Candida tropicalis Systemic Infection Redirects Leukocyte Infiltration to the Kidneys Attenuating Encephalomyelitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals
2.3. Candida tropicalis Strain and Inoculum
2.4. EAE Induction and Clinical Evaluation
2.5. Serum Biochemical Analysis
2.6. Fungal Load Determination
2.7. Splenic and Renal Cell Cultures
2.8. Cytokine Quantification
2.9. Histopathological Evaluation
2.10. Isolation of CNS Mononuclear Cells
2.11. Flow Cytometry
2.12. RT-PCR
2.13. Statistical Analysis
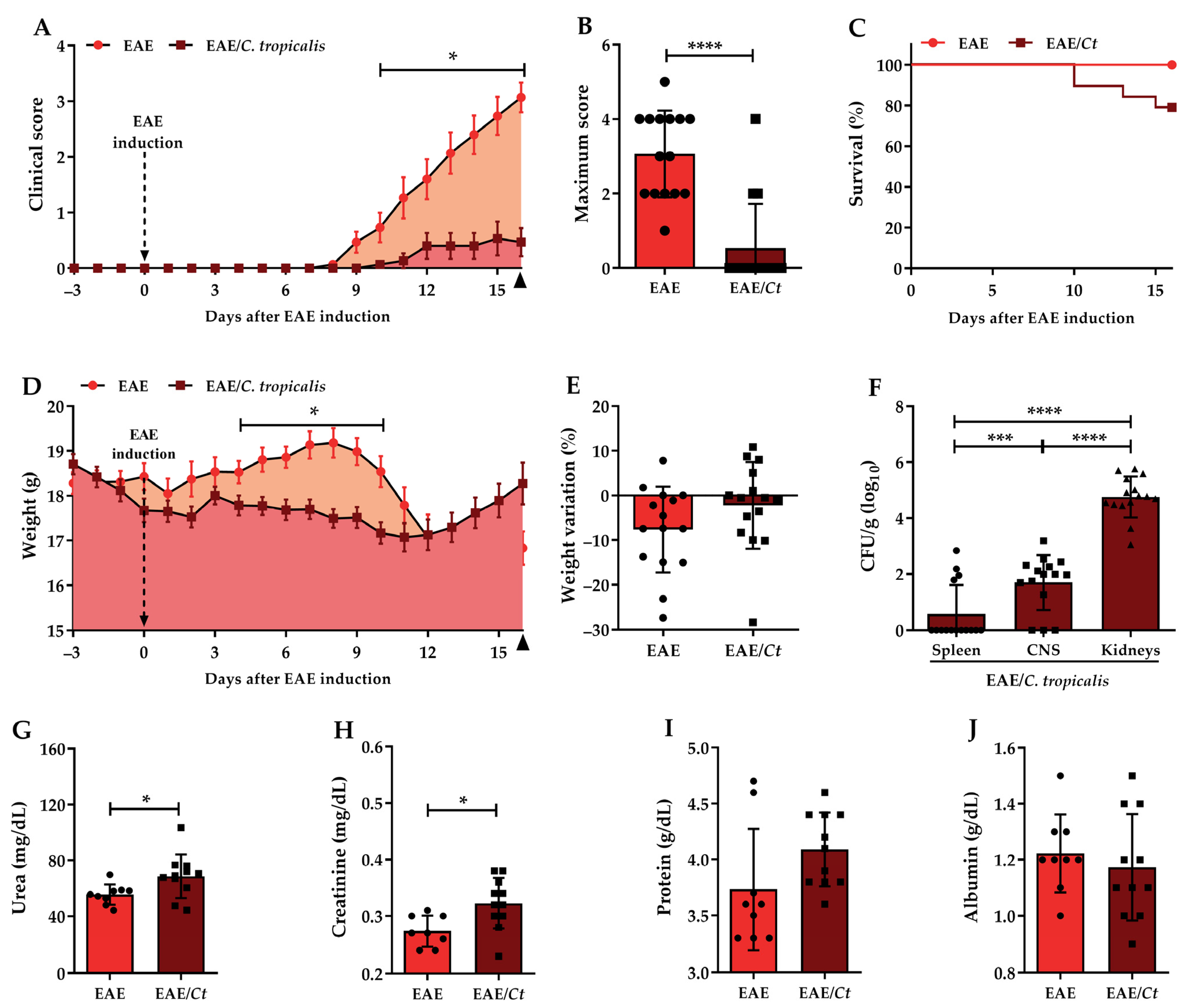
3. Results
3.1. Candida tropicalis Spreads to the CNS
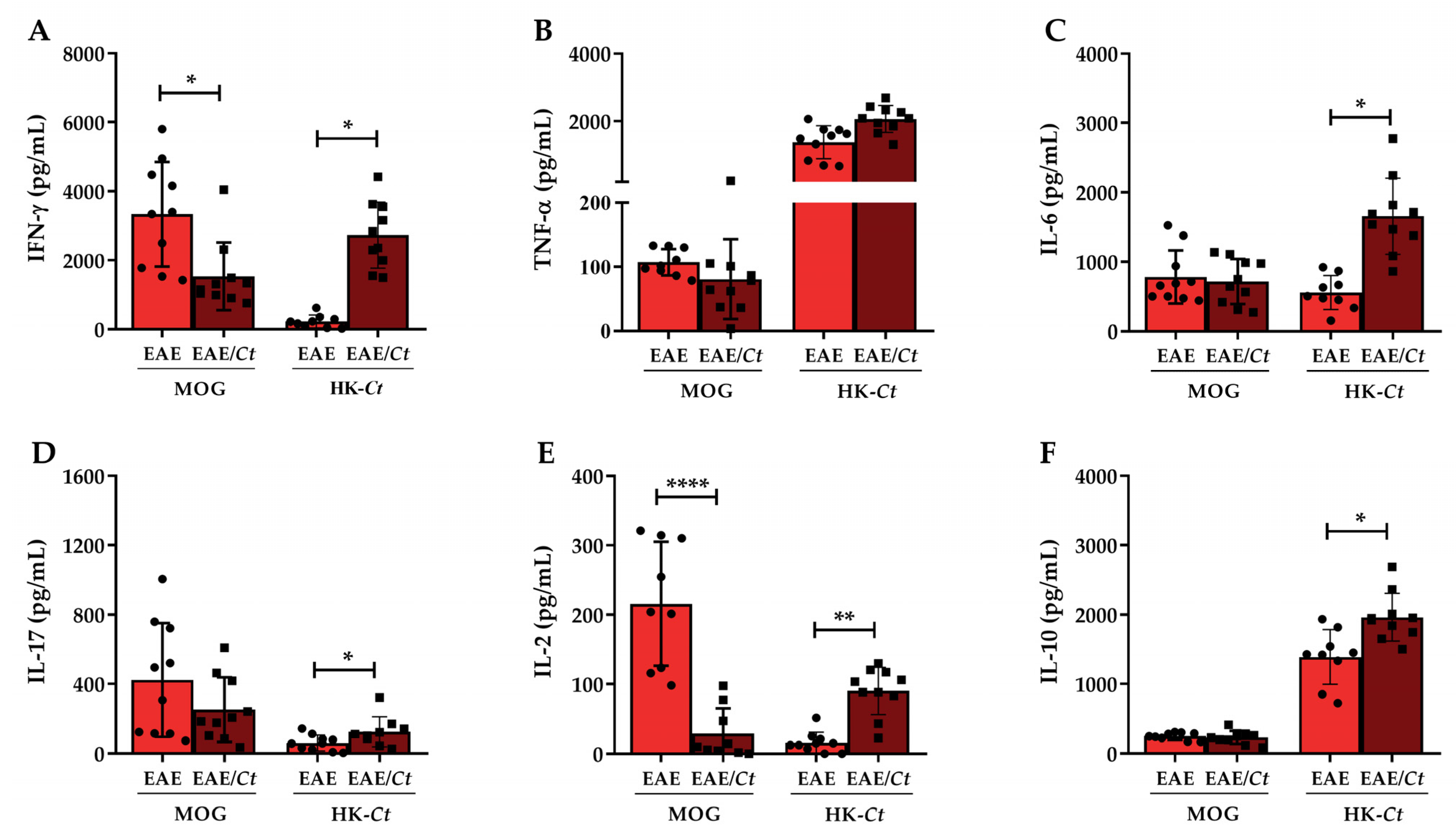
3.2. Cytokine Production in C. tropicalis Infected Mice
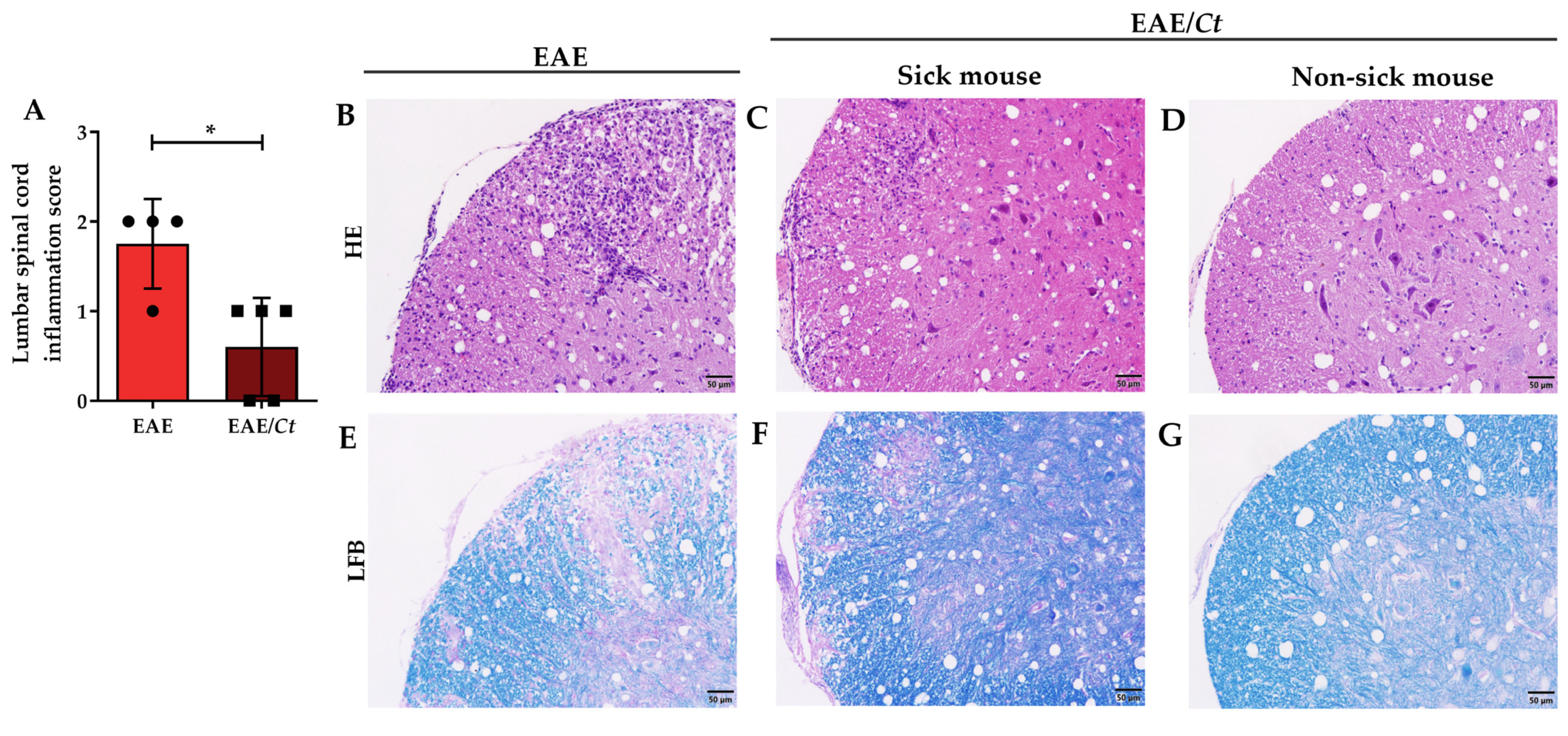
3.3. Effect of C. tropicalis Infection on EAE Development
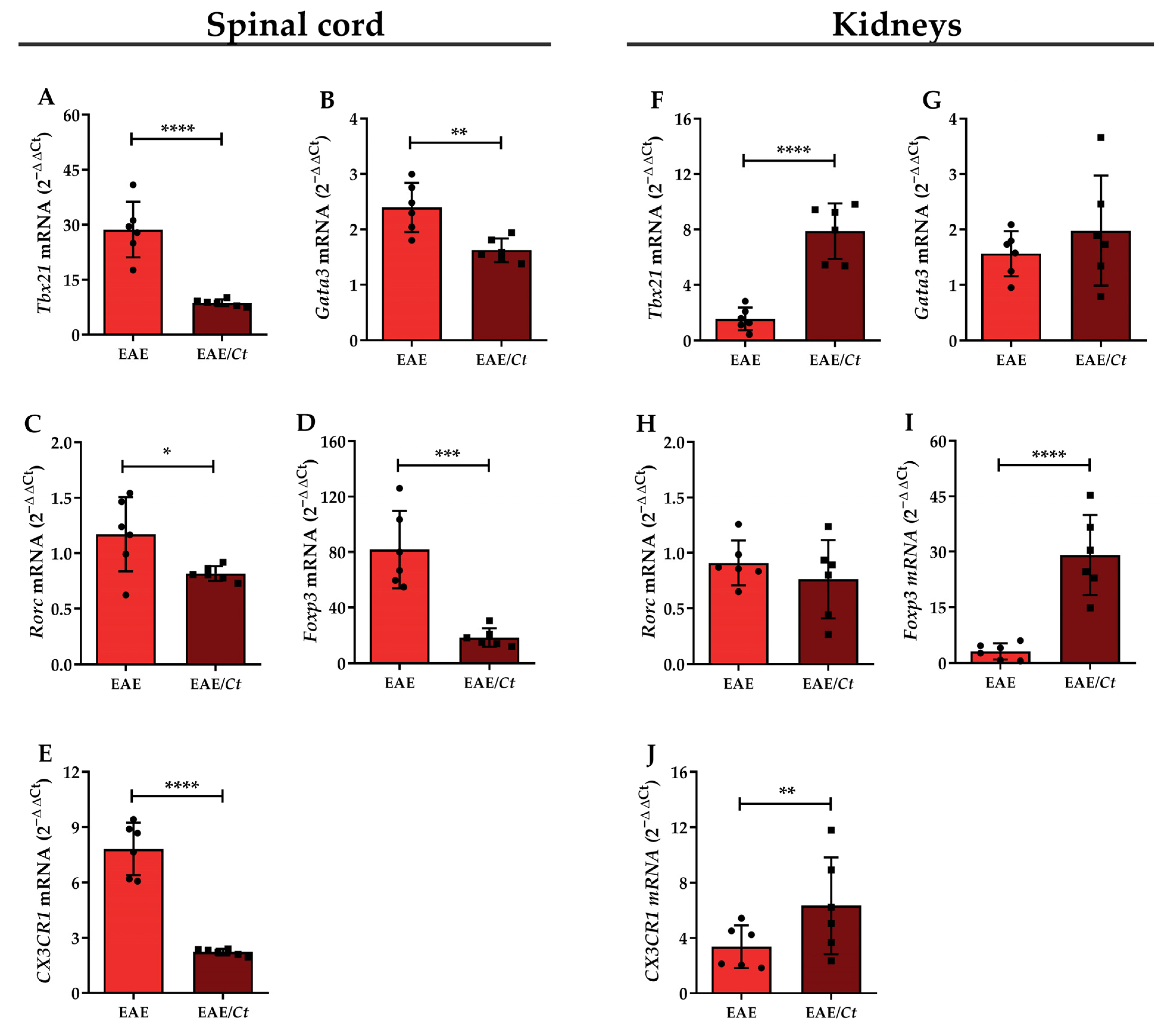
3.4. C. tropicalis Downmodulates Peripheral Production of MOG-Induced IFN-γ and IL-2
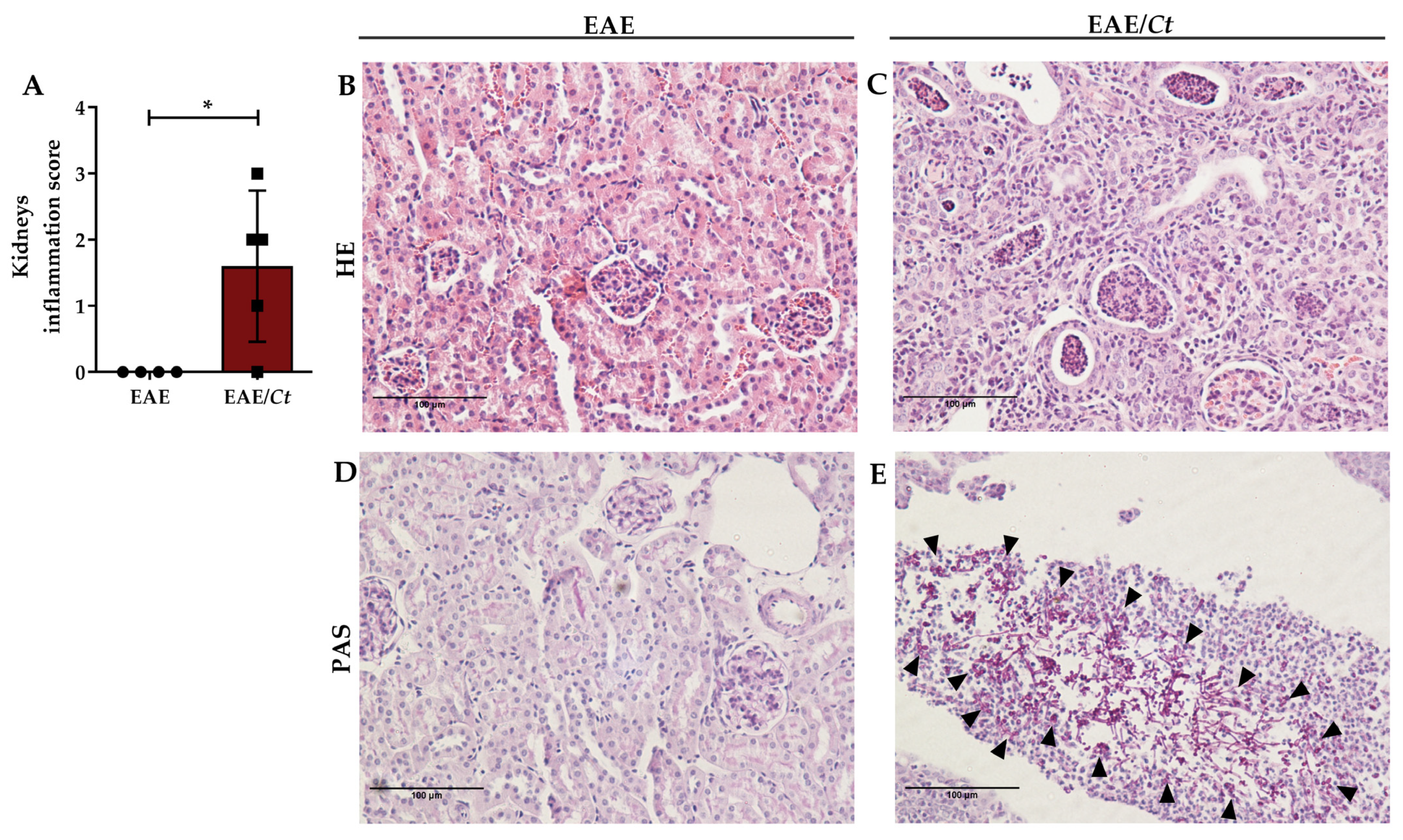
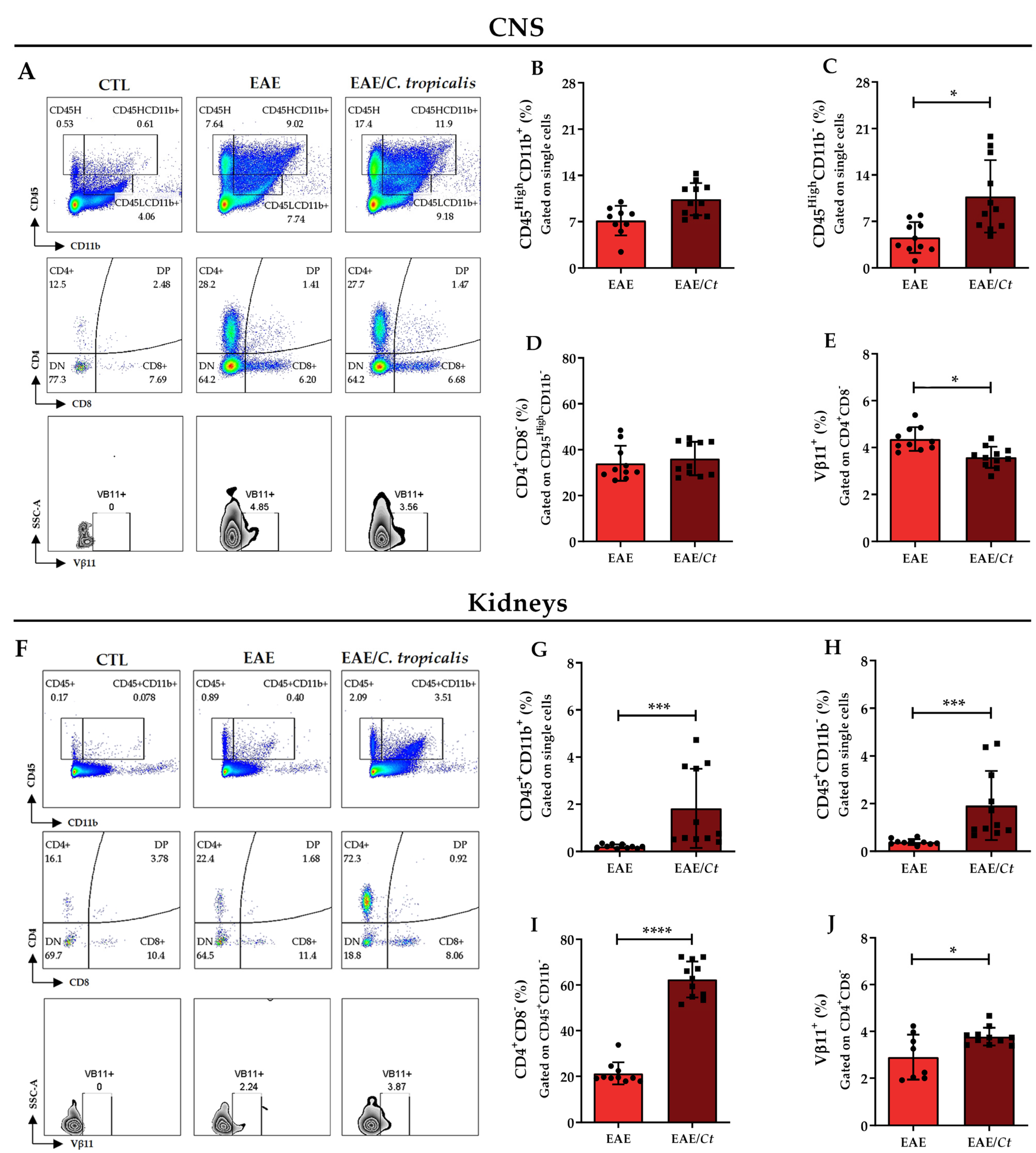
3.5. C. tropicalis Diverts Leukocyte Infiltration to the Kidneys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Brown, A.J.; Gow, N.A. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999, 7, 333–338. [Google Scholar] [CrossRef]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Geographic Variations in Species Distribution and Echinocandin and Azole Antifungal Resistance Rates among Candida Bloodstream Infection Isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 2011, 49, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Diba, K.; Makhdoomi, K.; Nasri, E.; Vaezi, A.; Javidnia, J.; Gharabagh, D.J.; Jazani, N.H.; Reza Chavshin, A.; Badiee, P.; Badali, H.; et al. Emerging Candida species isolated from renal transplant recipients: Species distribution and susceptibility profiles. Microb. Pathog. 2018, 125, 240–245. [Google Scholar] [CrossRef]
- Ngamchokwathana, C.; Chongtrakool, P.; Waesamaae, A.; Chayakulkeeree, M. Risk Factors and Outcomes of Non-albicans Candida Bloodstream Infection in Patients with Candidemia at Siriraj Hospital—Thailand’s Largest National Tertiary Referral Hospital. J. Fungi 2021, 7, 269. [Google Scholar] [CrossRef]
- Zuza-Alves, D.L.; Silva-Rocha, W.P.; Chaves, G.M. An Update on Candida tropicalis Based on Basic and Clinical Approaches. Front. Microbiol. 2017, 8, 1927. [Google Scholar] [CrossRef]
- Nucci, M.; Queiroz-Telles, F.; Tobón, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of Opportunistic Fungal Infections in Latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef]
- de Medeiros, M.A.P.; de Melo, A.P.V.; de Oliveira Bento, A.; de Souza, L.B.F.C.; de Assis Bezerra Neto, F.; Garcia, J.B.-L.; Zuza-Alves, D.L.; Francisco, E.C.; de Azevedo Melo, A.S.; Chaves, G.M. Epidemiology and prognostic factors of nosocomial candidemia in Northeast Brazil: A six-year retrospective study. PLoS ONE 2019, 14, e0221033. [Google Scholar] [CrossRef] [PubMed]
- da Matta, D.A.; Souza, A.C.R.; Colombo, A.L. Revisiting Species Distribution and Antifungal Susceptibility of Candida Bloodstream Isolates from Latin American Medical Centers. J. Fungi 2017, 3, 24. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, P.; Lu, L.; Xia, S.; Shen, W. Rare appearance of Candida tropicalis infection of the brain: Multiple micro-abscesses combined with diffuse hemorrhages. Radiol. Infect. Dis. 2014, 1, 33–36. [Google Scholar] [CrossRef]
- O’Brien, D.; Stevens, N.T.; Lim, C.H.; O’Brien, D.F.; Smyth, E.; Fitzpatrick, F.; Humphreys, H. Candida infection of the central nervous system following neurosurgery: A 12-year review. Acta Neurochir. 2011, 153, 1347–1350. [Google Scholar] [CrossRef]
- Rosa Duque, J.S.; To, K.K.W.; Chiang, A.K.S.; Chan, G.C.F.; Poon, R.W.S.; Yuen, K.-Y.; Ha, S.-Y.; Cheuk, D.K.L. Candida tropicalis renal microabscesses in a child with leukemia confirmed using nucleic acid amplification and recovery after prolonged antifungal and corticosteroid treatment. Int. J. Infect. Dis. 2019, 81, 110–113. [Google Scholar] [CrossRef]
- Chandra, A.; Rao, N.; Das, A.; Sen, M. A rare clinical entity as large intrarenal abscess in a typeII diabetic patient due to Candida tropicalis: A case report. Curr. Med. Mycol. 2019, 5, 54–57. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Semin. Neurol. 2005, 36, 115–127. [Google Scholar] [CrossRef]
- Baxter, A.G. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 2007, 7, 904–912. [Google Scholar] [CrossRef]
- Rodriguez, M. Effectors of Demyelination and Remyelination in the CNS: Implications for Multiple Sclerosis. Brain Pathol. 2007, 17, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Johnson, R.T. Infections and multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Benito-León, J.; Laurence, M. The role of fungi in the etiology of multiple sclerosis. Front. Neurol. 2017, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Pisa, D.; Alonso, R.; Carrasco, L. Fungal infection in a patient with multiple sclerosis. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Pisa, D.; Alonso, R.; Jiménez-Jiménez, F.J.; Carrasco, L. Fungal infection in cerebrospinal fluid from some patients with multiple sclerosis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Rahimian, P.; Kalhor, H.; Mordechai, E. Immunological cross reactivity between Candida albicans and human tissue. J. Clin. Lab. Immunol. 1996, 48, 1–15. [Google Scholar] [PubMed]
- Purzycki, C.B.; Shain, D.H. Fungal toxins and multiple sclerosis: A compelling connection. Brain Res. Bull. 2010, 82, 4–6. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Lim, J.K.; Lee, C.-C.R.; Murphy, P.M. Organ-Specific Innate Immune Responses in a Mouse Model of Invasive Candidiasis. J. Innate Immun. 2011, 3, 180–199. [Google Scholar] [CrossRef]
- Fraga-Silva, T.F.C.; Mimura, L.A.N.; Marchetti, C.M.; Chiuso-Minicucci, F.; Francą, T.G.D.; Zorzella-Pezavento, S.F.G.; Venturini, J.; Arruda, M.S.P.; Sartori, A. Experimental autoimmune encephalomyelitis development is aggravated by Candida albicans infection. J. Immunol. Res. 2015, 2015, 635052. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Silva, T.F.d.C.; Mimura, L.A.N.; Leite, L.d.C.T.; Borim, P.A.; Ishikawa, L.L.W.; Venturini, J.; Arruda, M.S.P.d.; Sartori, A. Gliotoxin Aggravates Experimental Autoimmune Encephalomyelitis by Triggering Neuroinflammation. Toxins 2019, 11, 443. [Google Scholar] [CrossRef]
- Sanches, M.D.; Mimura, L.A.N.; Oliveira, L.R.C.; Ishikawa, L.L.W.; Garces, H.G.; Bagagli, E.; Sartori, A.; Kurokawa, C.S.; Fraga-Silva, T.F.C. Differential Behavior of Non-albicans Candida Species in the Central Nervous System of Immunocompetent and Immunosuppressed Mice. Front. Microbiol. 2019, 9, 2968. [Google Scholar] [CrossRef]
- Casado, J.L.; Quereda, C.; Oliva, J.; Navas, E.; Moreno, A.; Pintado, V.; Cobo, J.; Corral, I. Candidal Meningitis in HIV-Infected Patients: Analysis of 14 Cases. Clin. Infect. Dis. 1997, 25, 673–676. [Google Scholar] [CrossRef][Green Version]
- Geers, T.A.; Gordon, S.M. Clinical Significance of Candida Species Isolated from Cerebrospinal Fluid Following Neurosurgery. Clin. Infect. Dis. 1999, 28, 1139–1147. [Google Scholar] [CrossRef]
- McCullers, J.A.; Vargas, S.L.; Flynn, P.M.; Razzouk, B.I.; Shenep, J.L. Candidal Meningitis in Children with Cancer. Clin. Infect. Dis. 2000, 31, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, Z.; Zhang, L.; Tian, Y.; Dong, D.; Peng, Y. Significance of hyphae formation in virulence of Candida tropicalis and transcriptomic analysis of hyphal cells. Microbiol. Res. 2016, 192, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Louria, D.B.; Busé, M.; Brayton, R.G.; Finkel, G. The pathogenesis of Candida tropicalis infections in mice. Med. Mycol. 1967, 5, 14–25. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Gutierrez, E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994, 179, 53–56. [Google Scholar] [CrossRef]
- Gutierrez, E.G.; Banks, W.A.; Kastin, A.J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993, 47, 169–176. [Google Scholar] [CrossRef]
- Murphy, Á.C.; Lalor, S.J.; Lynch, M.A.; Mills, K.H.G. Brain, Behavior, and Immunity Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2010, 24, 641–651. [Google Scholar] [CrossRef]
- Yanagisawa, S. Mapping of V beta 11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. Int. Immunol. 1997, 9, 227–237. [Google Scholar] [CrossRef][Green Version]
- Bettelli, E.; Pagany, M.; Weiner, H.L.; Linington, C.; Sobel, R.A.; Kuchroo, V.K. Myelin Oligodendrocyte Glycoprotein–specific T Cell Receptor Transgenic Mice Develop Spontaneous Autoimmune Optic Neuritis. J. Exp. Med. 2003, 197, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R. Multiple sclerosis: Human model for EAE? Eur. J. Immunol. 2009, 39, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lavi, E. The Role of Astrocytes, Microglia, and Endothelial Cells in Coronavirus-Induced Demyelination. In Experimental Models of Multiple Sclerosis; Springer: Boston, MA, USA, 2005; pp. 717–735. [Google Scholar] [CrossRef]
- Li, H.; Gong, H.; Qi, Y.; Li, J.; Ji, X.; Sun, J.; Tian, R.; Bao, H.; Song, X.; Chen, Q.; et al. In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species. Sci. Rep. 2017, 7, 16942. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Wu, V.-C.; Lin, W.-C.; Chen, Y.-M. Renal Candida tropicalis abscesses in a patient with acute lymphoblastic leukemia. Kidney Int. 2007, 72, 382. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Mice with Disseminated Candidiasis Die of Progressive Sepsis. J. Infect. Dis. 2005, 192, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Gozalbo, D. Role of IFN-gamma in immune responses to Candida albicans infections. Front. Biosci. 2014, 19, 1279. [Google Scholar] [CrossRef]
- Mencacci, A.; Cenci, E.; Del Sero, G.; Fé d’Ostiani, C.; Mosci, P.; Trinchieri, G.; Adorini, L.; Romani, L. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J. Immunol. 1998, 161, 6228–6237. [Google Scholar]
- Pandiyan, P.; Conti, H.R.; Zheng, L.; Peterson, A.C.; Mathern, D.R.; Hernández-Santos, N.; Edgerton, M.; Gaffen, S.L.; Lenardo, M.J. CD4+CD25+Foxp3+ Regulatory T Cells Promote Th17 Cells In Vitro and Enhance Host Resistance in Mouse Candida albicans Th17 Cell Infection Model. Immunity 2011, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Whibley, N.; MacCallum, D.M.; Vickers, M.A.; Zafreen, S.; Waldmann, H.; Hori, S.; Gaffen, S.L.; Gow, N.A.R.; Barker, R.N.; Hall, A.M. Expansion of Foxp3+ T-cell populations by Candida albicans enhances both Th17-cell responses and fungal dissemination after intravenous challenge. Eur. J. Immunol. 2014, 44, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 1999, 2, 363–367. [Google Scholar] [CrossRef]
- Romani, L.; Puccetti, P. Protective tolerance to fungi: The role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006, 14, 183–189. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts Beyond Expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Small, A.; King, J.R.; Rathjen, D.A.; Ferrante, A. The Role of Phagocytes in Immunity to Candida albicans. In Candida albicans; IntechOpen: London, UK, 2019. [Google Scholar]
- Jae-Chen, S.; Young-Joo, J.; Seon-Min, P.; Kang Seok, S.; Jung-Hyun, S.; Jung-Il, C. Mechanism underlying renal failure caused by pathogenic Candida albicans infection. Biomed. Rep. 2015, 3, 179–182. [Google Scholar] [CrossRef][Green Version]
- Persson, R.; Lee, S.; Ulcickas Yood, M.; Wagner, C.M.; Minton, N.; Niemcryk, S.; Lindholm, A.; Evans, A.M.; Jick, S.S. Infections in patients diagnosed with multiple sclerosis: A multi-database study. Mult. Scler. Relat. Disord. 2020, 41, 101982. [Google Scholar] [CrossRef]
- Castelo-Branco, A.; Chiesa, F.; Conte, S.; Bengtsson, C.; Lee, S.; Minton, N.; Niemcryk, S.; Lindholm, A.; Rosenlund, M.; Piehl, F.; et al. Infections in patients with multiple sclerosis: A national cohort study in Sweden. Mult. Scler. Relat. Disord. 2020, 45, 102420. [Google Scholar] [CrossRef]
- Viart, L.; Elalouf, V.; Petit, J.; Al Khedr, A.; Kristkowiak, P.; Saint, F. Facteurs pronostiques d’urétéro-hydronéphrose (UHN) chez les patients atteints de sclérose en plaques (SEP) [Prognostics factors of renal failure in multiple sclerosis]. Progrès Urol. 2012, 22, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Sandy, A.R.; Stoolman, J.; Malott, K.; Pongtornpipat, P.; Segal, B.M.; Maillard, I. Notch Signaling Regulates T Cell Accumulation and Function in the Central Nervous System during Experimental Autoimmune Encephalomyelitis. J. Immunol. 2013, 191, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sandor, M.; Heninger, E.; Fabry, Z. Mycobacteria-Induced Suppression of Autoimmunity in the Central Nervous System. J. Neuroimmune Pharmacol. 2010, 5, 210–219. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Swamydas, M.; Fischer, B.G.; Plantinga, T.S.; Johnson, M.D.; Jaeger, M.; Green, N.M.; Masedunskas, A.; Weigert, R.; Mikelis, C.; et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J. Clin. Investig. 2013, 123, 5035–5051. [Google Scholar] [CrossRef]
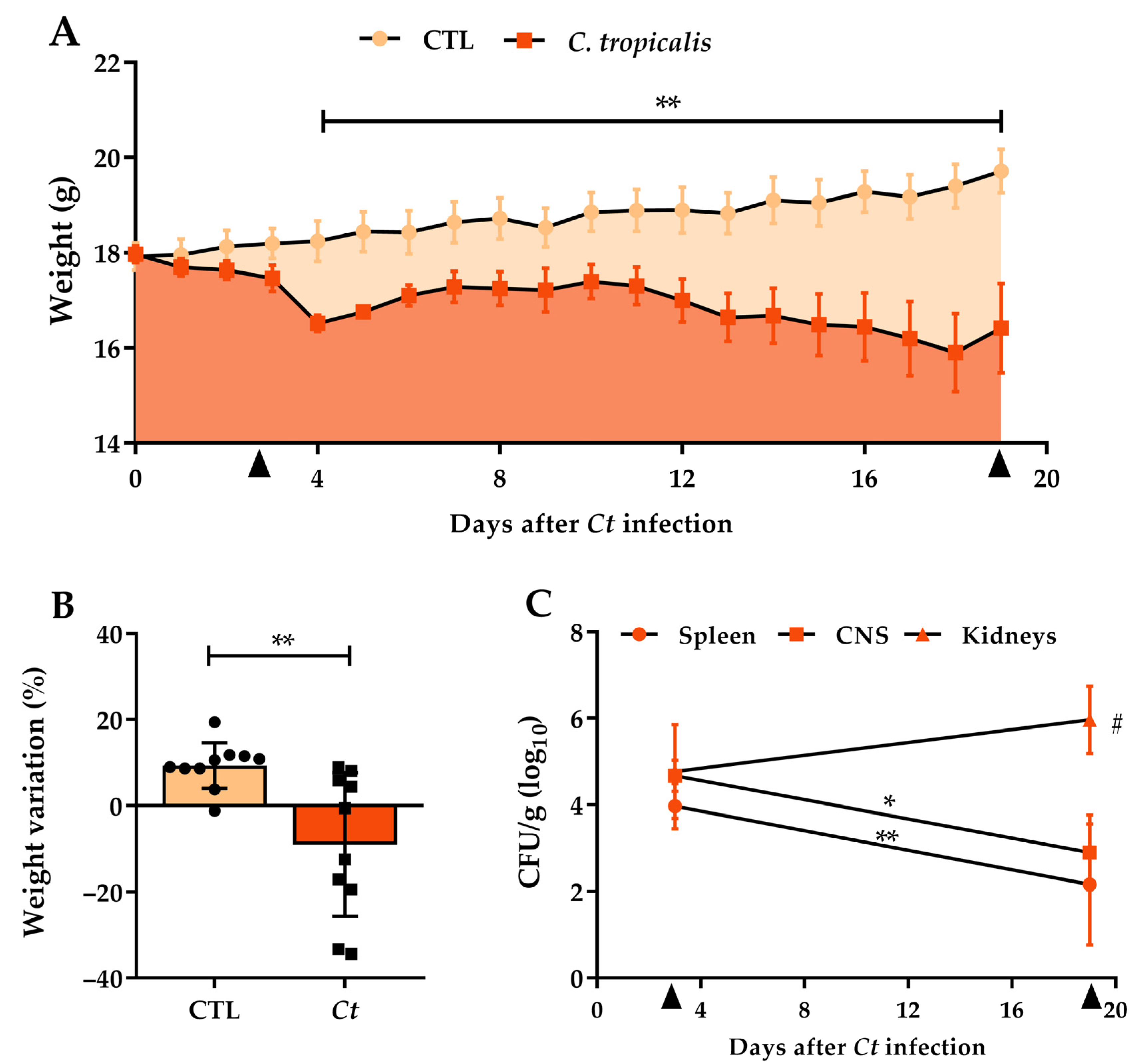
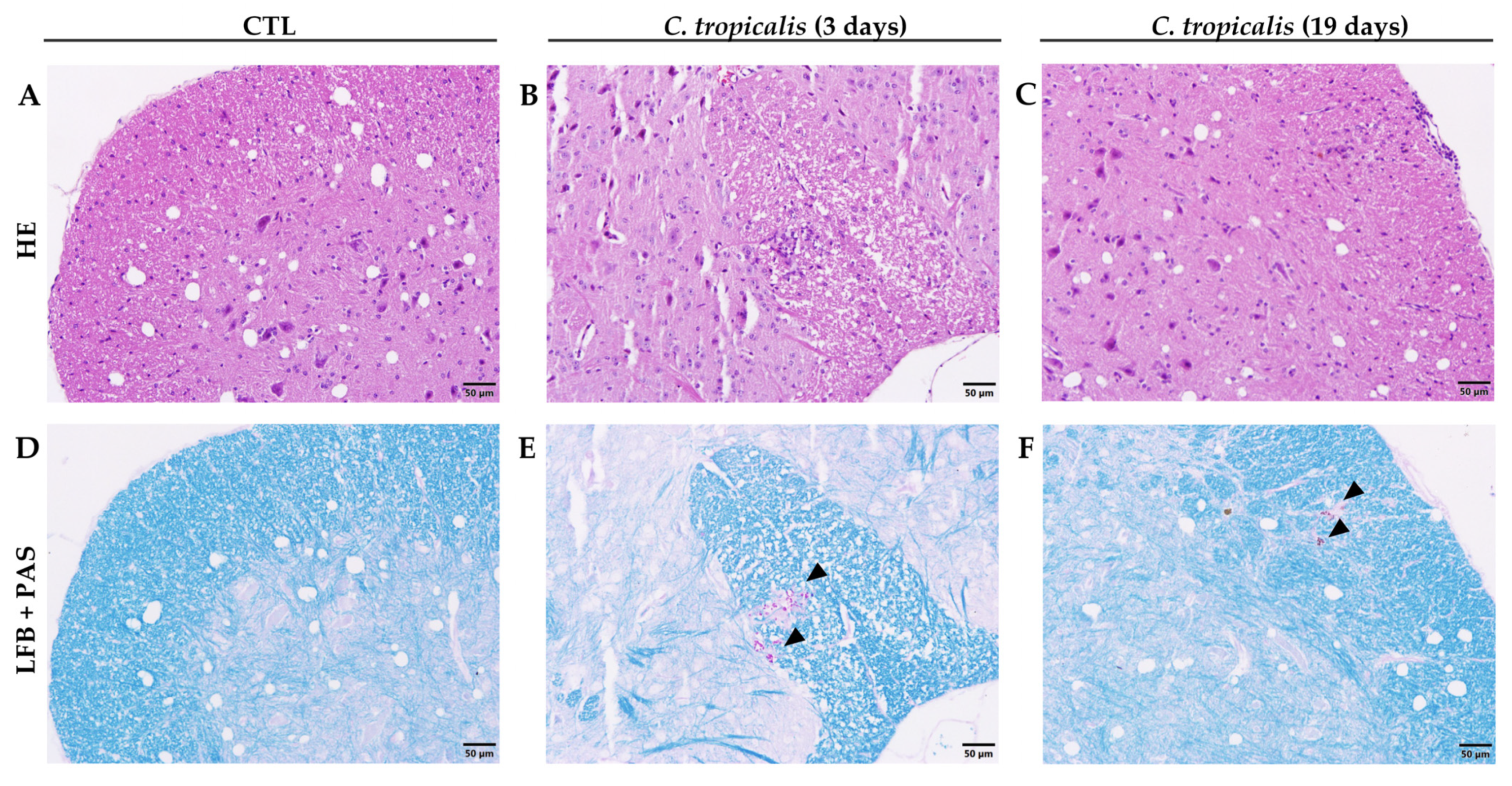
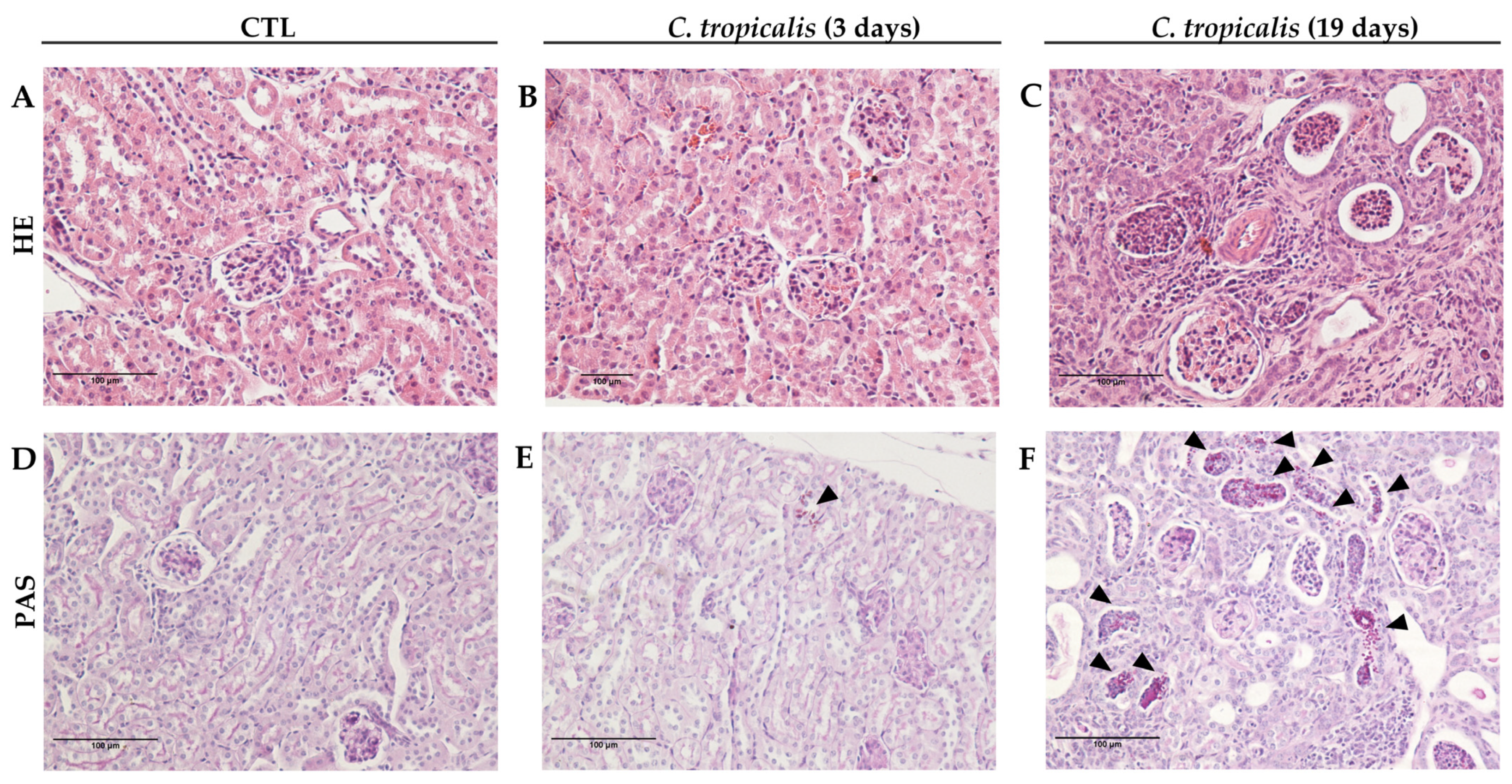
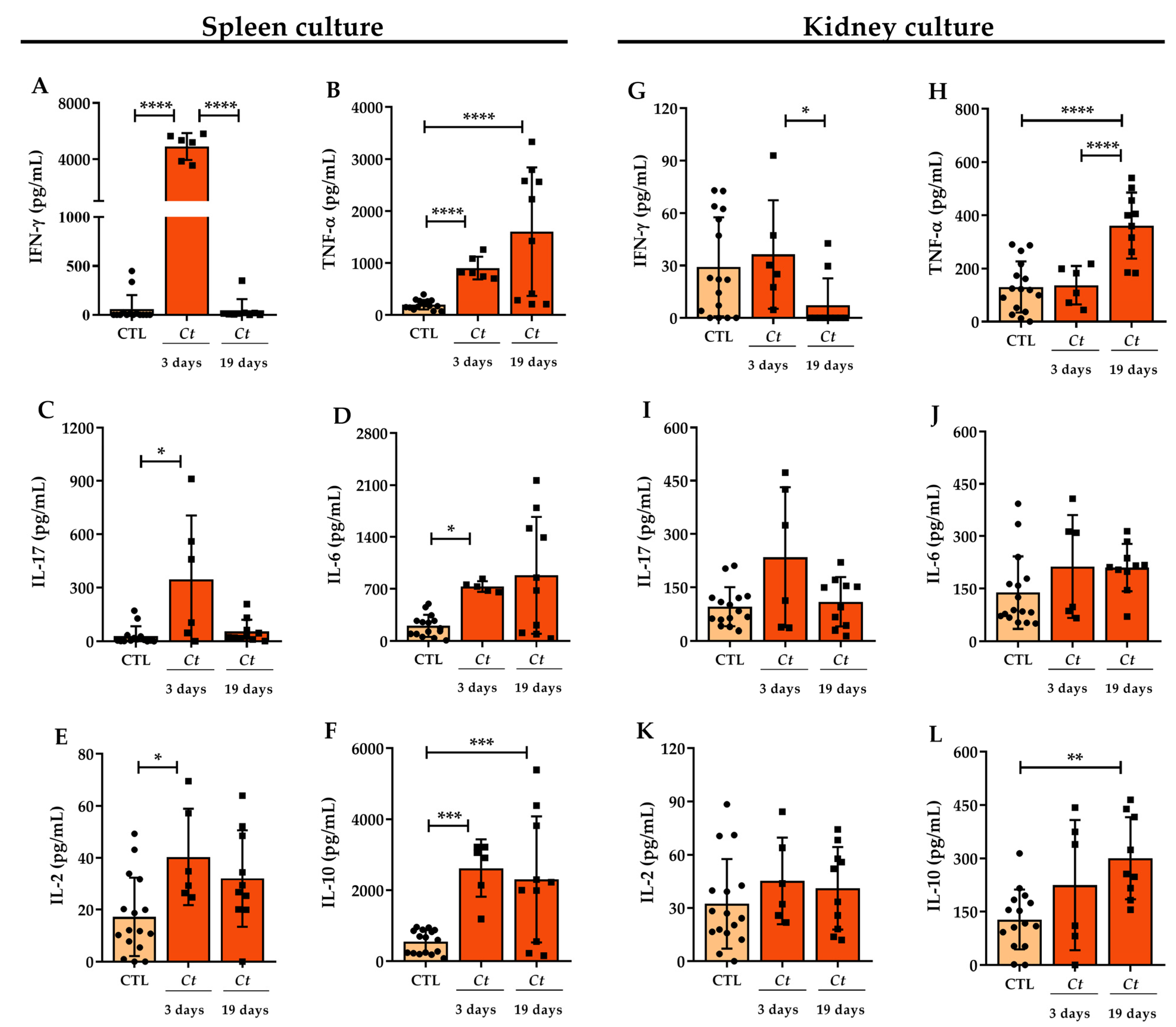
| Inoculum Concentration | Weight Variation (%) | Total Fungal Load (CFU/g log10) |
|---|---|---|
| 2.5 × 106 viable yeasts | −15.25 ± 2.27 | 2.99 ± 1.39 |
| 1 × 106 viable yeasts | −8.82 ± 4.21 | 2.76 ± 1.26 |
| Groups | Affected/Total | Incidence (%) |
|---|---|---|
| EAE | 15/15 | 100% |
| EAE/C. tropicalis | 3/15 | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munhoz-Alves, N.; Mimura, L.A.N.; Viero, R.M.; Bagagli, E.; Peron, J.P.S.; Sartori, A.; Fraga-Silva, T.F.d.C. Candida tropicalis Systemic Infection Redirects Leukocyte Infiltration to the Kidneys Attenuating Encephalomyelitis. J. Fungi 2021, 7, 757. https://doi.org/10.3390/jof7090757
Munhoz-Alves N, Mimura LAN, Viero RM, Bagagli E, Peron JPS, Sartori A, Fraga-Silva TFdC. Candida tropicalis Systemic Infection Redirects Leukocyte Infiltration to the Kidneys Attenuating Encephalomyelitis. Journal of Fungi. 2021; 7(9):757. https://doi.org/10.3390/jof7090757
Chicago/Turabian StyleMunhoz-Alves, Natália, Luiza Ayumi Nishiyama Mimura, Rosa Marlene Viero, Eduardo Bagagli, Jean Pierre Schatzmann Peron, Alexandrina Sartori, and Thais Fernanda de Campos Fraga-Silva. 2021. "Candida tropicalis Systemic Infection Redirects Leukocyte Infiltration to the Kidneys Attenuating Encephalomyelitis" Journal of Fungi 7, no. 9: 757. https://doi.org/10.3390/jof7090757
APA StyleMunhoz-Alves, N., Mimura, L. A. N., Viero, R. M., Bagagli, E., Peron, J. P. S., Sartori, A., & Fraga-Silva, T. F. d. C. (2021). Candida tropicalis Systemic Infection Redirects Leukocyte Infiltration to the Kidneys Attenuating Encephalomyelitis. Journal of Fungi, 7(9), 757. https://doi.org/10.3390/jof7090757








