Season, Vegetation Proximity and Building Age Shape the Indoor Fungal Communities’ Composition at City-Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Mycobiomes Characterization
2.3. Bioinformatics
2.4. Environmental Variables
2.5. Statistical Analysis
3. Results
3.1. Building Environments
3.2. Cultivable Fraction of Fungi & Environment
3.3. Factors Driving the Diversity of Indoor Fungal Community
3.4. Factors Structuring the Composition of Indoor Fungal Community
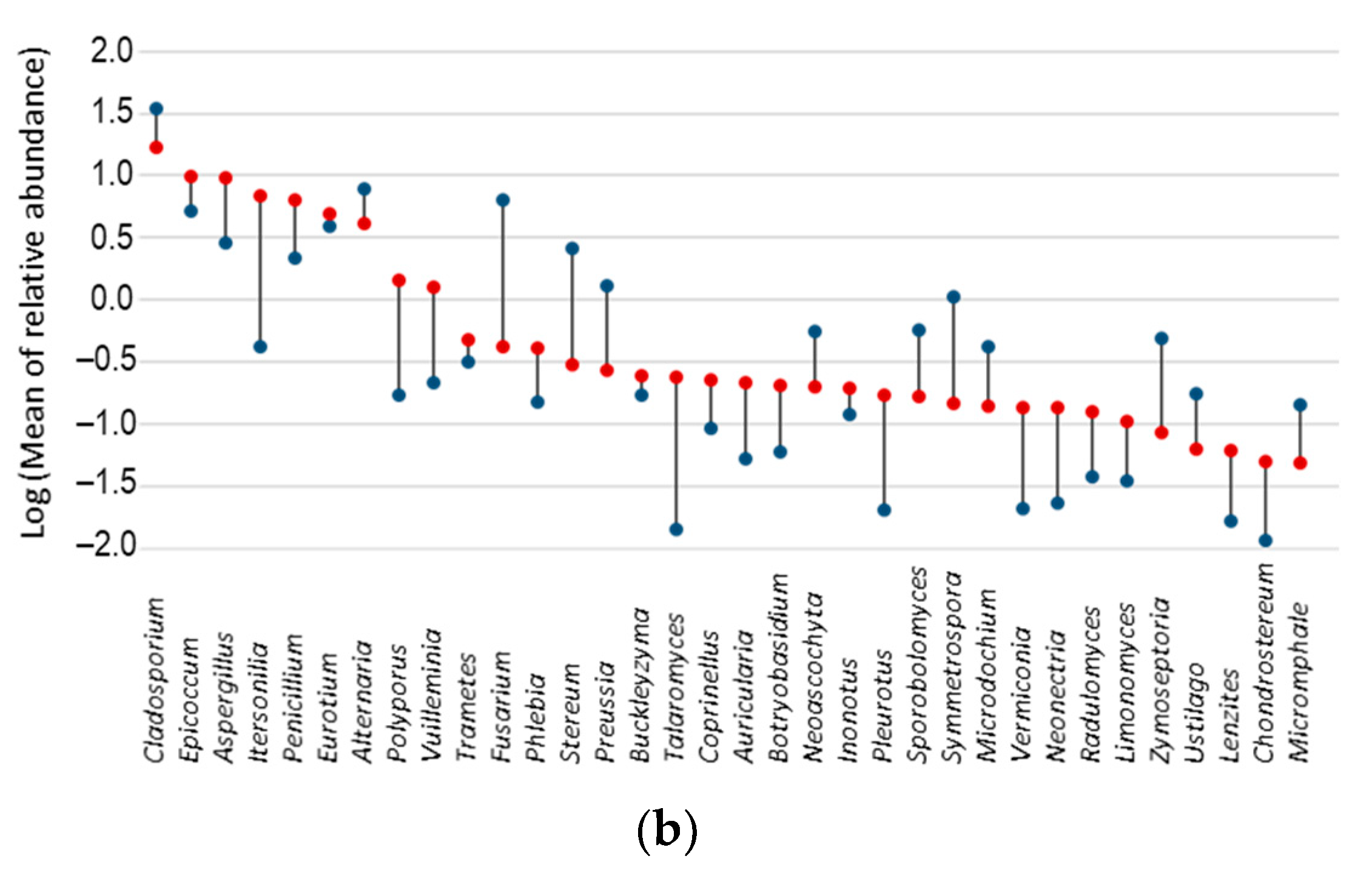
3.5. Fungal Indicator Taxa associated with Seasons
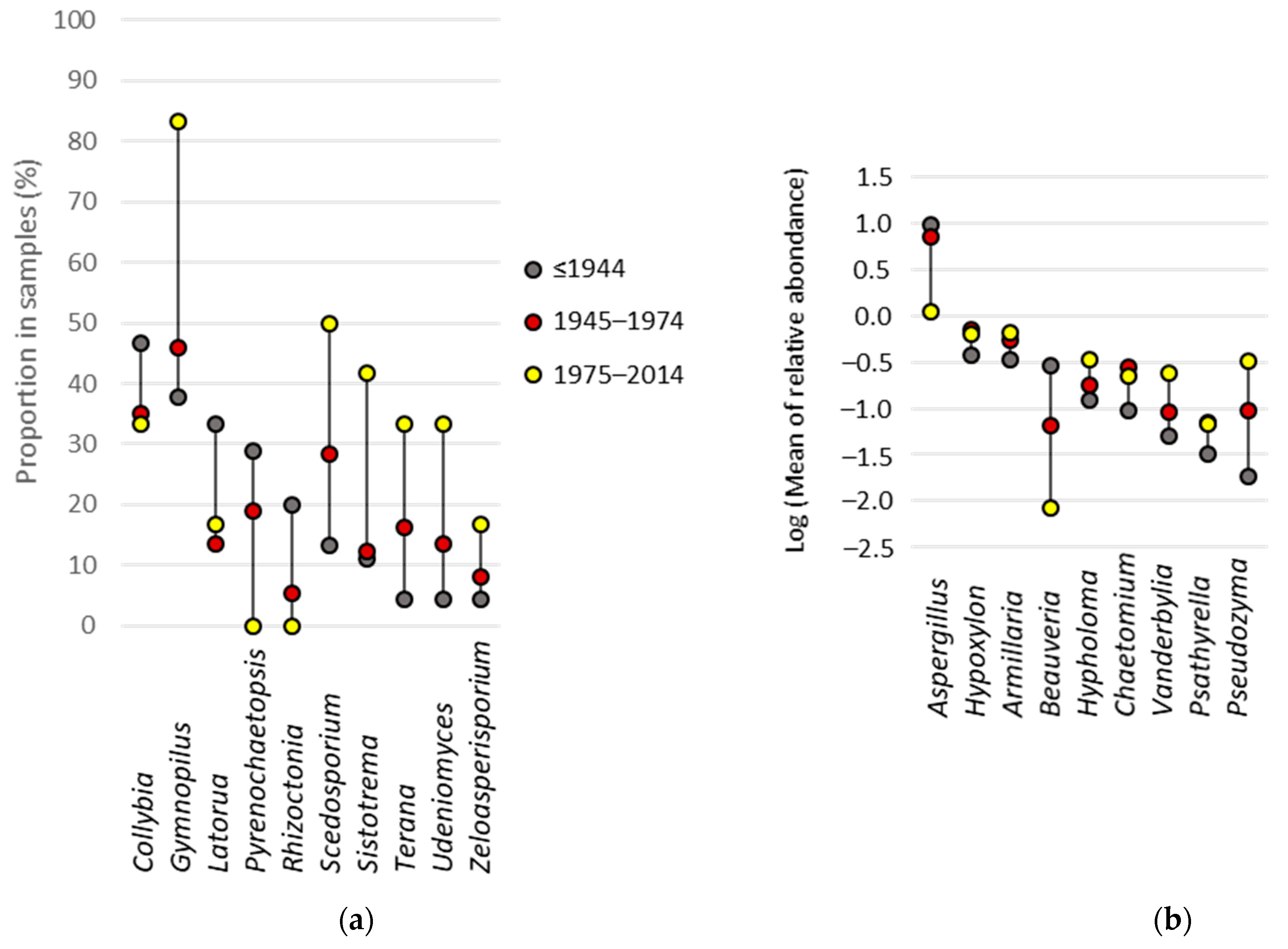
3.6. Fungal Indicator Taxa associated with the Construction Period of the Building
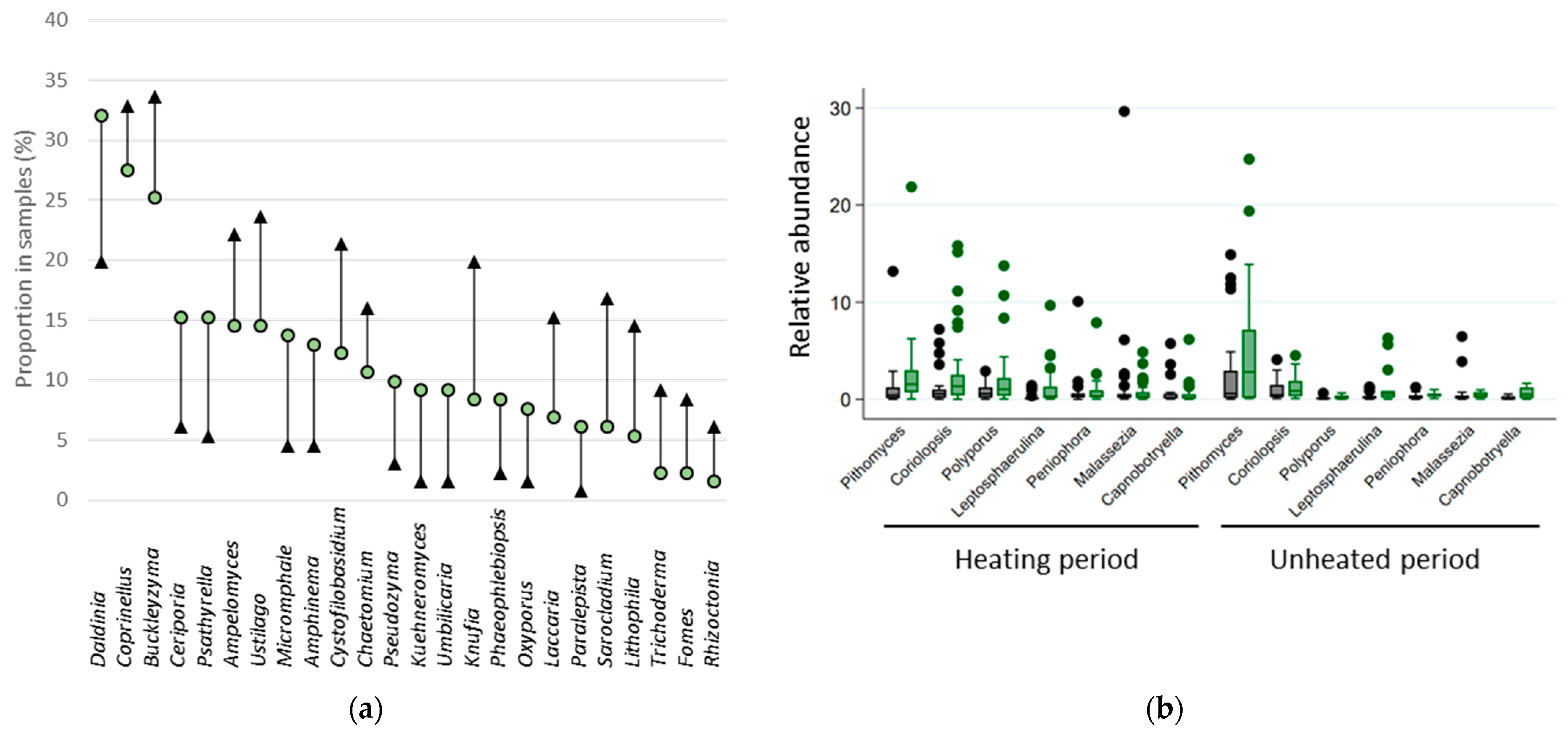
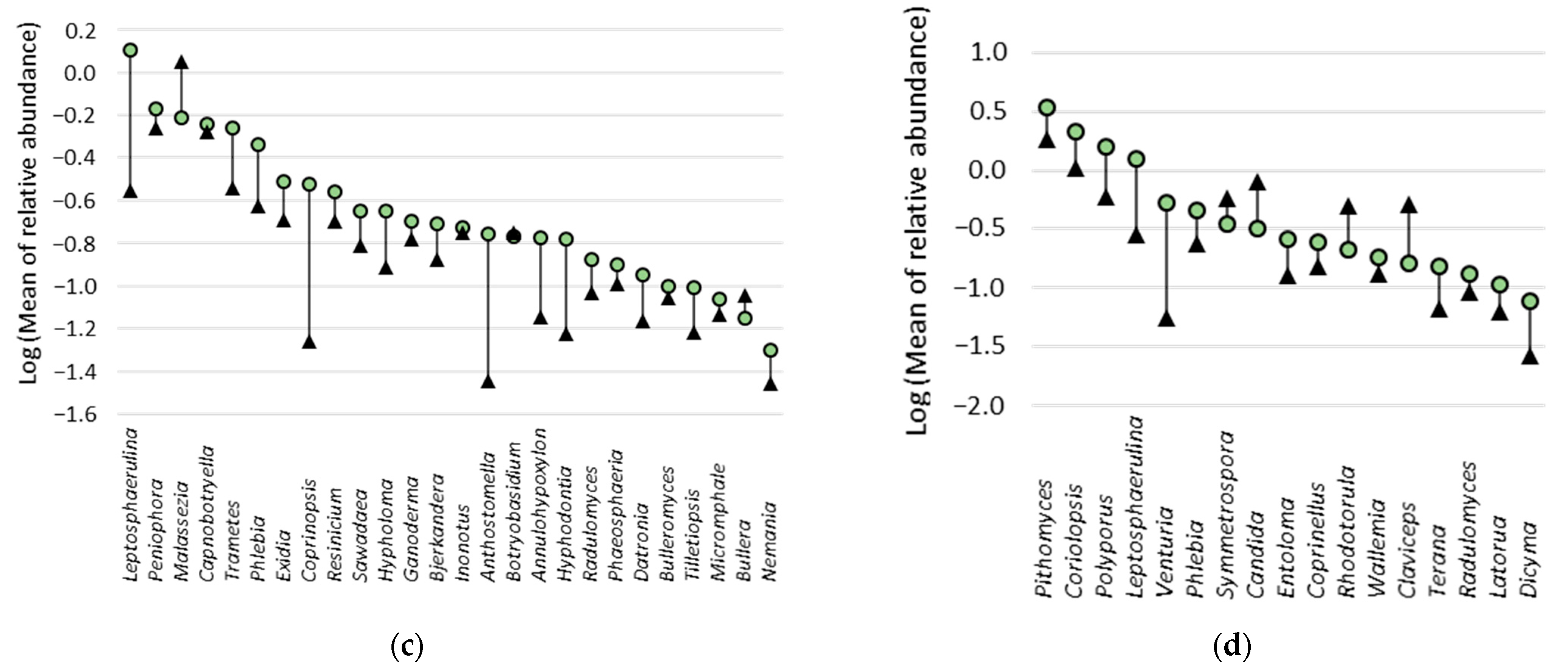
3.7. Fungal Indicator Taxa associated with the Building Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Reponen, T.; Lockey, J.; Bernstein, D.I.; Vesper, S.J.; Levin, L.; Hershey, G.K.K.; Zheng, S.; Ryan, P.; Grinshpun, S.A.; Villareal, M.; et al. Infant origins of childhood asthma associated with specific molds. J. Allergy Clin. Immunol. 2012, 130, 639–644. [Google Scholar] [CrossRef]
- Sharpe, R.A.; Bearman, N.; Thornton, C.R.; Husk, K.; Osborne, N.J. Indoor fungal diversity and asthma: A meta-analysis and systematic review of risk factors. J. Allergy Clin. Immunol. 2015, 135, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Karvala, K.; Toskala, E.; Luukkonen, R.; Lappalainen, S.; Uitti, J.; Nordman, H. New-onset adult asthma in relation to damp and moldy workplaces. Int. Arch. Occ. Environ. Health 2010, 83, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Ege, M.J.; Mayer, M.; Normand, A.C.; Genuneit, J.; Cookson, W.O.C.M.; Braun-Fahrlander, C.; Heederik, D.; Piarroux, R.; von Mutius, E.; Grp, G.T.S. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Dannemiller, K.C.; Mendell, M.J.; Macher, J.M.; Kumagai, K.; Bradman, A.; Holland, N.; Harley, K.; Eskenazi, B.; Peccia, J. Next-generation DNA sequencing reveals that low fungal diversity in house dust is associated with childhood asthma development. Indoor Air 2014, 24, 236–247. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Demoor, T.; Rauch, M.; Faruqi, A.A.; Jang, S.; Johnson, C.C.; Boushey, H.A.; Zoratti, E.; Ownby, D.; Lukacs, N.W.; et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 805–810. [Google Scholar] [CrossRef]
- Lynch, S.V.; Wood, R.A.; Boushey, H.; Bacharier, L.B.; Bloomberg, G.R.; Kattan, M.; O’Connor, G.T.; Sandel, M.T.; Calatroni, A.; Matsui, E.; et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J. Allergy Clin. Immunol. 2014, 134, 593–601. [Google Scholar] [CrossRef]
- Schaub, B.; Vercelli, D. Environmental protection from allergic diseases: From humans to mice and back. Curr. Opin. Immunol. 2015, 36, 88–93. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. mBio 2017, 8, e01492-17. [Google Scholar] [CrossRef]
- Vesper, S.; Barnes, C.; Ciaccio, C.E.; Johanns, A.; Kennedy, K.; Murphy, J.S.; Nunez-Alvarez, A.; Sandel, M.T.; Cox, D.; Dewalt, G.; et al. Higher Environmental Relative Moldiness Index (ERMI) values measured in homes of asthmatic children in Boston, Kansas City, and San Diego. J. Asthma 2013, 50, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Coombs, K.; Vesper, S.; Green, B.J.; Yermakov, M.; Reponen, T. Fungal microbiomes associated with green and non-green building materials. Int. Biodeterior. Biodegrad. 2017, 125, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gravesen, S.; Nielsen, P.A.; Iversen, R.; Nielsen, K.F. Microfungal contamination of damp buildings-Examples of risk constructions and risk materials. Environ. Health Perspect. 1999, 107, 505–508. [Google Scholar] [CrossRef]
- Hoang, C.P.; Kinney, K.A.; Corsi, R.L.; Szaniszlo, P.J. Resistance of green building materials to fungal growth. Int. Biodeterior. Biodegrad. 2010, 64, 104–113. [Google Scholar] [CrossRef]
- Hyvarinen, A.; Meklin, T.; Vepsalainen, A.; Nevalainen, A. Fungi and actinobacteria in moisture-damaged building materials-concentrations and diversity. Int. Biodeterior. Biodegrad. 2002, 49, 27–37. [Google Scholar] [CrossRef]
- Lax, S.; Cardona, C.; Zhao, D.; Winton, V.J.; Goodney, G.; Gao, P.; Gottel, N.; Hartmann, E.M.; Henry, C.; Thomas, P.M.; et al. Microbial and metabolic succession on common building materials under high humidity conditions. Nat. Commun. 2019, 10, 1767. [Google Scholar] [CrossRef]
- Pasanen, A.L.; Juutinen, T.; Jantunen, M.J.; Kalliokoski, P. Occurrence and moisture requirements of microbial-growth in building-materials. Int. Biodeterior. Biodegrad. 1992, 30, 273–283. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Arigela, R.; Gupta, S.K.; Raghunathan, R. Dynamic response of passive release of fungal spores from exposure to air. J. Aerosol Sci. 2019, 133, 37–48. [Google Scholar] [CrossRef]
- Niculita-Hirzel, H.; Yang, S.; Hager Jörin, C.; Perret, V.; Licina, D.; Goyette Pernot, J. Moulds contamination in energy efficient dwellings. Role of ventilation type and of rural /urban location. Int. J. Environ. Res. Public Health 2020, 17, 4936. [Google Scholar] [CrossRef]
- Lee, L.D.; Hachem, R.Y.; Berkheiser, M.; Hackett, B.; Jiang, Y.; Raad, I.I. Hospital environment and invasive aspergillosis in patients with hematologic malignancy. Am. J. Infect. Control 2012, 40, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Miller, D.; McMullen, E. Indoor air quality and health: Validity and determinants of reported home dampness and moulds. Int. J. Epidemiol. 1997, 26, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kettleson, E.M.; Adhikari, A.; Vesper, S.; Coombs, K.; Indugula, R.; Reponen, T. Key determinants of the fungal and bacterial microbiomes in homes. Environ. Res. 2015, 138, 130–135. [Google Scholar] [CrossRef]
- Norback, D.; Zock, J.P.; Plana, E.; Heinrich, J.; Tischer, C.; Bertelsen, R.J.; Sunyer, J.; Kunzli, N.; Villani, S.; Olivieri, M.; et al. Building dampness and mold in European homes in relation to climate, building characteristics and socio-economic status: The European Community Respiratory Health Survey ECRHS II. Indoor Air 2017, 27, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Weikl, F.; Tischer, C.; Probst, A.J.; Heinrich, J.; Markevych, I.; Jochner, S.; Pritsch, K. Fungal and bacterial communities in indoor dust follow different environmental determinants. PLoS ONE 2016, 11, e0154131. [Google Scholar] [CrossRef]
- Dharmage, S.; Bailey, M.; Raven, J.; Mitakakis, T.; Thien, F.; Forbes, A.; Guest, D.; Abramson, M.; Walters, E.H. Prevalence and residential determinants of fungi within homes in Melbourne, Australia. Clin. Exp. Allergy 1999, 29, 1481–1489. [Google Scholar] [CrossRef]
- Fairs, A.; Wardlaw, A.J.; Thompson, J.R.; Pashley, C.H. Guidelines on Ambient Intramural Airborne Fungal Spores. J. Investig. Allergy Clin. 2010, 20, 490–498. [Google Scholar]
- Du, C.Q.; Li, B.Z.; Yu, W.; Cai, J.; Wang, L.X.; Li, X.Y.; Yao, Y.H.; Li, B.C. Evaluating the effect of building construction periods on household dampness/mold and childhood diseases corresponding to different energy efficiency design requirements. Indoor Air 2021, 31, 541–556. [Google Scholar] [CrossRef]
- Schwab, S.; Rinquet, J.-L. Approche Globale Pour L’enveloppe du Bâtiment. Renovation Énergétique; Haute école d’ingénierie et d’architecture de Fribourg: Fribourg, Switzerland, 2016. [Google Scholar]
- Mahnert, A.; Ortega, R.A.; Berg, C.; Grebe, M.; Berg, G. Leaves of indoor ornamentals are biodiversity and functional hotspots for fungi. Front. Microbiol. 2018, 9, 2343. [Google Scholar] [CrossRef]
- Tong, X.; Leung, M.H.Y.; Wilkins, D.; Lee, P.K.H. City-scale distribution and dispersal routes of mycobiome in residences. Microbiome 2017, 5, 131. [Google Scholar] [CrossRef]
- Bernstein, R.S.; Sorenson, W.G.; Garabrant, D.; Reaux, C.; Treitman, R.D. Exposures to respirable, airborne Penicillium from a contaminated ventilation system-clinical, environmental and epidemiological aspects. Am. Ind. Hyg. Assoc. J. 1983, 44, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Barberan, A.; Ladau, J.; Leff, J.W.; Pollard, K.S.; Menninger, H.L.; Dunn, R.R.; Fierer, N. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Natl. Acad. Sci. USA 2015, 112, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Amend, A.S.; Seifert, K.A.; Samson, R.; Bruns, T.D. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 13748–13753. [Google Scholar] [CrossRef]
- Yamamoto, N.; Bibby, K.; Qian, J.; Hospodsky, D.; Rismani-Yazdi, H.; Nazaroff, W.W.; Peccia, J. Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J. 2012, 6, 1801–1811. [Google Scholar] [CrossRef]
- Grinn-Gofron, A.; Bosiacka, B. Effects of meteorological factors on the composition of selected fungal spores in the air. Aerobiologia 2015, 31, 63–72. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, H.; Delgado, J.; Abreu, I. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. Int. J. Biometeorol. 2009, 53, 61–73. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, H.; Delgado, J.L.; Abreu, I. Seasonal and intradiurnal variation of allergenic fungal spores in urban and rural areas of the North of Portugal. Aerobiologia 2009, 25, 85–98. [Google Scholar] [CrossRef]
- Pellissier, L.; Oppliger, A.; Hirzel, A.H.; Savova-Bianchi, D.; Mbayo, G.; Mascher, F.; Kellenberger, S.; Niculita-Hirzel, H. Airborne and grain dust fungal community compositions are shaped regionally by plant genotypes and farming practices. Appl. Environ. Microbiol. 2016, 82, 2121–2131. [Google Scholar] [CrossRef]
- Lymperopoulou, D.S.; Adams, R.I.; Lindow, S.E. Contribution of vegetation to the microbial composition of nearby outdoor air. Appl. Environ. Microbiol. 2016, 82, 3822–3833. [Google Scholar] [CrossRef]
- Kozdroj, J.; Fraczek, K.; Ropek, D. Assessment of bioaerosols in indoor air of glasshouses located in a botanical garden. Build. Environ. 2019, 166, 106436. [Google Scholar] [CrossRef]
- Hameed, A.A.A.; Khodr, M.I. Suspended particulates and bioaerosols emitted from an agricultural non-point source. J. Environ. Monit. 2001, 3, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Goyette Pernot, J.; Hager Jörin, C.; Niculita-Hirzel, H.; Perret, V.; Licina, D. Energy, indoor air quality, occupant behavior, self-reported symptoms and satisfaction in energy-efficient dwellings in Switzerland. Build. Environ. 2020, 171, 106618. [Google Scholar] [CrossRef]
- Schweiker, M.; Haldi, F.; Shukuya, M.; Robinson, D. Verification of stochastic models of window opening behaviour for residential buildings. J. Build. Perform. Simu. 2012, 5, 55–74. [Google Scholar] [CrossRef]
- Fabi, V.; Andersen, R.V.; Corgnati, S.; Olesen, B.W. Occupants’ window opening behaviour: A literature review of factors influencing occupant behaviour and models. Build. Environ. 2012, 58, 188–198. [Google Scholar] [CrossRef]
- Santos-Eggimann, B.; Karmaniola, A.; Seematter-Bagnoud, L.; Spagnoli, J.; Bula, C.; Cornuz, J.; Rodondi, N.; Vollenweider, P.; Waeber, G.; Pecoud, A. The Lausanne cohort Lc65+: A population-based prospective study of the manifestations, determinants and outcomes of frailty. BMC Geriatr. 2008, 8, 20. [Google Scholar] [CrossRef]
- Noss, I.; Wouters, I.M.; Visser, M.; Heederik, D.J.; Thorne, P.S.; Brunekreef, B.; Doekes, G. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl. Environ. Microbiol. 2008, 74, 5621–5627. [Google Scholar] [CrossRef]
- Frankel, M.; Timm, M.; Hansen, E.W.; Madsen, A.M. Comparison of sampling methods for the assessment of indoor microbial exposure. Indoor Air 2012, 22, 405–414. [Google Scholar] [CrossRef]
- Normand, A.C.; Ranque, S.; Cassagne, C.; Gaudart, J.; Sallah, K.; Charpin, D.A.; Piarroux, R. Comparison of Air Impaction and Electrostatic Dust Collector sampling methods to assess airborne fungal contamination in public buildings. Ann. Occup. Hyg. 2016, 60, 161–175. [Google Scholar] [CrossRef]
- Barrera, C.; Rocchi, S.; Degano, B.; Soumagne, T.; Laurent, L.; Bellanger, A.P.; Laplante, J.J.; Millon, L.; Dalphin, J.C.; Reboux, G. Microbial exposure to dairy farmers’ dwellings and COPD occurrence. Int. J. Environ. Health Res. 2018, 29, 387–399. [Google Scholar] [CrossRef]
- Pitt, J.I. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press Inc. Ltd.: London, UK, 1979; p. 634. [Google Scholar]
- Samson, R.; Pitt, J. Advances in Penicillium and Aspergillus Systematics; Plenum Press: New York, NY, USA, 1986; p. 483. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Koljalg, U.; Larsson, K.H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Hoiland, K.; Kjoller, R.; Larsson, E.; et al. UNITE: A database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 2005, 166, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Porras-Alfaro, A.; Liu, K.L.; Kuske, C.R.; Xie, G. From Genus to Phylum: Large-Subunit and Internal Transcribed Spacer rRNA operon regions show similar classification accuracies influenced by database composition. Appl. Environ. Microbiol. 2014, 80, 829–840. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 30 August 2022).
- Reboux, G.; Rocchi, S.; Laboissiere, A.; Ammari, H.; Bochaton, M.; Gardin, G.; Rame, J.M.; Millon, L. Survey of 1012 moldy dwellings by culture fungal analysis: Threshold proposal for asthmatic patient management. Indoor Air 2019, 29, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Basilico, M.D.L.Z.; Chiericatti, C.; Aringoli, E.E.; Althaus, R.L.; Basilico, J.C. Influence of environmental factors on airborne fungi in houses of Santa Fe city, Argentina. Sci. Total Environ. 2007, 376, 143–150. [Google Scholar] [CrossRef]
- MeteoSwiss. Climate Normals Pully. Available online: https://www.meteoswiss.admin.ch/product/output/climate-data/climate-diagrams-normal-values-station-processing/PUY/climsheet_PUY_np9120_e.pdf (accessed on 28 August 2022).
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air 2017, 27, 354–363. [Google Scholar] [CrossRef]
- Rocchi, S.; Valot, B.; Reboux, G.; Millon, L. DNA metabarcoding to assess indoor fungal communities: Electrostatic dust collectors and Illumina sequencing. J. Microbiol. Methods 2017, 139, 107–112. [Google Scholar] [CrossRef]
- Shin, S.K.; Kim, J.; Ha, S.M.; Oh, H.S.; Chun, J.; Sohn, J.; Yi, H. Metagenomic insights into the bioaerosols in the indoor and outdoor environments of childcare facilities. PLoS ONE 2015, 10, e0126960. [Google Scholar] [CrossRef]
- Taubel, M.; Karvonen, A.M.; Reponen, T.; Hyvarinen, A.; Vesper, S.; Pekkanen, J. Application of the environmental relative moldiness index in Finland. Appl. Environ. Microbiol. 2016, 82, 578–584. [Google Scholar] [CrossRef]
- Adam, R.I.; Bhangar, S.; Pasut, W.; Arens, E.A.; Taylor, J.W.; Lindow, S.E.; Nazaroff, W.W.; Bruns, T.D. Chamber bioaerosol study: Outdoor air and human occupants as sources of indoor airborne microbes. PLoS ONE 2015, 10, e0133221. [Google Scholar] [CrossRef]
- Sharpe, R.; Thornton, C.R.; Osborne, N.J. Modifiable factors governing indoor fungal diversity and risk of asthma. Clin. Exp. Allergy 2014, 44, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Frisvad, J.C.; Sondergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Howden-Chapman, P.; Saville-Smith, K.; Crane, J.; Wilson, N. Risk factors for mold in housing: A national survey. Indoor Air 2005, 15, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Niculita-Hirzel, H. Latest trends in pollutant accumulations at threatening levels in energy-efficient residential buildings with and without mechanical ventilation: A Review. Int. J. Environ. Res. Public Health 2022, 19, 3538. [Google Scholar] [CrossRef]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Hanson, B.; Zhou, Y.J.; Bautista, E.J.; Urch, B.; Speck, M.; Silverman, F.; Muilenberg, M.; Phipatanakul, W.; Weinstock, G.; Sodergren, E.; et al. Characterization of the bacterial and fungal microbiome in indoor dust and outdoor air samples: A pilot study. Environ. Sci.-Process Impacts 2016, 18, 713–724. [Google Scholar] [CrossRef]
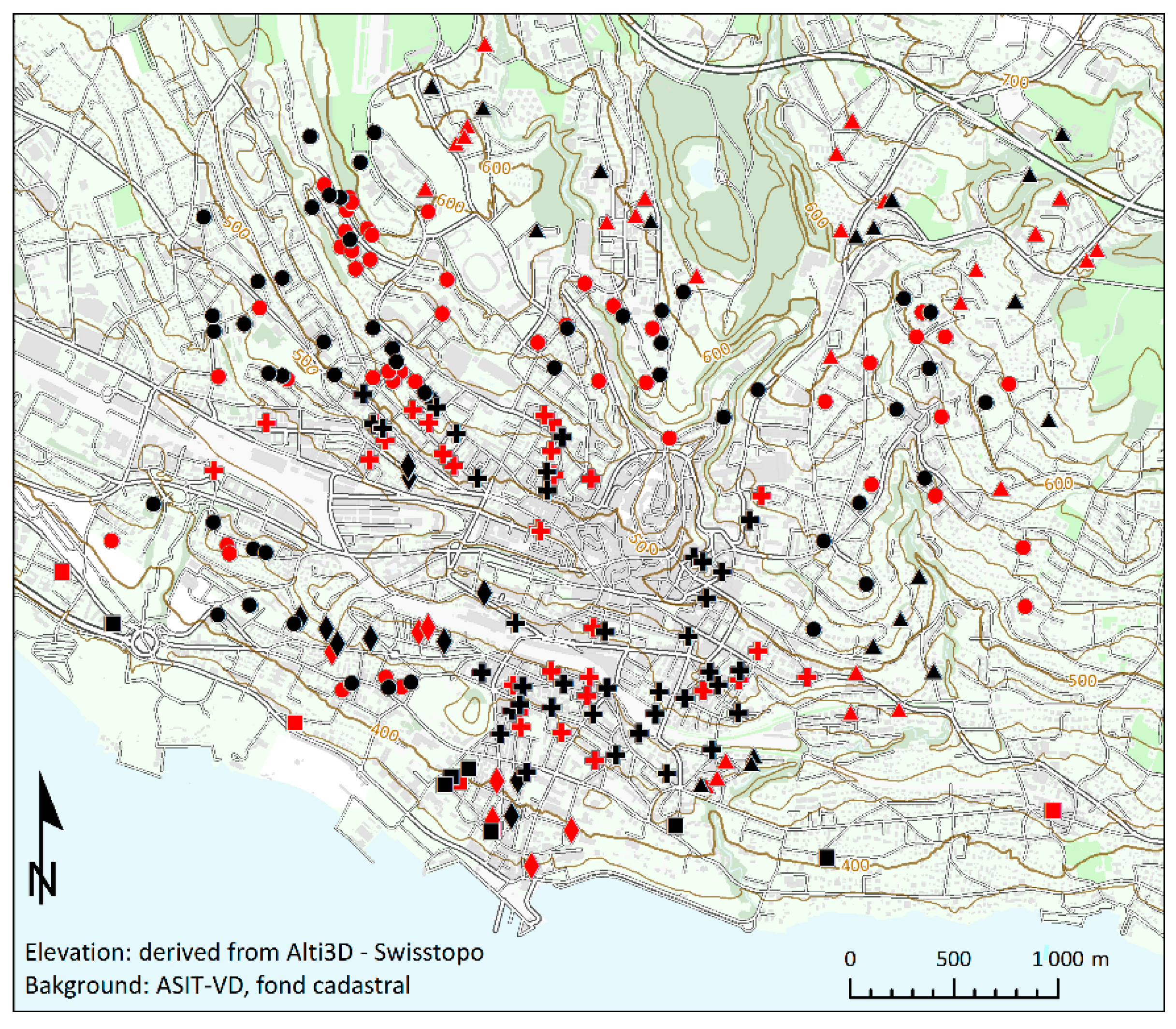
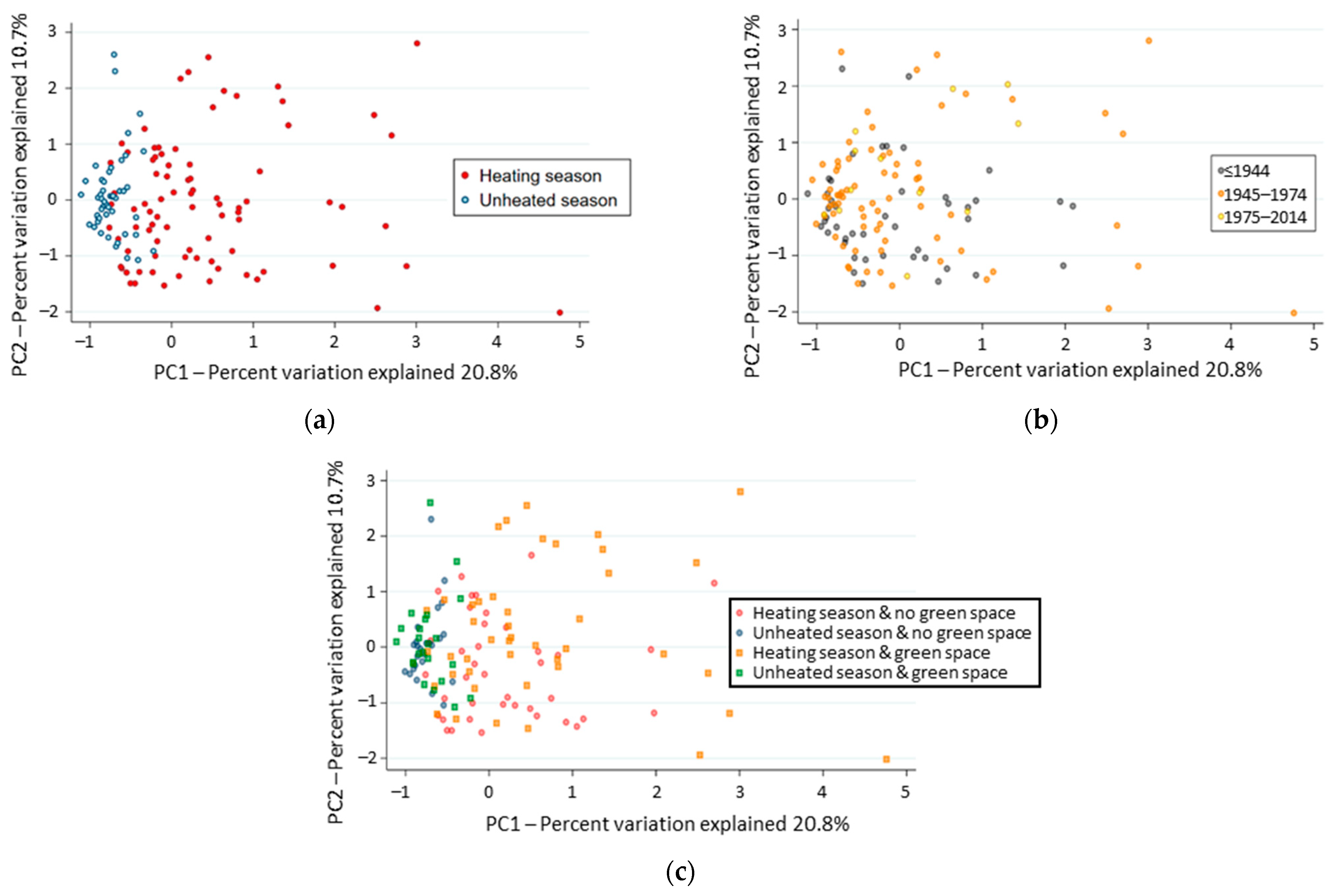
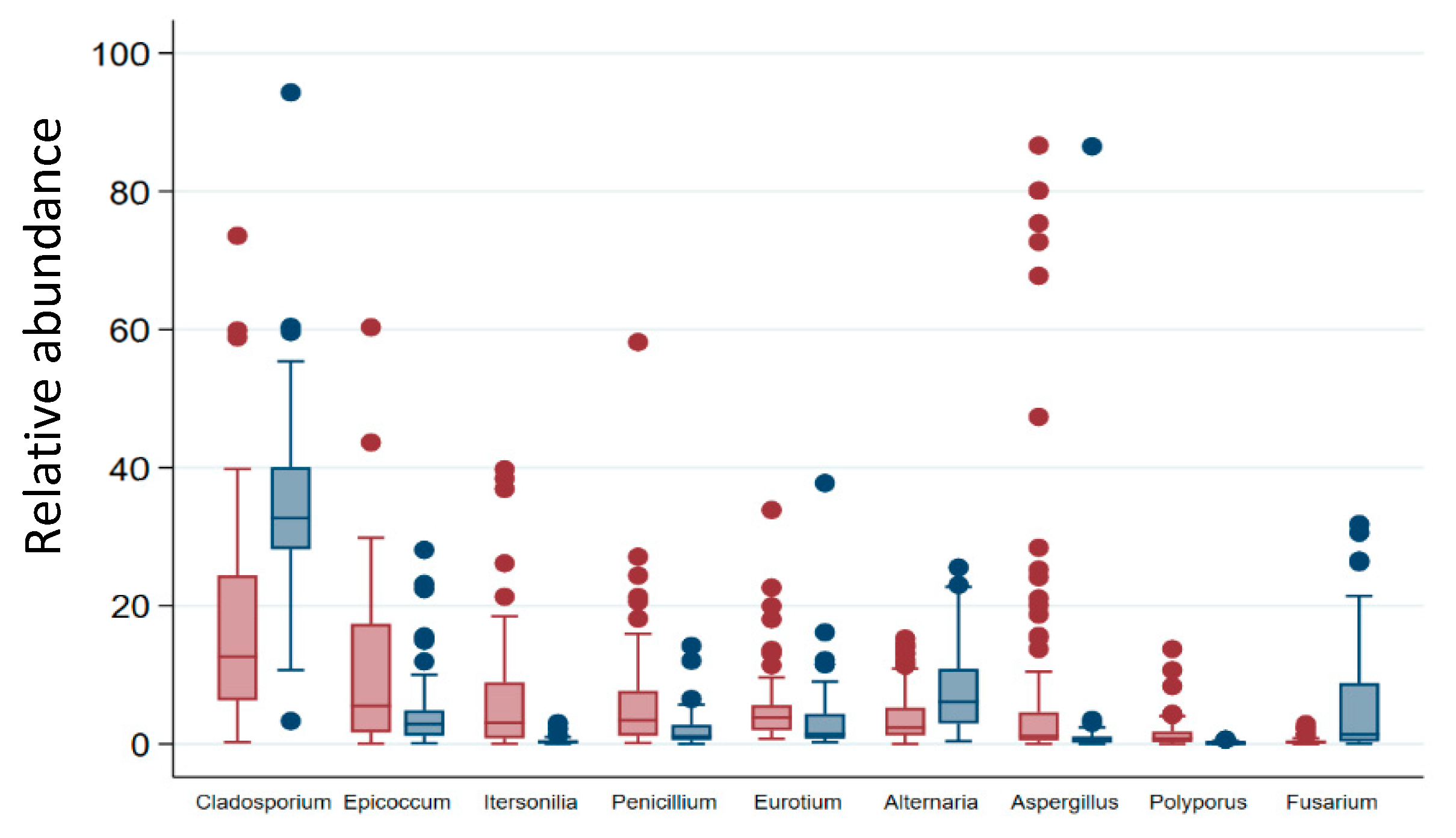
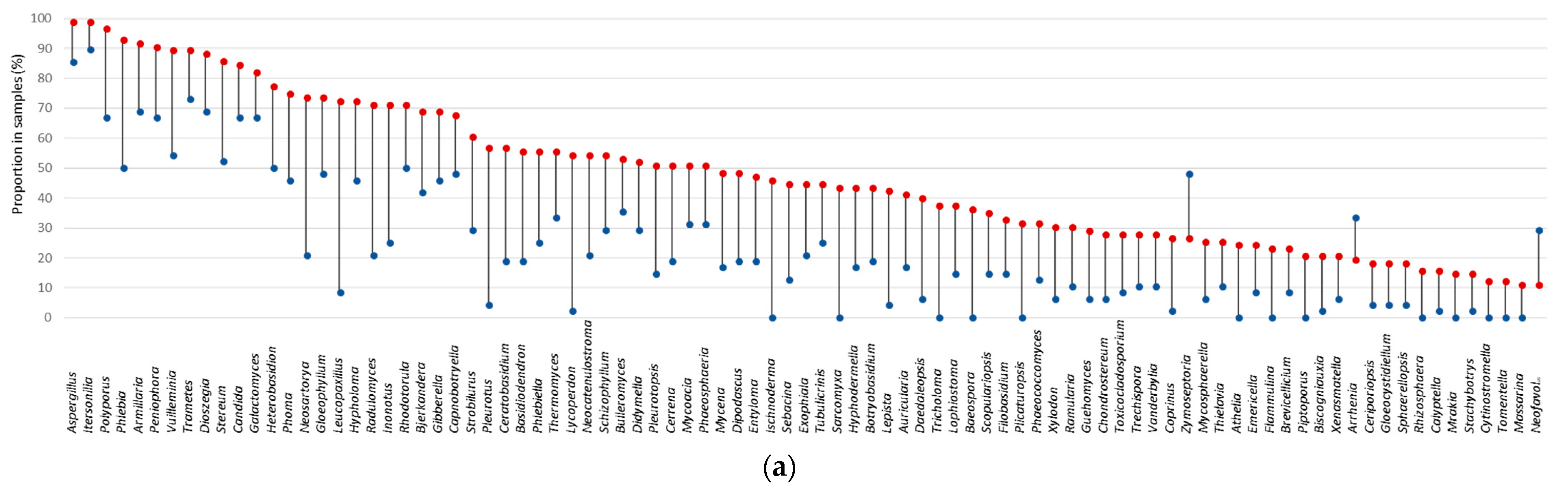
| Construction Period | Building Environment Cluster | Total N (% in Column) | ||||
|---|---|---|---|---|---|---|
| Periurban | Suburbs | Historical Town Centre | Town Centre near the Lake | Lake Shore | ||
| Seniors buildings in the cohort (N = 1881), N (% in line) | ||||||
| until 1944 | 61 (10.0) | 169 (28.8) | 276 (47.1) | 54 (9.2) | 26 (4.4) | 586 (31.2) |
| 1945–1974 | 313 (29.1) | 519 (48.2) | 149 (13.8) | 66 (6.1) | 30 (2.8) | 1077 (57.3) |
| 1975–2014 | 53(24.3) | 130 (59.6) | 27 (12.4) | 5 (2.3) | 3 (1.4) | 218 (11.6) |
| Seniors buildings analyzed by culture (N = 259), N (% in line) | ||||||
| until 1944 | 11 (11.6) | 29 (30.5) | 42 (44.2) | 9 (9.5) | 4 (4.2) | 95 (35.5) |
| 1945–1974 | 34 (23.3) | 69 (47.3) | 31 (21.2) | 7 (4.8) | 5 (3.4) | 146 (54.5) |
| 1975–2014 | 10 (37.0) | 13 (48.2) | 2 (7.4) | 2 (7.4) | 0 (0.0) | 27 (10.1) |
| Seniors buildings analyzed by sequencing (N = 131), N (% in line) | ||||||
| until 1944 | 4 (8.9) | 12 (26.7) | 22 (48.9) | 6 (13.3) | 1 (2.2) | 45 (34.3) |
| 1945–1974 | 15 (20.3) | 33 (44.6) | 16 (21.6) | 4 (5.4) | 6 (8.1) | 74 (56.5) |
| 1975–2014 | 2 (16.7) | 9 (75.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 12 (9.6) |
| Total | <1945 | 1945–1974 | 1975–2014 | p Value | |
| Heating period | |||||
| Number of samples analyzed by culture | 183 | 65 | 100 | 18 | |
| Total CFUs per day, Mean ± SD | 26 ± 20 | 24 ± 17 | 25 ± 17 | 40 ± 35 | 0.008 |
| Aspergillus CFUs per day, Mean ± SD | 3 ± 12 | 3 ± 11 | 3 ± 10 | 7 ± 23 | 0.497 |
| Penicillium CFUs per day, Mean ± SD | 10 ± 21 | 9 ± 19 | 9 ± 17 | 22 ± 37 | 0.034 |
| Number of samples analyzed by metabarcoding | 83 | 27 | 48 | 8 | |
| Shannon index, Mean ± SD | 3.5 ± 0.8 | 3.5 ± 0.9 | 3.5 ± 0.7 | 3.6 ± 0.6 | 0.947 |
| Fungal richness, Mean ± SD | 94 ± 34 | 99 ± 31 | 91 ± 37 | 102 ± 29 | 0.495 |
| Unheated period | |||||
| Number of samples analyzed by culture | 76 | 25 | 43 | 8 | |
| Total CFUs per day, Mean ± SD | 14 ± 15 | 19 ± 22 | 12 ± 11 | 9 ± 5 | 0.141 |
| Aspergillus CFUs per day, Mean ± SD | 4 ± 11 | 6 ± 15 | 3 ± 7 | 1 ± 2 | 0.453 |
| Penicillium CFUs per day, Mean ± SD | 4 ± 11 | 7 ± 17 | 4 ± 6 | 1 ± 2 | 0.267 |
| Number of samples analyzed by metabarcoding | 48 | 18 | 26 | 4 | |
| Shannon index, Mean ± SD | 3.1 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.7 | 3.2 ± 0.4 | 0.910 |
| Fungal richness, Mean ± SD | 62 ± 32 | 60 ± 28 | 63 ± 33 | 73 ± 49 | 0.790 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculita-Hirzel, H.; Wild, P.; Hirzel, A.H. Season, Vegetation Proximity and Building Age Shape the Indoor Fungal Communities’ Composition at City-Scale. J. Fungi 2022, 8, 1045. https://doi.org/10.3390/jof8101045
Niculita-Hirzel H, Wild P, Hirzel AH. Season, Vegetation Proximity and Building Age Shape the Indoor Fungal Communities’ Composition at City-Scale. Journal of Fungi. 2022; 8(10):1045. https://doi.org/10.3390/jof8101045
Chicago/Turabian StyleNiculita-Hirzel, Hélène, Pascal Wild, and Alexandre H. Hirzel. 2022. "Season, Vegetation Proximity and Building Age Shape the Indoor Fungal Communities’ Composition at City-Scale" Journal of Fungi 8, no. 10: 1045. https://doi.org/10.3390/jof8101045
APA StyleNiculita-Hirzel, H., Wild, P., & Hirzel, A. H. (2022). Season, Vegetation Proximity and Building Age Shape the Indoor Fungal Communities’ Composition at City-Scale. Journal of Fungi, 8(10), 1045. https://doi.org/10.3390/jof8101045






