only be synthesized by bacteria, archaea, fungi, and plants [10]. Therefore, enzymes involved in the biosynthesis of BCAAs can be effective targets for fungicides. For example, sulfonylureas inhibit acetylhydroxyl synthase, the first enzyme in the branched chain amino acid synthesis pathway, and are thus used as feedstocks for fungicides [11] and herbicides [12,13,14]. Biosynthesis of branched amino acids is highly conserved in fungi, bacteria, and plants [14]. It involves a common pathway catalyzed by the same four enzymes [10] that leads to conversion of pyruvate and α-ketobutyrate to valine and isoleucine and a branch that leads to the conversion of α-ketoisovalerate, the immediate precursor of valine, to leucine [15]. Leucine synthesis begins with 3-methyl-2-oxobutanoate. The first step is the condensation of 3-methyl-2-oxobutanoate and acetyl-CoA catalyzed by isopropylmalate synthase (IPMS) to form 2-isopropylmalate. The second step is catalyzation by isopropylmalate isomerase (IPMI), also known as isopropylmalate dehydratase, and involves conversion of 2-isopropylmalate (2-IPM) to 3-isopropylmalate (3-IPM). In the third step, 4-methyl-2-oxopentanoate is synthesized by the oxidative decarboxylation of isopropyl malate dehydrogenase (IPMDH). The reversible reaction catalyzed isopropylmalate isomerase encoded by the LEU1 gene is an indispensable step in leucine synthesis [10], similar to the reactions catalyzed by the aconitase family monomeric proteins, which contain [4Fe-4S] clusters [16].
BCAAs serve as building blocks for all life forms and act as important nutritional signals that mediate protein synthesis, glucose homeostasis, and nutrient-sensitive signaling pathways [14]. LEU1 gene expression is highly conserved in different species, and mainly affects the leucine biosynthesis pathway. Leu1 acts as an isopropylmalate isomerase to isomerize α-isopropylmalic acid into β-isopropylmalic acid. The functions of LEU1 homologs have been studied in many organisms. In S. cerevisiae, the expression product of the LEU1 gene acts as an isopropylmalate isomerase and is regulated by Leu3p [15]. Mitochondrial Fe-S cluster (ISC) synthesis is regulated by the leucine biosynthetic pathway [17]; the role of Leu1 in iron metabolism has also been demonstrated in Cryptococcus neoformans [18]. In a subsequent study, LEU1 was suggested to combine with the regulator of the H+-ATPase in the vacuolar and endosomal membranes (REVA) to jointly regulate V-ATPase activity, which may affect the tolerance of F. raminearum to Ca2+ [19].
Several genes involved in leucine biosynthesis have also been studied in Magnaporthe oryzae, a model filamentous fungus. The results showed that deletion of leucine biosynthesis genes affected the development and pathogenicity of fungi [20,21]. According to Wei et al., leucine biosynthesis may be related to the degradation of glycogen and lipid droplets, which is related to the energy supply in fungi [21]. In F.graminearum, FgLEU2A/B are two paralogous downstream genes of FgLEU1; knockout of these genes was shown to inhibit the synthesis of 3-isopropylmalate dehydrogenase (IPMD), leading to severe damage to mycelial growth and pathogenicity [22]. These results indicate that inhibition of leucine synthesis affects the normal life activities of the fungus.
Considering the importance of leucine biosynthesis and its potential as a target for fungicides, we characterized the gene FgLEU1 (FGSG-09589) in the F.graminearum genome in this study. We then utilized polyethylene glycol-mediated fungal transformation to yield a FgLEU1-deficient mutant strain and examined the functional role of FgLEU1 in vegetative growth, asexual and sexual development, plant infection, and other traits of F. graminearum.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
F. graminearum PH-1 was used as the wild-type (WT) strain for constructing the mutants (ΔLEU1) and complementary (ΔLEU1-C) strains. All the strains were cultured at 25 °C on potato dextrose agar medium (PDA), complete medium (CM), fructose gelatin agar (FGA), or yeast extract peptone dextrose (YEPD). For mycelial growth assays, all strains were grown at 25 °C on PDA medium and CM medium for 4 days. The colony diameter was measured using the crossed wire method. The average of the data obtained was processed. For the aerial hyphal growth assay, 5 mL of sterilized PDA medium was added to the test tube (1.5 cm in diameter). All strains were inoculated and grown for 5 days at 25 °C in test tubes. Hyphal quality was measured by filtration collection of solid materials after culture for 5 d in PDB, rinsed with sterilized water, dried in an oven at 65 °C, followed by determination of mycelial dry weight. All experiments were repeated three times. To induce asexual reproduction, mycelia were shaken in liquid carboxymethyl cellulose (CMC) medium at 180 rpm. Fungal mycelia were harvested from potato dextrose broth (PDB) and used for extracting total genomic DNA and RNA. Escherichia coli DH5a was used for normal bacterial transformation and was cultured at 37 °C. To test cell wall integrity, the colony diameter was measured using a CM plate with 0.4 mg/mL Congo Red (CR) added. Exogenous amino acids were added to the FGA at different concentrations, as indicated in the figures. Five 5 mm mycelial plugs of the wild-type strain and mutants were collected from the edge of a 3-day-old colony and incubated in the plates. Carrot agar was used to assess sexual reproduction. The experiments were repeated three times, with three replicates each.
2.2. Generation of FgLEU1 Deletion Mutants
We found the sequence of FgLEU1 (FGSG_09589) from the F. graminearum database. To detect FgLEU1, we first PCR-amplified FgLEU1-A and FgLEU1-B using the primer set FgLEU1-A-F/ FgLEU1-A-R and FgLEU1-B-F/ FgLEU1-B-R (Table S1) and F. graminearum PH-1 genomic DNA as templates. The hygromycin B phosphotransferase (HPH) gene was amplified from plasmid pCB1003 with primer pairs HYG-F/HY-R and YG-F/HYGR (Table S1). The fusion cassette containing the gene-flanking sequences and the HPH gene was obtained by split-marker PCR, and the resultant PCR product was purified and stored at −20 °C for protoplast transformation PCR conditions: 200 mM primers, 200 mM dNTP, 1 unit of Phanta Max Super-Fidelity DNA Polymerase (Vazyme Biotech Co, Jiangsu, China, CAT: P505-d1), and the PCR fragments were transformed into the protoplasts of PH-1 using PEG to generate ΔFgLEU1 mutants. TB3 medium supplemented with hygromycin B (300 mg/L) was used for preliminary screening of the transformations. Single spore isolation and PCR identification were performed. Southern blotting analyses of FgLEU1 deletion mutants were performed using DIG High Prime DNA Labeling and Detection Starter Kit I, according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany). The probes were PCR-amplified using the primer pair tLEU1F/R (Table S1). The strains were stored in sterile water at −20 °C for further experiments.
2.3. Gene Complementation
To prepare a complementation strain, a 4.5 kb DNA fragment containing the full-length FgLEU1 sequence, and its promoter sequence was amplified by PCR with the primer pair FgLEU1-CF/R. Then, the recombinant plasmid pYF11 using the yeast in vivo recombination approach (Roche Diagnostics, Mannheim, Germany), obtain recombinant plasmid pYF11-FgLEU1. The recombinant plasmid was transformed into protoplasts of the ΔLEU1 mutants to produce ΔLEU1 (ΔLEU1-C) complemented strains, which were selected using 200 mg/mL G418 and were identified by PCR using the primer pair ΔLEU1-CF/R (Table S1).
2.4. Asexual and Sexual Reproduction Assays
For conidiation assays, two 5 mm mycelial plugs of each strain were placed in 30 mL CMC medium (1 g KH2PO4, 2 g NaNO3, 1 g yeast extract, 0.5 g MgSO4·7H2O, and 15 g sodium carboxymethyl cellulose in 1000 mL distilled H2O) for 3 days in a shaker at 180 rpm at 25 °C. The number of conidia was counted for each strain using a hemocytometer. To determine the germination rate of conidia, 5-day-old conidia were resuspended in yeast extract peptone dextrose (YEPD) medium (yeast extract, 10.0 g; peptone, 20.0 g; glucose, 20.0 g; distilled water, 1000 mL), in a final volume of 80 mL, then shaken at 180 rpm, 25 °C for 4–6 h, followed by examination under a fluorescence microscope (Eclipse 90i, Nikon, Tokyo, Japan). Each experiment was performed in triplicate.
For sexual reproduction assays, each strain was inoculated on carrot medium for 7 days, aerial hyphae were pressed down with 1 mL of sterile 2.5% Tween 60 solution for sexual reproduction assays and cultured for 21 days at 25 °C under black light. Perithecium formation was examined using a microscope (Leica MZ10 F, Germany). Each experiment was repeated three times.
2.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
To determine the expression levels of the genes to be determined, the same amounts of conidia of strains were inoculated into PDA and glucose yeast extract peptone (GYEP) medium and cultured in a shaker at 25 °C and 180 rpm for 3 days. The total RNA of wild-type PH-1 and deletion mutants was isolated from the hyphae using TransZol Up (TransGen Biotech, Beijing, China). qRT-PCR experiments were performed according to the manufacturer’s instructions. The 2−ΔΔCT method was used to quantify the transcription levels relative to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as a reference. The primers used are shown in Table S1.
2.6. Plant Infection and DON Production Assays
To assess the virulence of F. graminearum on wheat heads, conidia were harvested from 5-day-old CMC cultures and resuspended in sterile distilled water at 4 × 105 spores/mL [23]. Briefly, a 10 μL conidial suspension was injected into the middle lower spikelet of wheat heads from susceptible wheat cv. Jimai 22 during flowering [24], and the inoculated wheat heads were sprayed with sterile distilled water and then wrapped in small plastic bags for 48 h to maintain high humidity. The wheat heads were then photographed and assayed 14 days after inoculation [25]. To assess the virulence of F. graminearum on corn silk, a hyphal plug (5 mm in diameter) was inoculated on four fresh corn silks placed on plates at 25 °C and high humidity. The results were observed at 5 dpi, and each experiment was performed in triplicate. To determine DON production and ergosterol levels. DON toxin used ergosterol as internal reference, and 25 g of autoclaved healthy wheat kernels were inoculated with eight mycelial plugs of each strain for 21 days at 25 °C. DON samples were quantified by liquid chromatography and tandem mass spectrometry, as described previously [26]. Each experiment was performed in triplicate.
2.7. Statistical Analysis
DPS data processing system was used for data analysis. Data were presented as the mean ± standard error values, and the differences among variables were analyzed using Duncan’s multiple range test with p < 0.05.
3. Results
3.1. Identification of FgLEU1 in F. graminearum
One putative LEU1 (FGSG_09589, designated as FgLEU1) in F. graminearum was retrieved by a BLAST search in National Center for Biotechnology Information (NCBI Blast: https://blast.ncbi.nlm.nih.gov/Blast.cgi) with M. oryzae LEU1 (MGG_01553) as the query. The FgLEU1 gene is predicted to encode a 777-amino acid protein showing 62.47% identity with S. cerevisiae and 63.42% identity with M. oryzae. SMART-PFAM analysis revealed that FgLEU1 contains two different regions: aconitate hydratase and an Aconitase C-terminal domain. Phylogenetic analysis using the neighbor-joining method indicated that LEU1 is highly conserved among F. graminearum and other fungi (Figure 1).
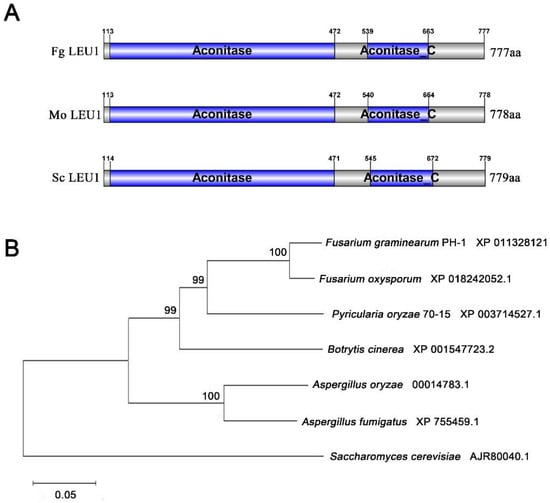
Figure 1.
Sequence and phylogenetic analysis of LEU1. (A) Sequence and phylogenetic analysis of LEU1 in Fusarium graminearum (Fg), Magnaporthe oryzae (Mo), and Saccharomyces cerevisiae (Sc). Aconitase: An enzyme that catalyzes the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle through a non-redox-active process. Aconitase C: The Aconitase C-terminal domain, which undergoes conformational change in the enzyme mechanism. (B) A phylogenetic tree of LEU1 with homologues from other fungal species was constructed using the neighbor-joining method in MEGA version 7.0. Numbers at the nodes in the rooted tree represent the bootstrap value after 1000 replications. GenBank accession numbers are presented after the fungal species names. The bar indicates 0.05 distance units.
To determine the biological function of FgLEU1 in F. graminearum, gene replacement constructs were generated and transformed into the wild-type (WT) strain PH-1 to generate FgLEU1 deletion mutants (ΔLEU1), using homologous recombination; molecular verification of FgLEU1 is shown in Figure S1. We obtained at least three independent deletion mutants for FgLEU1 and further verified them by Southern blot analysis (Supplementary Figure S1). pYF11-LEU1 complementation constructs were generated using the primer pairs CF/CR, and were transformed into the ΔLEU1 mutant protoplasts to produce the complementation strain, ΔLEU1-C. The expression levels of FgLEU1 were assessed using qRT-PCR analysis (Supplementary Figure S1). ΔLEU1 mutants did not express the corresponding target genes, whereas the expression levels of FgLEU1 were restored in the complementation strains.
3.2. FgLEU1 Is Responsible for Hyphal Growth, Conidiation, and Conidial Germination
To determine the role of FgLEU1 in hyphal growth, ΔLEU1 mutants were inoculated onto PDA, fructose gelatin agar (FGA) and yeast extract peptone dextrose (YEPD) plates for growth assays. Compared with the wild-type strain PH-1, on PDA, the colony diameter of the ΔLEU1 mutant was reduced by approximately 18.4%, hyphal quality was reduced by approximately 91.6% (Figure 2A,B), and aerial hyphae in germ tubes were significantly shorter than those in PH-1(Figure 2C,D). ΔLEU1 mutants could not grow on the nutritionally deficient FGA plate but grew normally on YEPD medium with sufficient nutrition.
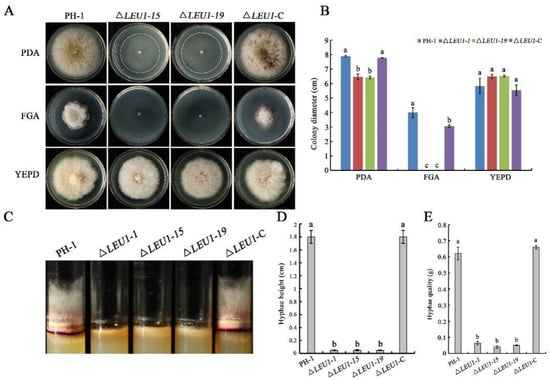
Figure 2.
Hyphal growth of PH-1, ΔLEU1, and ΔLEU1-C strains. (A) Colonies of the wild type (PH-1), FgLEU1 deletion mutants, and complemented strains were cultured on PDA, FGA, and YEPD agar plates after 4 days at 25 °C. (B) Height of aerial mycelium in test tubes containing PDA medium after 4 days at 25 °C. (C) Colony diameters of strains cultured on PDA, FGA, and YEPD plates. (D) Colony height of strains cultured in tubes with PDA. (E) Hyphal quality of strains cultured in potato dextrose broth (PDB) at 25 °C for 5 days at 180 rpm. Different letters on the bars for each treatment indicate significant differences at p < 0.05 by Duncan’s multiple range test. Measurements represent the average of three independent experiments. All experiments were repeated three times with three replicates at each time.
Compared to the WT strain, conidiation in 5-day-old cultures of ΔLEU1 mutants in carboxymethyl cellulose (CMC) medium was reduced by approximately 78.9% (Table 1), but their morphology did not change significantly. When incubated on yeast extract peptone dextrose (YEPD) medium, approximately 60.34% of the PH-1 conidia germinated at 4 h post-incubation (hpi). Under the same conditions, the germination rate of ΔLEU1 conidia was 42.32%, and the length of the germ tubes was slightly shorter than that in PH-1 (Figure 3A). Taken together, these results indicated that the presence of FgLEU1 is required for cells for sexual reproduction.

Table 1.
Conidiation, conidial germination perithecia counts, and DON production in the PH-1, ΔLEU1, and ΔLEU1-C strains.
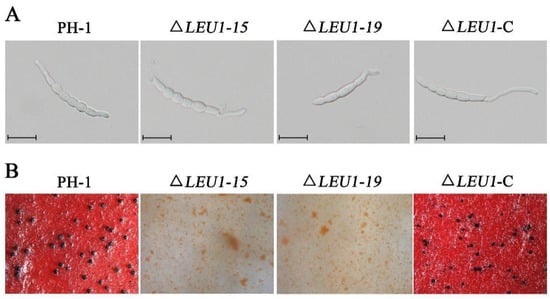
Figure 3.
Conidial germination and sexual reproduction of FgLEU1 deletion mutants. (A) All strains were incubated in liquid YEPD for 4 h at 25 °C with 180 rpm and examined for germination. Scale bar = 20 μm. (B) Sexual development of the strains. Perithecia were visualized as black structures on carrot agar plates. Photographs were taken at 21 days post self-fertilization. All experiments were repeated three times with three replicates at each time.
3.3. FgLEU1 Is Necessary for Sexual Reproduction
Sexual reproduction plays a critical role in the infection cycle of F. graminearum [27]. Therefore, we assessed the sexual reproduction of the mutants and wild type (PH-1) on carrot agar plates at 2–3 weeks post-fertilization. PH-1 and ΔLEU1-C produced abundant and normal perithecia, whereas the mutants did not produce perithecia (Figure 3B). These results indicate that FgLEU1 plays a crucial role in sexual reproduction and thus affects the disease cycle seriously.
3.4. FgLEU1 Is Essential for Pathogenicity and DON Production
To determine the role of FgLEU1 in pathogenicity, pathogenicity assays were conducted to determine the effect of ∆LEU1 on flowering wheat heads and corn silks. On wheat heads inoculated with 4.0 × 105 conidia/mL either PH-1 or ΔLEU1-C, typical scab symptoms spread from the inoculated to uninoculated spikelets within 14 days post inoculation (dpi). To our surprise, the mutant strain was completely unable to infect the spikelet, and the disease index score was 0 (Figure 4A,C). This was also confirmed by the results of corn silk infection assays; PH-1 and ΔLEU1-C could form typical brown lesions at 5 dpi, but the mutants could not (Figure 4B,D). These results indicate that FgLEU1 plays an important role in pathogenicity.
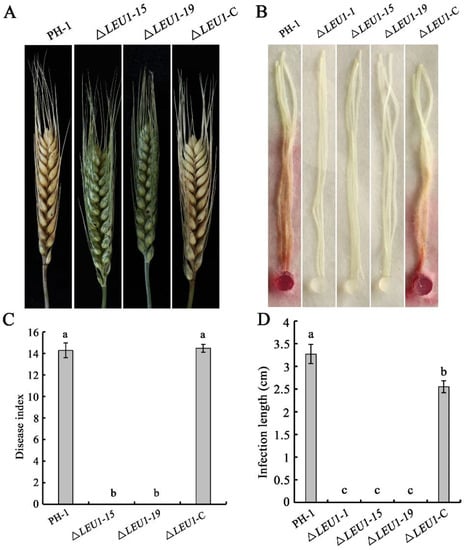
Figure 4.
Virulence of the WT strain and FgLEU1 deletion mutants on wheat heads and corn silks. (A) Disease symptom on flowering wheat heads caused by PH-1, ∆LEU1, and ∆LEU1-C strains after injection with conidial suspension (10 μL, 4 × 105 conidia/mL). Images were taken at 14 days post-inoculation (dpi). (B) Brown necrosis caused by PH-1, ∆LEU1, and ∆LEU1-C strain mycelial plugs on corn silks. Images were taken at 5 dpi at 25 °C. (C) Disease indices of F. graminearum determined at 14 dpi. At least 20 wheat heads were examined in each replicate. Error bars represent the standard errors of the means. Different letters on the bars for each treatment indicate significant differences at p < 0.05 by Duncan’s multiple range test. (D) The length of brown necrotic tissue upon infection was determined at 5 dpi. At least 20 corn silks were examined in each replicate. Different letters on the bars for each treatment indicate significant differences at p < 0.05 by Duncan’s multiple range test.
To determine the effect of FgLEU1 on DON production, we assayed DON production in toxin-producing rice medium inoculated with all strains for 21 days. The results showed that the ∆LEU1 mutant produced significantly less DON than PH-1 and ∆LEU1-C strains (Table 1). To confirm this finding, the expression of Tri5, Tri6, and Tri10 (trichothecene biosynthesis), which are essential for DON synthesis, was analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). As shown in Table 2, the expression of Tri genes was significantly downregulated in ΔLEU1 mutants compared to those in the WT strain. As expected, supplementation with leucine alleviated this change. These results indicate that FgLEU1 is involved in regulating Tri expression and DON synthesis. Based on these results, we concluded that leucine is involved in the DON synthesis pathway.

Table 2.
Relative expression level of Tri (trichothecene biosynthesis) genes in the PH-1, ΔLEU1, and mutants supplemented with leucine.
3.5. Deletion of FgLEU1 Results in Reduced Red Pigmentation
Deletion of FgLEU1 also led to reduced accumulation of red pigment; PH-1 and ∆LEU1-C formed pink colonies at 3 dpi, whereas ∆LEU1 formed slightly pale yellowish colonies on the bottom of the plates (Figure 5A). In PDB flasks, the filtrate of the wild-type and complemented strains appeared red, whereas the color of the mutant strain was almost unchanged (Figure 5B). These results indicated that FgLEU1 is involved in the production of the red pigment, aurofusarin.
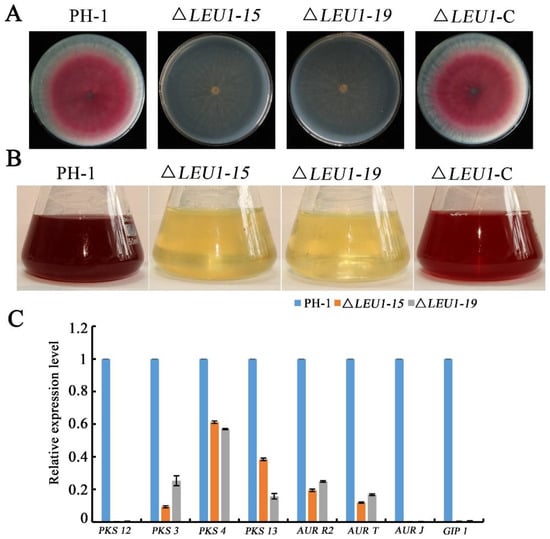
Figure 5.
Pigment production and relative expression level of aurofusarin biosynthesis genes by the wild-type strain PH-1 and FgLEU1 deletion mutants. (A) Red pigment aurofusarin formation by the wild-type PH-1, FgLEU1 deletion mutants, and complementation strains cultured on PDA at 25 °C for 4 days. (B) Red pigment aurofusarin formation by the wild-type PH-1, FgLEU1 deletion mutants, and complementation strains cultured in liquid medium in flasks containing 50 mL of PDB at 180 rpm, 25 °C for 5 days. (C) Relative expression levels of aurofusarin biosynthesis-related genes. GAPDH was used as an internal control. Gene expression in PH-1 was set to 1.0 (p < 0.05).
To further confirm this conclusion, we performed qRT-PCR to determine the expression levels of PKS12, PKS3, PKS4, PKS13, AURT, AURJ, AURR2, and GIP1 genes related to the biosynthesis of aurofusarin. As shown in Figure 5C, deletion of the FgLEU1 gene led to the downregulation of these genes, thus confirming that FgLEU1 deletion affects pigment biosynthesis.
3.6. FgLEU1 Plays a Crucial Role in Leu Biosynthesis in F. graminearum
As shown in Figure 2, FgLEU1 deletion resulted in amino acid auxotrophy in F. graminearum. ∆LEU1 could not survive normally on PDA, a semi-synthetic medium. It could not survive in the absence of amino acids on FGA. In contrast, the auxotrophy of ΔLEU1 could be completely recovered when cultured on the full-nutrition medium, YEPD. Therefore, we examined whether the growth defects caused by nutrient deficiency could be repaired by exogenous leucine added to fructose gelatin broth (FGB) medium. The results of mycelial biomass analysis showed that the nutritional deficiency of mutants could be partially recovered by exogenous leucine addition. When the terminal leucine concentration was greater than 0.25 mM, the growth defects of the mutants were almost completely eliminated (Figure 6).
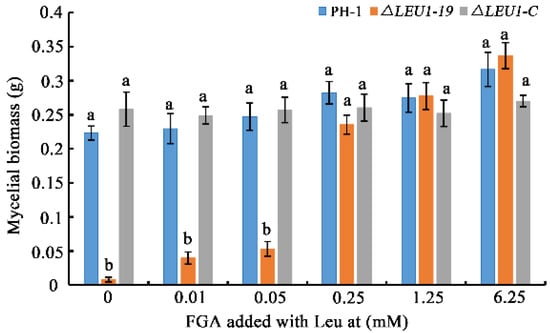
Figure 6.
The mycelial biomass of wild-type, ΔLEU1, and ∆LEU1-C strains with different concentrations of leucine. Different concentrations of leucine (final concentrations 0, 0.01, 0.05, 0.25, 1.25, and 6.25 mM) were added to fructose gelatin broth medium and the strains were cultured at 25 °C, 180 rpm, for 6 days. Mycelium was collected from the medium, dried at 65 °C and weighed after two days. Error bars represent the standard errors of the means. Different letters on the bars for each treatment indicate significant differences at p < 0.05 by Duncan’s multiple range test.
3.7. Effects of FgLEU1 Deletion on the Maintenance of Cell Wall Integrity
To investigate the possible role of FgLEU1 in maintaining cell wall integrity (CWI), we assessed the mycelial growth in all strains in the presence of cell wall-damaging agents. Congo red (CR) specifically binds substances in the cell wall, blocks normal cell wall assembly, produces cell wall stress, and inhibits fungal growth [28]. All strains were incubated in CM medium supplemented with 300 μg/mL of CR. ΔLEU1 showed increased tolerance to CR compared to PH-1 and ∆LEU1-C (Figure 7A,B). For further confirmation, all strains were treated with lysozyme, driselase, and snailase for 4 h at 30 °C. The mycelium of PH-1 was enzymatically cleaved and released many protoplasts, whereas most mycelia of ΔLEU1 were not enzymatically cleaved and remained intact (Figure 7C). These results suggest that FgLEU1 is associated with the maintenance of CWI.
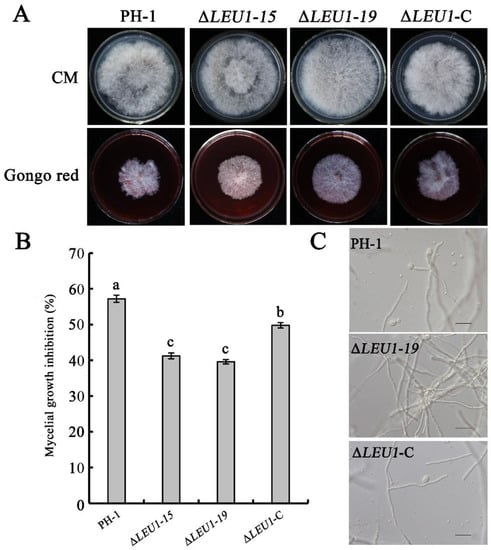
Figure 7.
Role of FgLEU1 in resistance to cell wall-damaging agents and cell wall-degrading enzymes. (A) Comparison of PH-1, ΔLEU1, and ΔLEU1-C after 4 days of incubation at 25 °C on CM plates with or without 300 mg/mL Congo Red (CR). The plates were photographed at 4 days post cultivation. (B) Mean rates of mycelial growth inhibition by CR on PH-1, ΔLEU1, or ΔLEU1-C strains at 4 days post cultivation. Different letters on the bars for each treatment indicate significant differences at p < 0.05 by Duncan’s multiple range test. Bar = 20 µm. Bars denote standard errors from three experiments. Values on bars followed by the same letter for each treatment indicate absence of a significant difference at p < 0.05. (C) After treatment with lysozyme, driselase, and snailase for 4 h at 30 °C, the mycelia of PH-1 and ΔLEU1-C mutants were well digested and numerous protoplasts were released, but this was not observed for ΔLEU1.
4. Discussion
The physiological and metabolic processes of plant pathogenic fungi are receiving extensive attention for the selection of targets in fungicide development. Branched chain amino acids (BCAAs) include valine, leucine, and isoleucine, which can only be synthesized by bacteria, archaea, fungi, and plants; animals, including humans, can only obtain these from their diet [10,29]. It is thus promising to examine enzymes in the BCAA biosynthetic pathway as potential targets. Amino acid synthesis plays an important role in the growth and development of plant pathogenic fungi and in host plant infestation [30,31]. This has been confirmed in several previous studies on F. graminearum. For example, enzymes involved in the biosynthesis of arginine (Arg)(Amt1), leucine (Leu), isoleucine (Ile), valine (Val) (Ilv1, Ilv2, Ilv3, Ilv5, Ilv6), and glutathione (Oxp1, Oxp2), affect the growth and development of F. graminearum [14,32,33,34,35,36]. These results all indicate the link between amino acid malnutrition and morphological growth and the pathogenicity of phytopathogenic fungi. In this study, we demonstrated that targeting alternative leucine biosynthesis of FgLEU1 in F. graminearum leads to a significant reduction in the growth of mutant ΔLEU1 strains, thereby inhibiting conidiogenesis. These results are consistent with previous findings.
LEU1 is dispensable for vegetative growth in S. cerevisiae, M. oryzae, and C. neoformans [18,20,37]. Based on our experimental results that ΔLEU1 could not colonize the nutrient-deficient (nutrient-poor) FGA medium and that exogenous supplementation with Leu could effectively rescue the nutritional deficiency of the mutant, we concluded that leucine plays a conserved role in the morphogenesis of F. graminearum. Moreover, we found that 0.25 mM Leu is required for the normal growth of the strain.
The hyphae of F. graminearum form overwintering structures together with the host plant tissue, in the spring field, the airborne ascospores then become the main primary inoculum that infect the host flowering structures. The process of sexual development to produce ascospores plays a crucial role in the disease cycle [1,38]. The inability of the ΔLEU1 mutant to produce perithecia and subsequent ascospores suggesting that its presence is required for cells for sexual reproduction in F. graminearum. Deletion of the FgLEU1 gene leads to blockage of the disease transmission pathway, suggesting that FgLEU1 may be a useful fungicide target for controlling FHB.
Liu et al. [22] reported that LEU2 is involved in the pathogenicity of F. graminearum; MoLEU1, MoLEU2, and MoLEU4 are regulated by MoLEU3, which also affects the pathogenicity of M. oryzae [21]. Plant infection assays revealed that the ΔLEU1 mutant had significantly reduced infestability in wheat heads and corn silk. Reduced pathogenicity is influenced by many factors. For example, we found that the number and germination rate of mutant conidia were reduced (Table 1) and mycelial growth was inhibited under low nutrient conditions. During the spore germination and infestation stages, the nutrients required by the pathogenic fungus were derived exclusively from the internal stores, and the fungus only carries out its own nutrient synthesis after infestation [30]. The lack of nutrients in plants does not allow the pathogenic fungal growth. However, synthesis of leucine in the mutant strain is blocked; thus, the pathogenic fungus cannot grow properly to complete the infestation. The study by Tang et al. [20] agrees with this view.
Both DON and pigment production are important virulence factors. DON is a secondary metabolite produced by F.graminearum, and previous studies have shown that DON production significantly contributes to the fungal infestation in wheat spikes [39,40].
This study showed that DON production was reduced in ΔLEU1 mutants (Table 1), suggesting that deletion of FgLEU1 interfered with DON production in F. graminearum. Furthermore, qRT-PCR analysis confirmed that the expression levels of trichothecene synthase genes, Tri5, Tri6, and Tri10 were reduced in the ΔLEU1 mutant; exogenous addition of leucine increased their expression, but its effect was not significant (Table 2).
Pigment production is accompanied by the growth stage of F. graminearum. As pigment deposition in the cell wall causes mycelium color changes, color can also be an indicator of growth in growth studies [41]. Mycelial color changes were also observed in the mutant strains in this study, and significant downregulation of pigmentation genes was observed in the FgLEU1 deficient strain. These results are consistent with the reduced pathogenicity of the mutant, indicating that the FgLEU1 gene plays an important role in the growth of F. graminearum. Further, FgLEU1 deletion mutants showed reduced sensitivity to cell wall disruptors, which is consistent with the high tolerance to cell wall-lysing enzymes. These results differ from previous reports on mutants of BCAA synthesis genes in filamentous fungi [14,18,22,35].
Que et al. [42] previously proposed the metabolic pathway of leucine in M. oryzae. Combined with the results of qRT-PCR in this study, we concluded that the expression of LEU3 is not regulated by genes such as LEU1, LEU2, and LEU4. Deletion of LEU1 can significantly upregulate LEU2, LEU4, and BAT2 genes, indicating that LEU1 can affect the expression of its upstream and downstream genes.
In summary, we identified and elucidated the biological functions of FgLEU1 in the phytopathogenic fungus, F. graminearum. The results showed that FgLEU1 is essential for aerial hyphal growth, conidiation, virulence, pigmentation, and full pathogenicity in F. graminearum. These findings provide further evidence indicating the importance of leucine biosynthesis in the development of filamentous phytopathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8101090/s1, Figure S1: The FgLEU1 gene deletion strategy and confirmation in F. graminearum; Figure S2. Sexual reproductive morphology of the wild-type and revertant strains. Table S1: Primers used in this study.
Author Contributions
L.Z. and J.Y. designed the experiments, supervised the work, and revised and edited the manuscript; S.S. performed the majority of the experiments, and wrote the draft manuscript; M.W. and C.L. analyzed the data; Y.L. participated in the article modification; Y.T. and T.W. performed data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wheat Innovation Team of Shandong Province Modern Agricultural Industry Technology System (SDAIT-01-09), Natural science gene of Shandong Province (ZR2021QC141), and Shandong Excellent Youth Fund (ZR2021YQ20).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Ann. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Güldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef]
- Fan, X.; He, F.; Ding, M.; Geng, C.; Chen, L.; Zou, S.; Liang, Y.; Yu, J.; Dong, H. Thioredoxin Reductase Is Involved in Development and Pathogenicity in Fusarium graminearum. Front. Microbiol. 2019, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Dai, Y.; Wang, Y.; Lee, Y.W.; Shi, J.; Xu, J. Biodegradation of Deoxynivalenol by a Novel Microbial Consortium. Front. Microbiol. 2019, 10, 2964. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Ji, F.; Xu, L.; Yu, M.; Shi, J.; Xu, J. Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 2019, 276, 436–442. [Google Scholar] [CrossRef]
- Binder, S. Branched-Chain Amino Acid Metabolism in Arabidopsis thaliana. Arab. Book. 2010, 8, e0137. [Google Scholar] [CrossRef]
- Boigegrain, R.A.; Liautard, J.P.; Köhler, S. Targeting of the virulence factor acetohydroxyacid synthase by sulfonylureas results in inhibition of intramacrophagic multiplication of Brucella suis. Antimicrob Agents Chemother. 2005, 49, 3922–3925. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, X. Acetohydroxyacid synthases: Evolution, structure, and function. Appl. Microbiol. Biotechnol. 2016, 100, 8633–8649. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, S.; Cheng, D.; Li, Y.; Liu, J.; Xie, Y.; Li, Y.; Li, Z. Design, synthesis and herbicidal activity study of aryl 2,6-disubstituted sulfonylureas as potent acetohydroxyacid synthase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Y.; Zhang, Y.; Yu, M.; Jiang, H.; Xu, J.; Shi, J. FgIlv3a is crucial in branched-chain amino acid biosynthesis, vegetative differentiation, and virulence in Fusarium graminearum. J. Microbiol. 2019, 57, 694–703. [Google Scholar] [CrossRef]
- Kohlhaw, G.B. Leucine biosynthesis in fungi: Entering metabolism through the back door. Microbiol. Mol. Biol. Rev. 2003, 67, 1–15, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Gruer, M.J.; Artymiuk, P.J.; Guest, J.R. The aconitase family: Three structural variations on a common theme. Trends Biochem. Sci. 1997, 22, 3–6. [Google Scholar] [CrossRef]
- Bedekovics, T.; Li, H.; Gajdos, G.B.; Isaya, G. Leucine biosynthesis regulates cytoplasmic iron-sulfur enzyme biogenesis in an Atm1p-independent manner. J. Biol. Chem. 2011, 286, 40878–40888. [Google Scholar] [CrossRef]
- Do, E.; Hu, G.; Caza, M.; Oliveira, D.; Jung, W.H. Leu1 plays a role in iron metabolism and is required for virulence in Cryptococcus neoformans. Fungal Genet. Biol. 2015, 75, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Gao, T.; Gu, C.; Sun, F.; Yu, L.; Hu, J. Characterization of the complex involved in regulating V-ATPase activity of the vacuolar and endosomal membrane. J. Bioenerg. Biomembr. 2017, 49, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, H.; Zheng, Q.; Chen, X.; Wang, R.; Yang, S.; Zhao, G.; Liu, J.; Norvienyeku, J.; Wang, Z. Isopropylmalate isomerase MoLeu1 orchestrates leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2019, 103, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Yu, Q.; Dong, B.; Zhang, Y.; Liu, X.H.; Lin, F.C.; Liang, S. MoLEU1, MoLEU2, and MoLEU4 regulated by MoLEU3 are involved in leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzae. Environ. Microbiol. Rep. 2019, 11, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Q.; Wang, J.; Wang, X.; Xu, J.; Shi, J. Two FgLEU2 Genes with Different Roles in Leucine Biosynthesis and Infection-Related Morphogenesis in Fusarium graminearum. PLoS ONE 2016, 11, e0165927. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhou, B.; Gao, Z.; Liang, Y. Effects of Phospholipase C on Fusarium graminearum Growth and Development. Curr. Microbiol. 2015, 71, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Liu, C.; Sun, S.; Liu, A.; Liang, Y.; Yu, J. The roles of FgPEX2 and FgPEX12 in virulence and lipid metabolism in Fusarium graminearum. Fungal Genet. Biol. 2020, 135, 103288. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhu, Q.; Liang, Y.; Li, J.; Fan, X.; Yu, X.; He, F.; Xu, H.; Liang, Y.; Yu, J. Differential roles of three FgPLD genes in regulating development and pathogenicity in Fusarium graminearum. Fungal Genet. Biol. 2017, 109, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mirocha, C.J.; Kolaczkowski, E.; Xie, W.; Yu, H.; Jelen, H. Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography/M\mass spectrometry. J. Agric. Food Chem. 1998, 46, 1414–1418. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, R.; Zhang, J.; Ma, J.; Wu, Z.; Wang, G.; Wang, C.; Xu, J.R. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS ONE 2013, 8, e66980. [Google Scholar] [CrossRef]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.C.; Omer, S.; Sheldon, J.R.; Welch, I.; Heinrichs, D.E. Role of BrnQ1 and BrnQ2 in branched-chain amino acid transport and virulence in Staphylococcus aureus. Infect. Immun. 2015, 83, 1019–1029. [Google Scholar] [CrossRef]
- Divon, H.H.; Fluhr, R. Nutrition acquisition strategies during fungal infection of plants. FEMS Microbiol. Lett. 2007, 266, 65–74. [Google Scholar] [CrossRef]
- Hoffmeister, D.; Keller, N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, C.; Hou, R.; Zhou, X.; Li, G.; Zhang, S.; Xu, J.R. The AMT1 arginine methyltransferase gene is important for plant infection and normal hyphal growth in Fusarium graminearum. PLoS ONE 2012, 7, e38324. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Wang, J.; Ji, F.; Yin, X.; Shi, J. Involvement of threonine deaminase FgIlv1 in isoleucine biosynthesis and full virulence in Fusarium graminearum. Curr. Genet. 2015, 61, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Q.; Xu, J.; Wang, J.; Shi, J. Acetohydroxyacid synthase FgIlv2 and FgIlv6 are involved in BCAA biosynthesis, mycelial and conidial morphogenesis, and full virulence in Fusarium graminearum. Sci. Rep. 2015, 5, 16315. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Xu, J.; Shi, J. FgIlv5 is required for branched-chain amino acid biosynthesis and full virulence in Fusarium graminearum. Microbiology 2014, 160, 692–702. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.; Wu, H.; Fang, W.; Liang, Q.; Zheng, Y.; Olsson, S.; Zhang, D.; Zhou, J.; Wang, Z.; et al. The 5-oxoprolinase is required for conidiation, sexual reproduction, virulence and deoxynivalenol production of Fusarium graminearum. Curr. Genet. 2018, 64, 285–301. [Google Scholar] [CrossRef]
- Chen, X.; Nielsen, K.F.; Borodina, I.; Kielland-Brandt, M.C.; Karhumaa, K. Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism. Biotechnol. Biofuels 2011, 4, 21. [Google Scholar] [CrossRef]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef]
- Seong, K.Y.; Pasquali, M.; Zhou, X.; Song, J.; Hilburn, K.; McCormick, S.; Dong, Y.; Xu, J.R.; Kistler, H.C. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 2009, 72, 354–367. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Bai, G.H.; Plattner, R.D.; Proctor, R.H. Analysis of aberrant virulence of Gibberella zeae following transformation-mediated complementation of a trichothecene-deficient (Tri5) mutant. Microbiology 2000, 146 Pt 8, 2059–2068. [Google Scholar] [CrossRef]
- Cambaza, E. Comprehensive Description of Fusarium graminearum Pigments and Related Compounds. Foods 2018, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Yue, X.; Yang, N.; Xu, Z.; Tang, S.; Wang, C.; Lv, W.; Xu, L.; Talbot, N.J.; Wang, Z. Leucine biosynthesis is required for infection-related morphogenesis and pathogenicity in the rice blast fungus Magnaporthe Oryzae. Curr. Genet. 2020, 66, 155–171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).