The Synergy of Arbuscular Mycorrhizal Fungi and Exogenous Abscisic Acid Benefits Robinia pseudoacacia L. Growth through Altering the Distribution of Zn and Endogenous Abscisic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. AM Fungi Inoculum Preparation
2.2. Plant Cultivation and Soil Preparation
2.3. Experimental Design and Plant Growth
2.4. Plant Sampling and Biomass Measurements
2.5. AM Colonization
2.6. Analysis of Abscisic Acid Contents
2.7. Analysis of Total Zn Contents and Water-Soluble Zn Complex Contents
2.8. Analysis of Proline Contents
2.9. Analysis of P5CS, ProDH and OAT Activities
2.10. Analysis of Lipid Peroxidation and Reactive Oxygen Species (ROS)
2.11. Analysis of Antioxidant Enzyme Activities
2.12. Statistical Analysis
3. Results
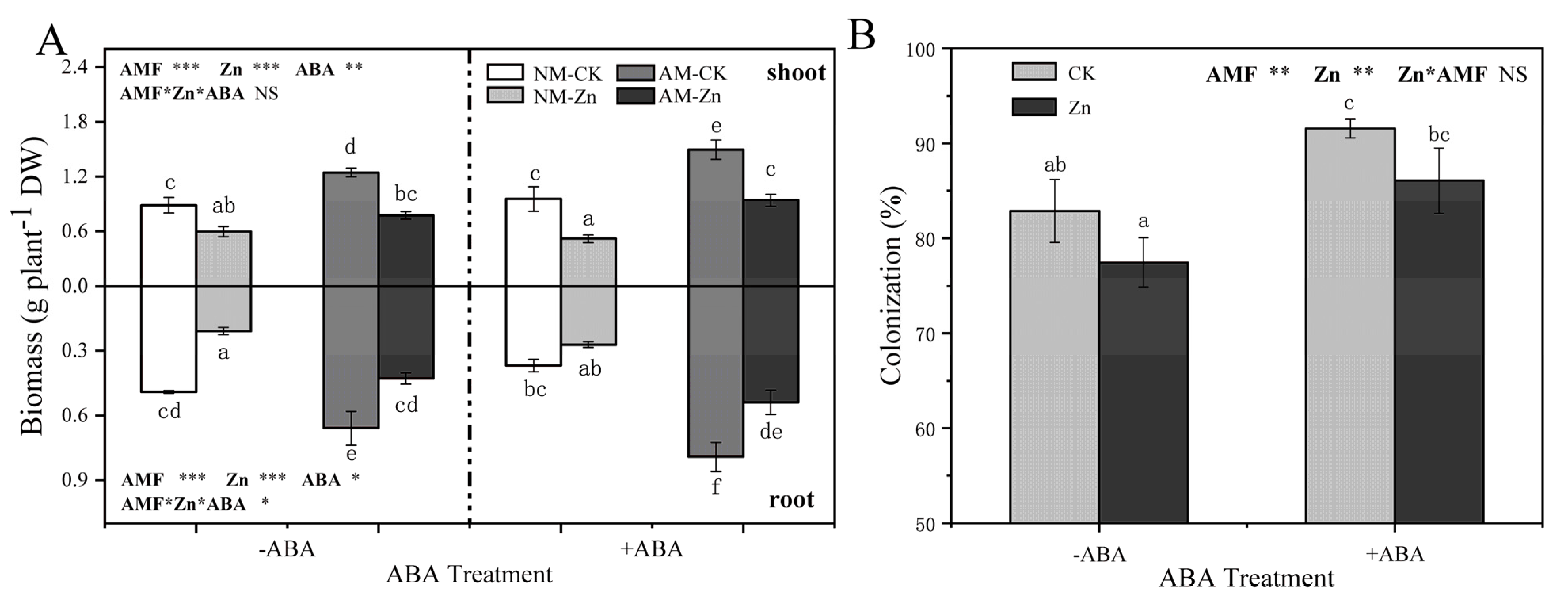
3.1. Biomass and Colonization
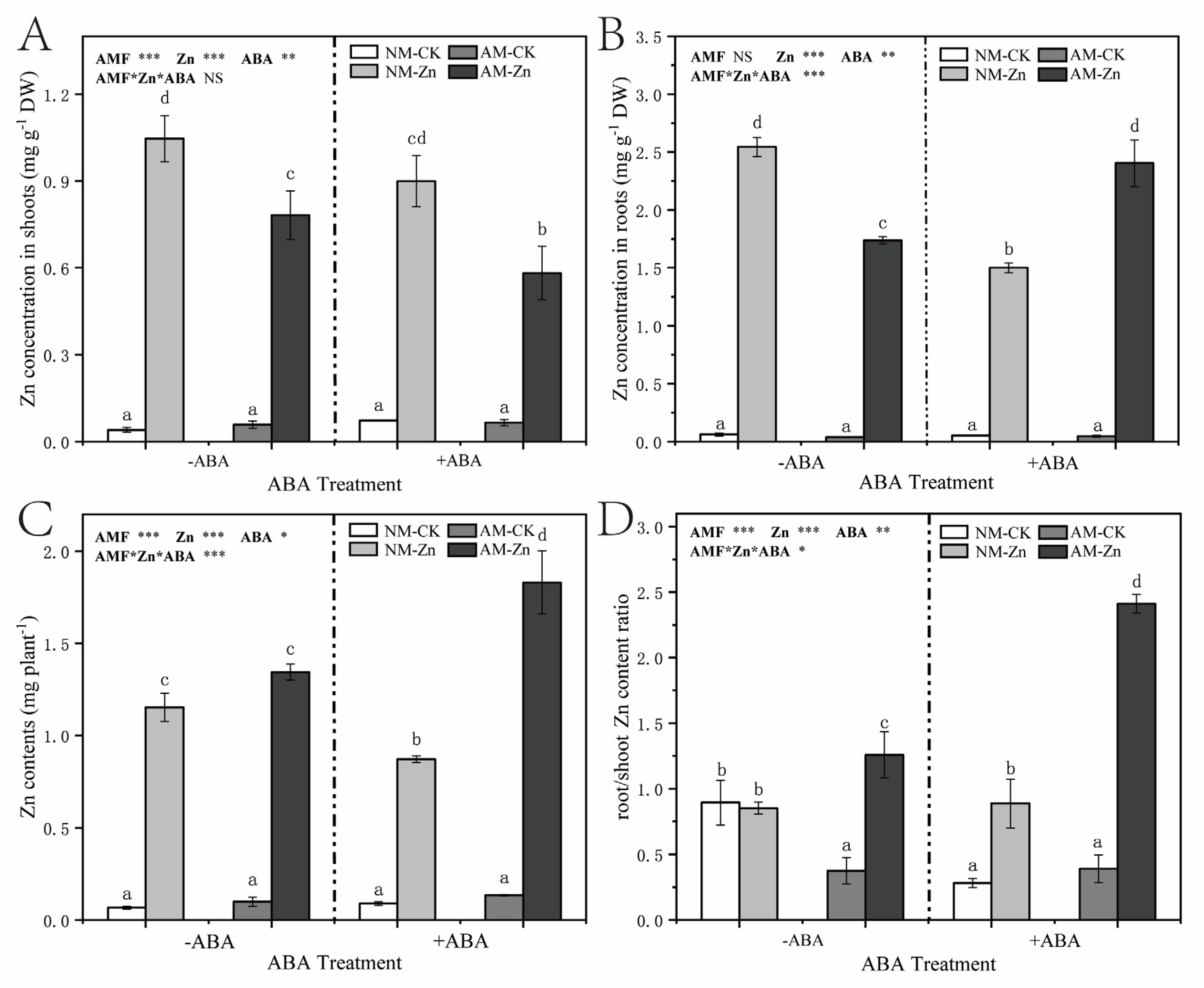
3.2. Total Zn Contents and Water-Soluble Zn Complex Contents
3.3. ABA Contents
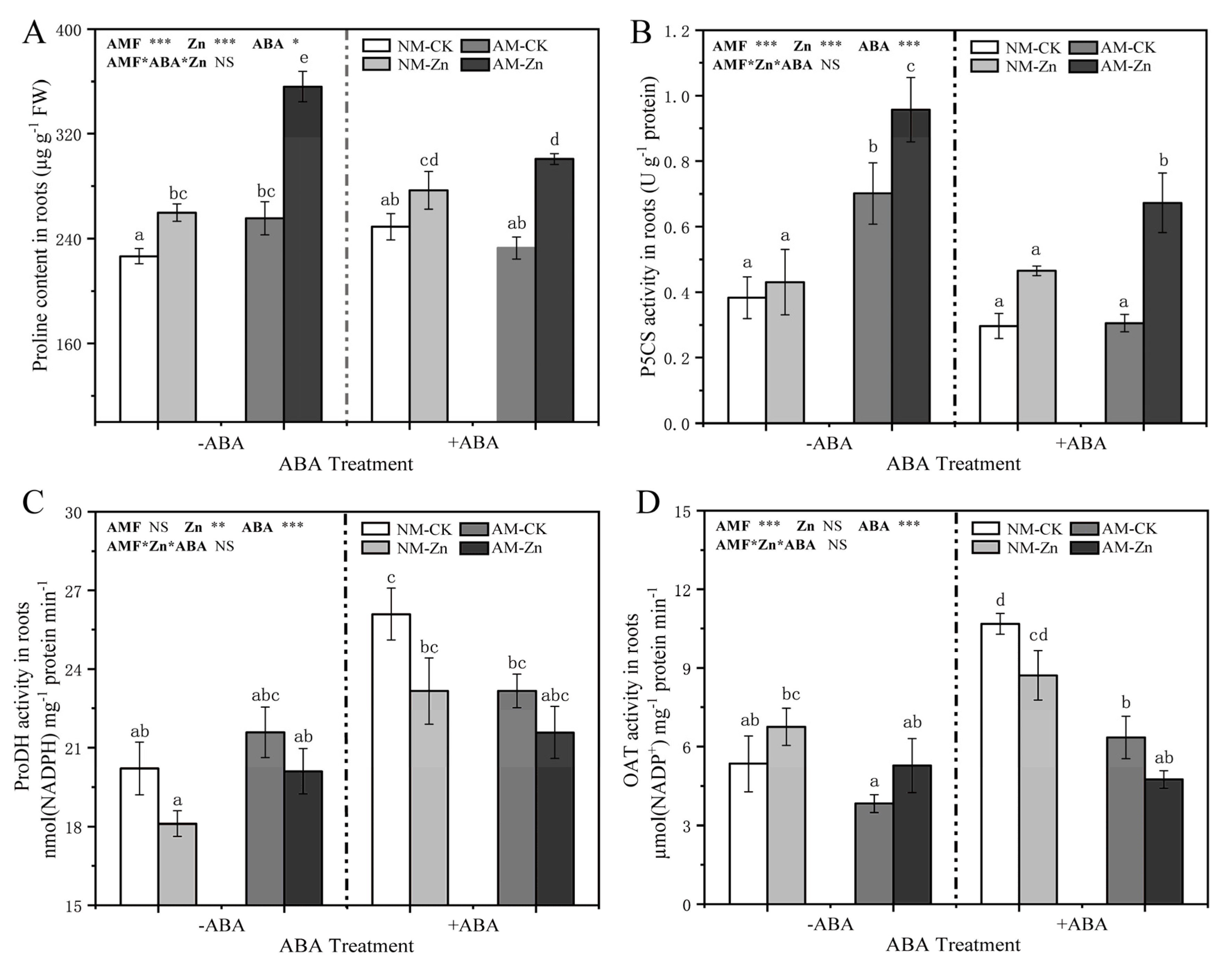
3.4. Proline Contents and Proline Metabolism-Related Enzyme Activity
3.5. Antioxidant System Parameters
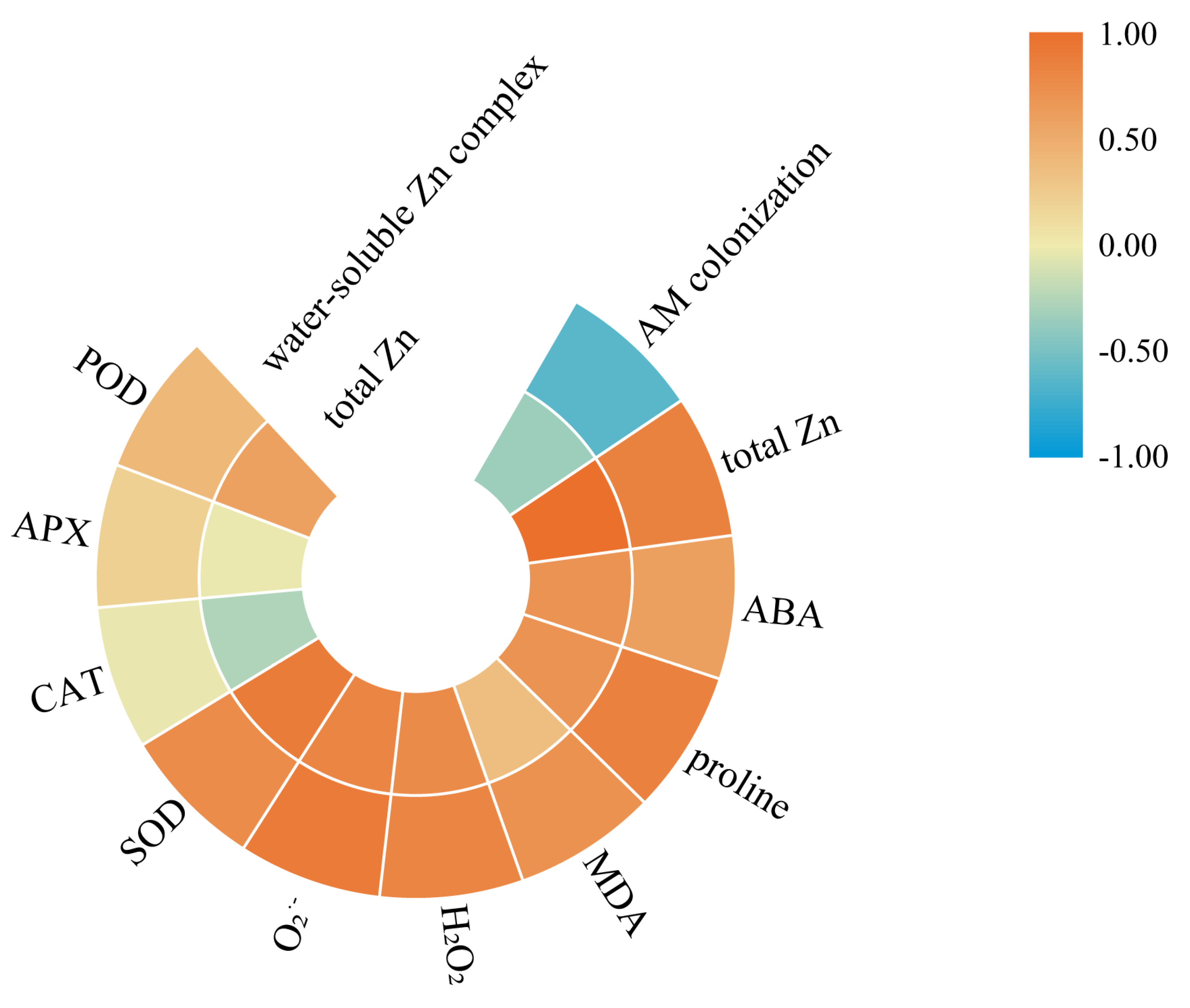
3.6. The Correlation Analysis
4. Discussion
4.1. ABA Enhanced Excess Zn Tolerance by Improving Arbuscular Mycorrhizal Symbiosis
4.2. ABA Played Different Roles in AM and Non-AM Plants under Zn Stress
4.3. ABA Application Stimulated the Antioxidant Response of Mycorrhizal Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.Y.; Shi, H.D.; Bai, Z.K.; Zhou, W.; Liu, K.; Wang, M.H.; He, Y.J. Heavy metal concentrations of soils near the large opencast coal mine pits in China. Chemosphere 2020, 244, 125360. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Lin, Y.F.; Aarts, M.G. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- De Oliveira, V.H.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of Cd and Zn in Populus trichocarpa. Environ. Exp. Bot. 2020, 171, 103925. [Google Scholar] [CrossRef]
- Zhang, F.G.; Liu, M.H.; Li, Y.; Che, Y.Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Barbafieri, M.; Morelli, E.; Tassi, E.; Pedron, F.; Remorini, D.; Petruzzelli, G. Overcoming limitation of “recalcitrant areas” to phytoextraction process: The synergistic effects of exogenous cytokinins and nitrogen treatments. Sci. Total Environ. 2018, 639, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.H.; Chen, L.; Xing, W.; Ran, S.M.; Wei, Z.H.; Amee, M.; Wassie, M.; Niu, H.; Tang, D.Y.; Sun, J.; et al. Phytohormones-induced senescence efficiently promotes the transport of cadmium from roots into shoots of plants: A novel strategy for strengthening of phytoremediation. J. Hazard. Mater. 2020, 388, 122080. [Google Scholar] [CrossRef]
- Shi, W.G.; Liu, W.Z.; Yu, W.J.; Zhang, Y.H.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.B. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus× canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Xie, Z.H. Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, P.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch. Agron. Soil Sci. 2019, 65, 1316–1330. [Google Scholar] [CrossRef]
- Hu, B.B.; Deng, F.L.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, J.X.; Chen, Z.H. Evolution of abscisic acid signaling for stress responses to toxic metals and metalloids. Front. Plant Sci. 2020, 11, 909. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.G.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus× canescens exposed to excess zinc. Plant Cell Environ. 2015, 38, 207–223. [Google Scholar] [CrossRef]
- Glassman, S.I.; Casper, B.B. Biotic contexts alter metal sequestration and AMF effects on plant growth in soils polluted with heavy metals. Ecology 2012, 93, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.R.; Song, Y.Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.H.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.D.; Shen, H.; Li, X.L.; Gu, F.; Christie, P. Effects of EDTA application and arbuscular mycorrhizal colonization on growth and zinc uptake by maize (Zea mays L.) in soil experimentally contaminated with zinc. Plant Soil 2004, 261, 219–229. [Google Scholar] [CrossRef]
- Souza, S.C.; Souza, L.A.; Schiavinato, M.A.; de Oliveira Silva, F.M.; de Andrade, S.A.L. Zinc toxicity in seedlings of three trees from the Fabaceae associated with arbuscular mycorrhizal fungi. Ecotoxicol. Environ. Saf. 2020, 195, 110450. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhao, Z.J.; Chen, L.; Wang, L.Q.; Ji, L.Z.; Xiao, Y. Influences of arbuscular mycorrhizae, phosphorus fertiliser and biochar on alfalfa growth, nutrient status and cadmium uptake. Ecotoxicol. Environ. Saf. 2020, 196, 110537. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.J.; Ai, S.Y.; Chen, K.; Wang, X.R. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef]
- Herrera-Medina, M.J.; Steinkellner, S.; Vierheilig, H.; Ocampo Bote, J.A.; Garcia Garrido, J.M. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007, 175, 554–564. [Google Scholar] [CrossRef]
- Martín-Rodríguez, J.Á.; León-Morcillo, R.; Vierheilig, H.; Ocampo, J.A.; Ludwig-Müller, J.; García-Garrido, J.M. Ethylene-dependent/ethylene-independent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytol. 2011, 190, 193–205. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.Q.; Zhang, X.L.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, waters status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, H.Q.; Lou, X.; Tang, M. Mycorrhizal and non-mycorrhizal Medicago truncatula roots exhibit differentially regulated NADPH oxidase and antioxidant response under Pb stress. Environ. Exp. Bot. 2019, 164, 10–19. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungus. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Zeng, F.R.; Ali, S.; Qiu, B.Y.; Wu, F.B.; Zhang, G.P. Effects of chromium stress on the subcellular distribution and chemical form of Ca, Mg, Fe, and Zn in two rice genotypes. J. Plant Nutr. Soil Sci. 2010, 173, 135–148. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sánchez, E.; López-Lefebre, L.R.; García, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike). J. Plant Physiol. 2001, 158, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.N.M.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, D.; Sun, G.C.; Wang, Z.X. Effects of superoxide radicals on ACC synthase activity in chilling-stressed etiolated mungbean seedlings. Plant Growth Regul. 2007, 51, 83–91. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Datta, S.K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Acta Physiol. Plant. 2008, 30, 325–331. [Google Scholar] [CrossRef]
- Gao, J.F. Experimental Guidance of Plant Physiology; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by Ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Christie, P.; Li, X.L.; Chen, B.D. Arbuscular mycorrhiza can depress translocation of zinc to shoots of host plants in soils moderately polluted with zinc. Plant Soil 2004, 261, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Lingua, G.; Franchin, C.; Todeschini, V.; Castiglione, S.; Biondi, S.; Burlando, B.; Parravicini, V.; Torrigiani, P.; Berta, G. Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ. Pollut. 2008, 153, 137–147. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.Z.; Wang, Q.Q.; Cao, H.Y.; Yang, G.L.; Deng, L.L.; Wang, Y.J.; Zhou, Y.Y.; Znastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2020, 402, 123919. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Patti, A.F.; Cavagnaro, T.R. Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 2013, 371, 299–312. [Google Scholar] [CrossRef]
- Xu, L.J.; Li, T.; Wu, Z.X.; Feng, H.Y.; Yu, M.; Zhang, X.; Chen, B.D. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Appl. Soil Ecol. 2018, 125, 213–221. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Chen, S.M.; Li, Y.Y.; Zheng, F.H.; He, B.; Gu, M.H. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. 2020, 27, 8719–8731. [Google Scholar] [CrossRef]
- Ren, A.T.; Zhu, Y.; Chen, Y.L.; Ren, H.X.; Li, J.Y.; Abbott, L.K.; Xiong, Y.C. Arbuscular mycorrhizal fungus alters root-sourced signal (abscisic acid) for better drought acclimation in Zea mays L. seedlings. Environ. Exp. Bot. 2019, 167, 103824. [Google Scholar] [CrossRef]
- Wu, S.L.; Zhang, X.; Sun, Y.Q.; Wu, Z.X.; Li, T.; Hu, Y.J.; Lv, J.T.; Li, G.; Zhang, Z.S.; Zhang, J.; et al. Chromium immobilization by extra-and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Tyerman, S.D.; Cavagnaro, T.R. The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: Linking plant physiology and gene expression. Plant Soil 2017, 420, 375–388. [Google Scholar] [CrossRef]
- Shen, G.M.; Niu, J.K.; Deng, Z.X. Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol. Biochem. 2017, 118, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Remans, T.; Opdenakker, K.; Guisez, Y.; Carleer, R.; Schat, H.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to excess Zn reveals a Zn-specific oxidative stress signature. Environ. Exp. Bot. 2012, 84, 61–71. [Google Scholar] [CrossRef]
- Sarkar, A.; Asaeda, T.; Wang, Q.Y.; Kaneko, Y.; Rashid, M.H. Response of Miscanthus sacchariflorus to zinc stress mediated by arbuscular mycorrhizal fungi. Flora 2017, 234, 60–68. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Zouari, M.; Ahmed, C.B.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; Abdallah, F.B.; Rouina, B.B. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Song, L.L. The role of ABA in the responses of wild type and abscisic acid mutants of Arabidopsis thaliana to excess zinc. Acta Physiol. Plant. 2020, 42, 74. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.Q.; Song, Y.Y.; Yang, Y.R.; Chen, H.; Tang, M. Subcellular compartmentalization and chemical forms of lead participate in lead tolerance of Robinia pseudoacacia L. with Funneliformis mosseae. Front. Plant Sci. 2017, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
| ABA Treatment | Inoculation Treatment | Zinc Treatment | Leaves (µg g−1 FW) | Roots (µg g−1 FW) | Root/Leaf of ABA Content Ration |
|---|---|---|---|---|---|
| −ABA | NM | Zn0 | 0.66 ± 0.13 a | 1.15 ± 0.11 bc | 4.69 ± 0.25 d |
| Zn1000 | 0.94 ± 0.11 bc | 0.74 ± 0.06 a | 1.83 ± 0.21 a | ||
| AM | Zn0 | 1.07 ± 0.04 c | 1.10 ± 0.02 bc | 2.45 ± 0.22 ab | |
| Zn1000 | 0.73 ± 0.12 ab | 1.30 ± 0.02 bc | 2.98 ± 0.44 b | ||
| +ABA | NM | Zn0 | 0.61 ± 0.04 a | 1.22 ± 0.13 bc | 3.86 ± 0.30 c |
| Zn1000 | 1.53 ± 0.08 d | 1.03 ± 0.22 b | 1.90 ± 0.30 a | ||
| AM | Zn0 | 0.78 ± 0.06 ab | 1.21 ± 0.15 bc | 2.91 ± 0.31 b | |
| Zn1000 | 0.69 ± 0.03 a | 1.34 ± 0.12 c | 4.78 ± 0.11 d | ||
| Significance | |||||
| AMF | ** | ** | NS | ||
| Zn | *** | NS | *** | ||
| ABA | NS | * | ** | ||
| AMF*Zn | *** | *** | *** | ||
| AMF*ABA | *** | NS | *** | ||
| ABA*Zn | *** | NS | *** | ||
| AMF*Zn*ABA | * | NS | NS | ||
| Treatment | MDA (µmol g−1 FW) | H2O2 (µmol g−1 FW) | O2·− Generation Rate (nmol min−1 g−1 Protein) | SOD Activity (U mg−1 Protein) | CAT Activity (U mg−1 Protein) | POD Activity (U mg−1 Protein) | APX Activity (U mg−1 Protein) | ||
|---|---|---|---|---|---|---|---|---|---|
| −ABA | NM | Zn0 | 30.67 ± 1.42 b | 7.73 ± 0.73 bc | 384.15 ± 22.46 d | 1.52 ± 0.30 a | 1.70 ± 0.05 a | 57.24 ± 5.08 a | 1.60 ± 0.11 a |
| Zn1000 | 54.07 ± 4.99 c | 8.44 ± 0.42 c | 520.52 ± 17.32 e | 2.16 ± 0.07 ab | 2.87 ± 0.08 cd | 71.64 ± 0.98 bc | 2.01 ± 0.04 cd | ||
| AM | Zn0 | 17.94 ± 1.79 a | 6.62 ± 0.049 ab | 129.78 ± 20.38 a | 2.11 ± 0.15 ab | 2.39 ± 0.13 abc | 65.33 ± 6.74 ab | 1.73 ± 0.06 ab | |
| Zn1000 | 27.83 ± 4.47 b | 7.89 ± 0.57 c | 202.54 ± 29.38 bc | 3.00 ± 0.36 cd | 3.47 ± 0.56 de | 82.46 ± 2.79 d | 2.23 ± 0.02 d | ||
| +ABA | NM | Zn0 | 23.83 ± 1.03 ab | 8.19 ± 0.25 c | 238.14 ± 16.18 c | 2.05 ± 0.12 ab | 3.05 ± 0.42 cd | 66.61 ± 0.84 ab | 1.90 ± 0.13 bc |
| Zn1000 | 56.00 ± 3.27 c | 7.93 ± 0.14 c | 246.28 ± 7.88 c | 2.32 ± 0.35 bc | 1.86 ± 0.07 ab | 80.25 ± 0.51 cd | 1.63 ± 0.04 a | ||
| AM | Zn0 | 15.60 ± 1.55 a | 5.66 ± 0.55 a | 116.77 ± 15.54 a | 2.42 ± 0.15 bc | 4.21 ± 0.38 e | 86.84 ± 3.61 de | 2.22 ± 0.18 d | |
| Zn1000 | 17.05 ± 2.99 a | 7.49 ± 0.21 bc | 182.66 ± 8.52 b | 3.30 ± 0.27 d | 2.57 ± 0.09 bc | 101.77 ± 2.34 e | 1.82 ± 0.03 abc | ||
| Significance | |||||||||
| AMF | *** | *** | *** | *** | *** | *** | *** | ||
| Zn | *** | *** | *** | *** | NS | *** | NS | ||
| ABA | ** | NS | *** | * | * | *** | NS | ||
| AMF*Zn | *** | ** | NS | NS | NS | NS | NS | ||
| AMF*ABA | NS | NS | *** | NS | NS | * | NS | ||
| ABA*Zn | NS | NS | *** | NS | *** | NS | *** | ||
| AMF*Zn*ABA | ** | * | ** | NS | NS | NS | NS | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, X.; Zhang, X.; Zhang, Y.; Tang, M. The Synergy of Arbuscular Mycorrhizal Fungi and Exogenous Abscisic Acid Benefits Robinia pseudoacacia L. Growth through Altering the Distribution of Zn and Endogenous Abscisic Acid. J. Fungi 2021, 7, 671. https://doi.org/10.3390/jof7080671
Lou X, Zhang X, Zhang Y, Tang M. The Synergy of Arbuscular Mycorrhizal Fungi and Exogenous Abscisic Acid Benefits Robinia pseudoacacia L. Growth through Altering the Distribution of Zn and Endogenous Abscisic Acid. Journal of Fungi. 2021; 7(8):671. https://doi.org/10.3390/jof7080671
Chicago/Turabian StyleLou, Xiao, Xiangyu Zhang, Yu Zhang, and Ming Tang. 2021. "The Synergy of Arbuscular Mycorrhizal Fungi and Exogenous Abscisic Acid Benefits Robinia pseudoacacia L. Growth through Altering the Distribution of Zn and Endogenous Abscisic Acid" Journal of Fungi 7, no. 8: 671. https://doi.org/10.3390/jof7080671
APA StyleLou, X., Zhang, X., Zhang, Y., & Tang, M. (2021). The Synergy of Arbuscular Mycorrhizal Fungi and Exogenous Abscisic Acid Benefits Robinia pseudoacacia L. Growth through Altering the Distribution of Zn and Endogenous Abscisic Acid. Journal of Fungi, 7(8), 671. https://doi.org/10.3390/jof7080671





