Fungal Biodiversity in Salt Marsh Ecosystems
Abstract
1. Introduction
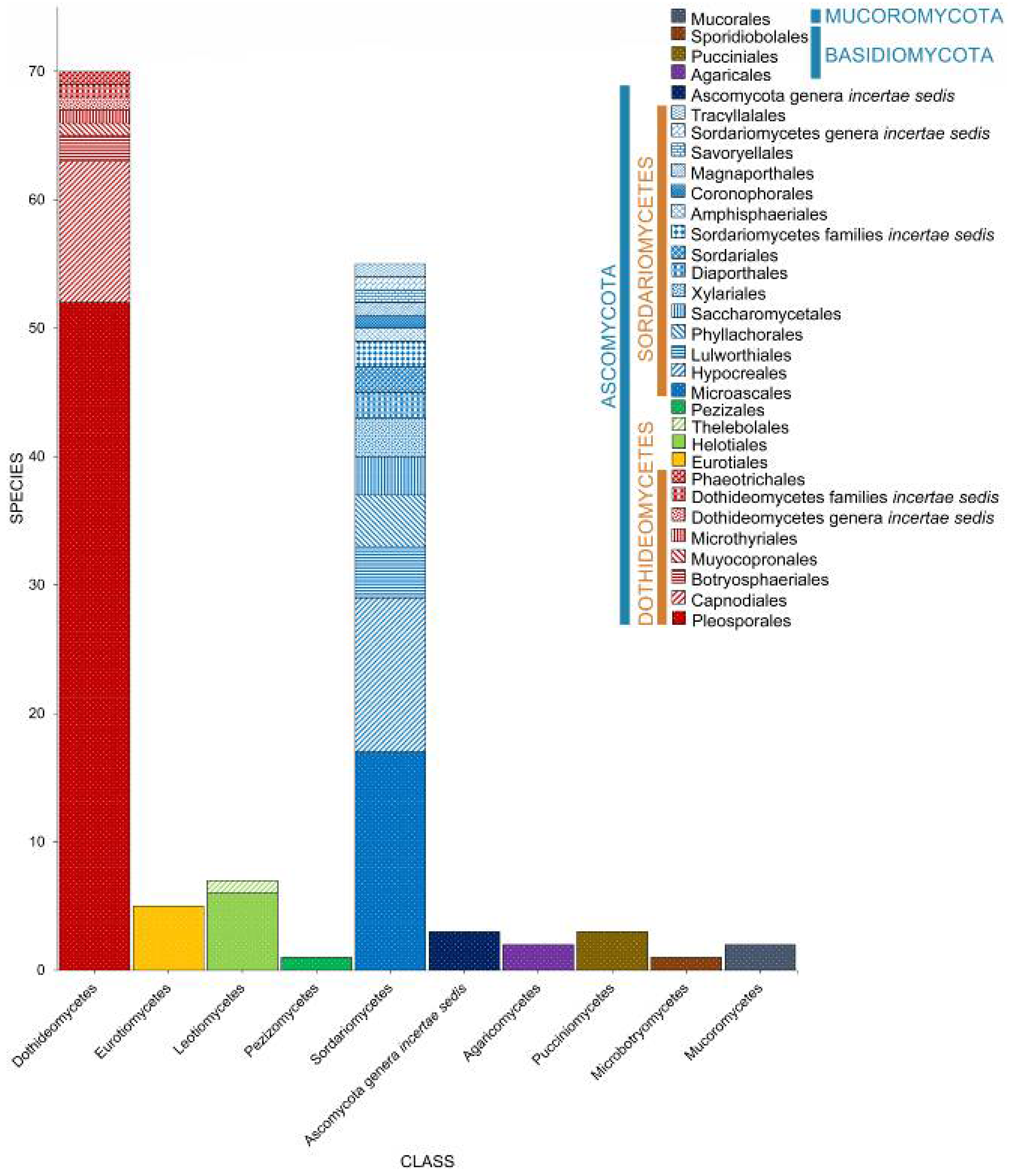
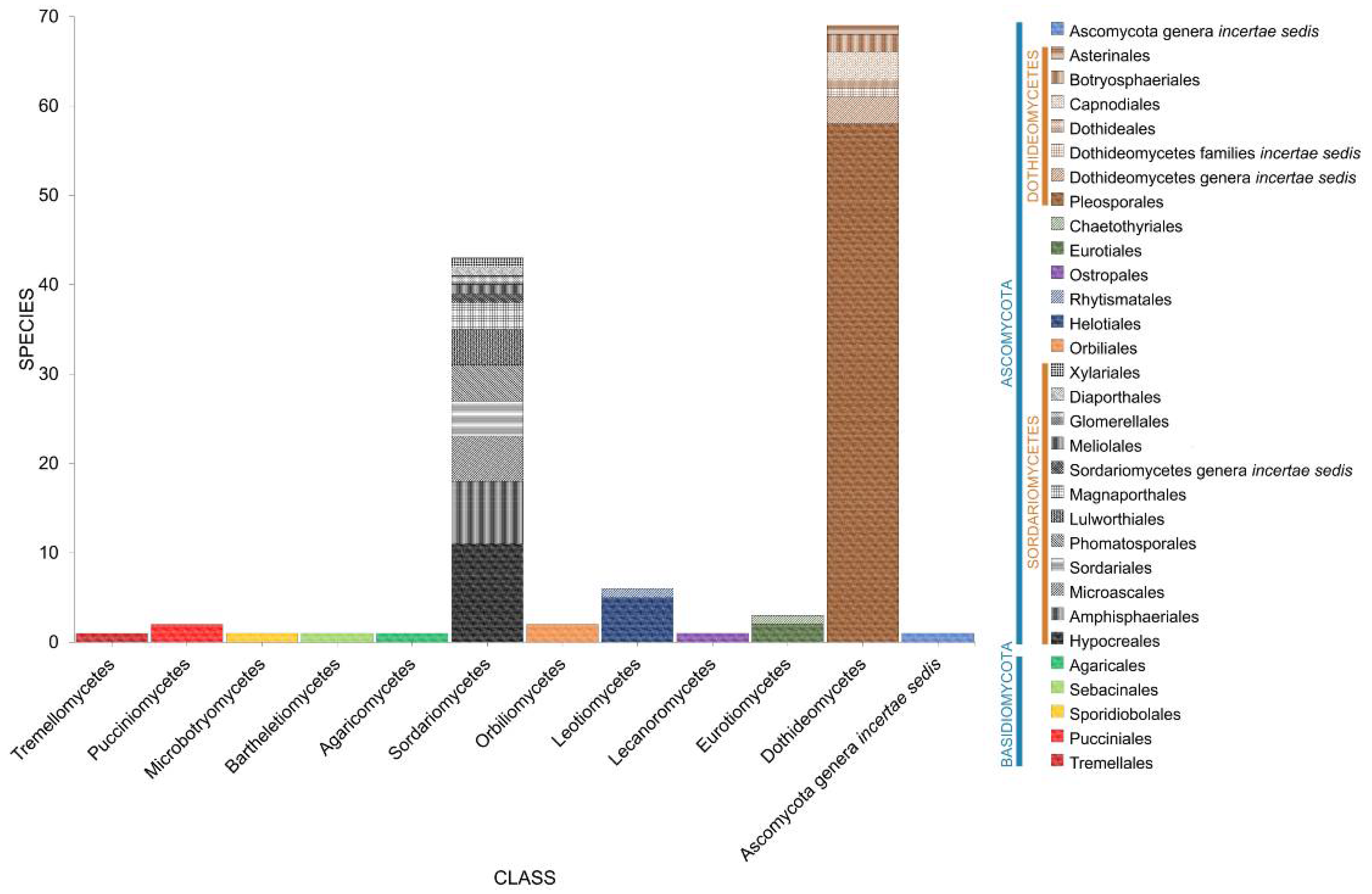
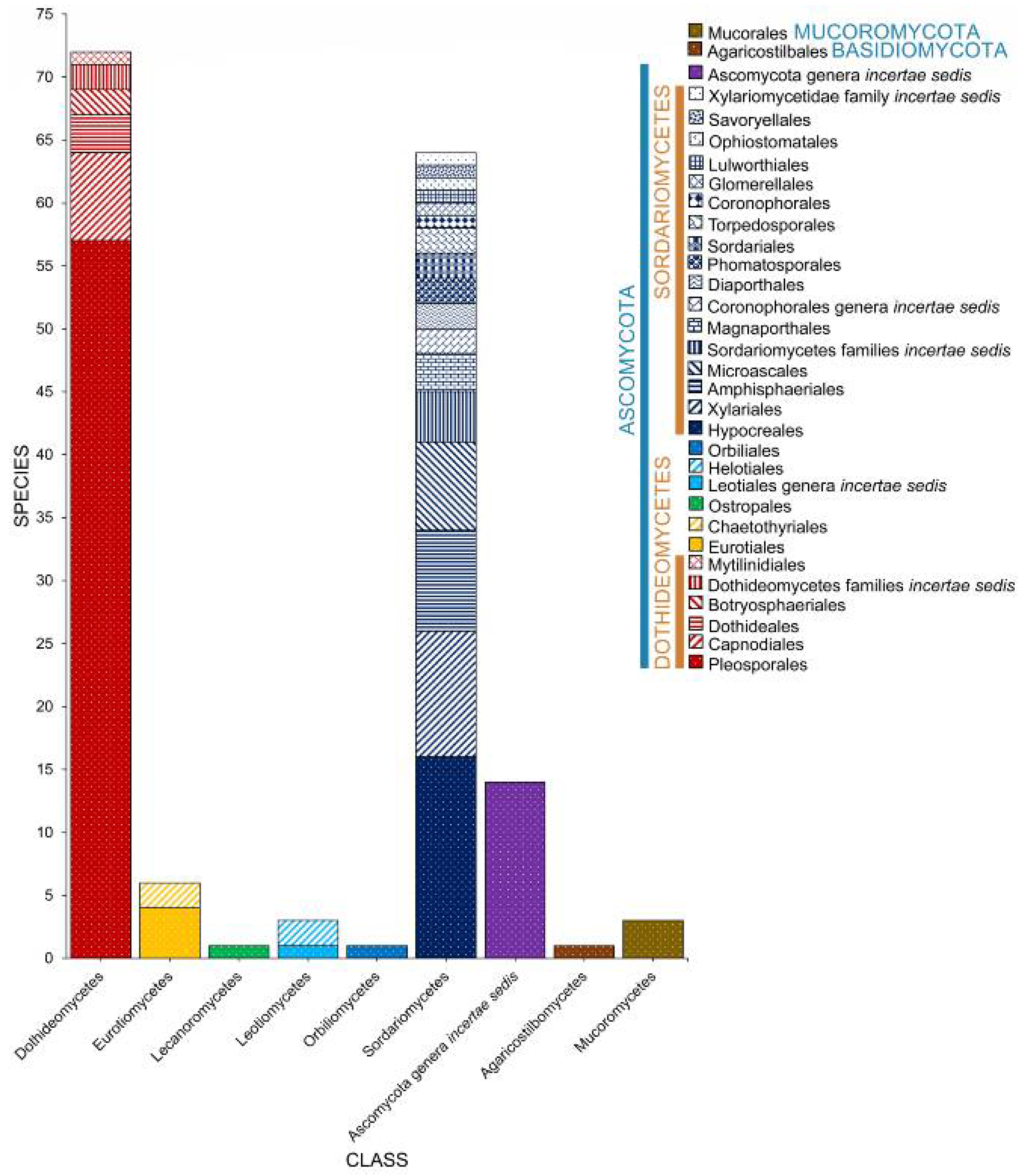
2. Taxonomic Classification of Salt Marsh Fungi
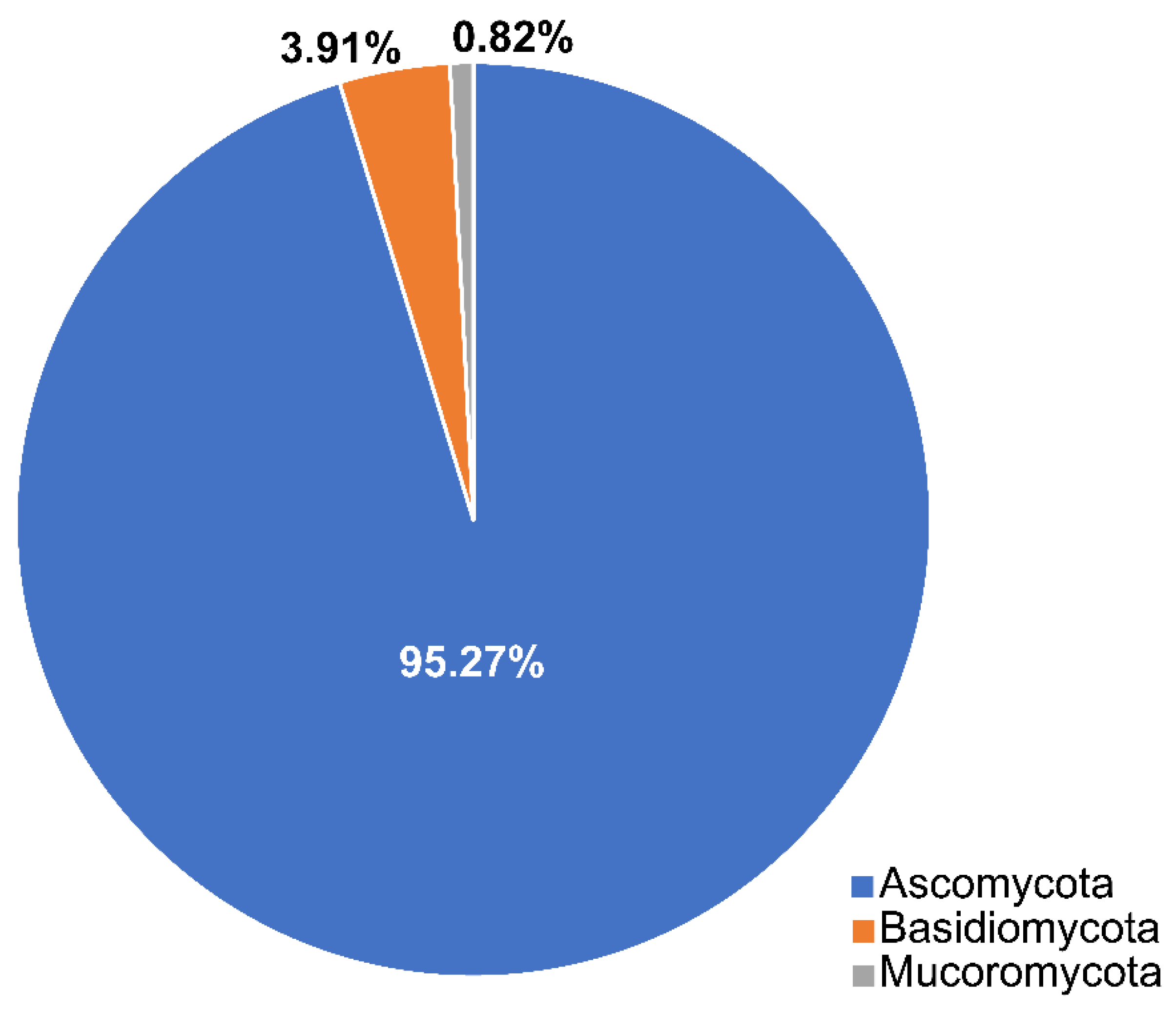
2.1. Phyla
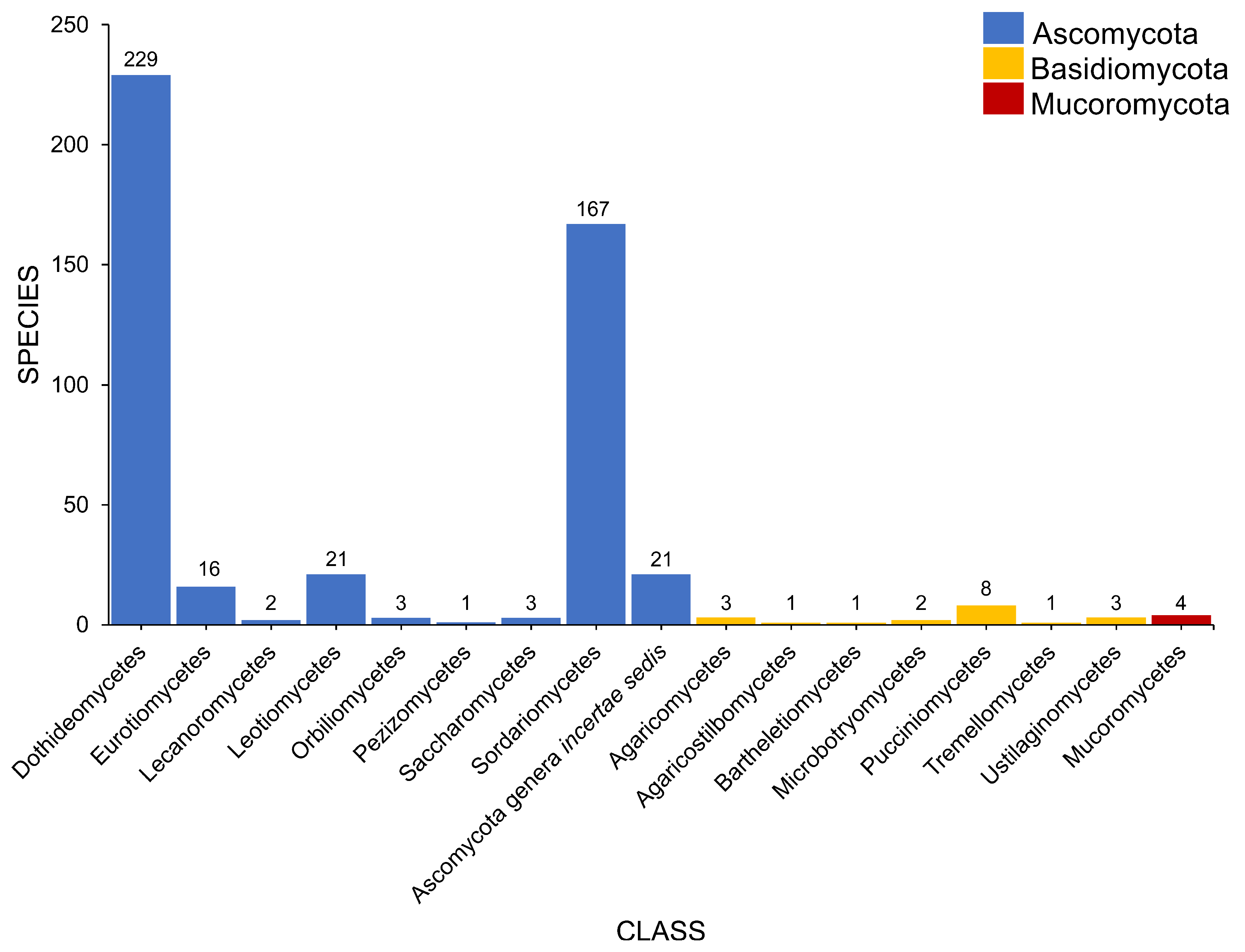
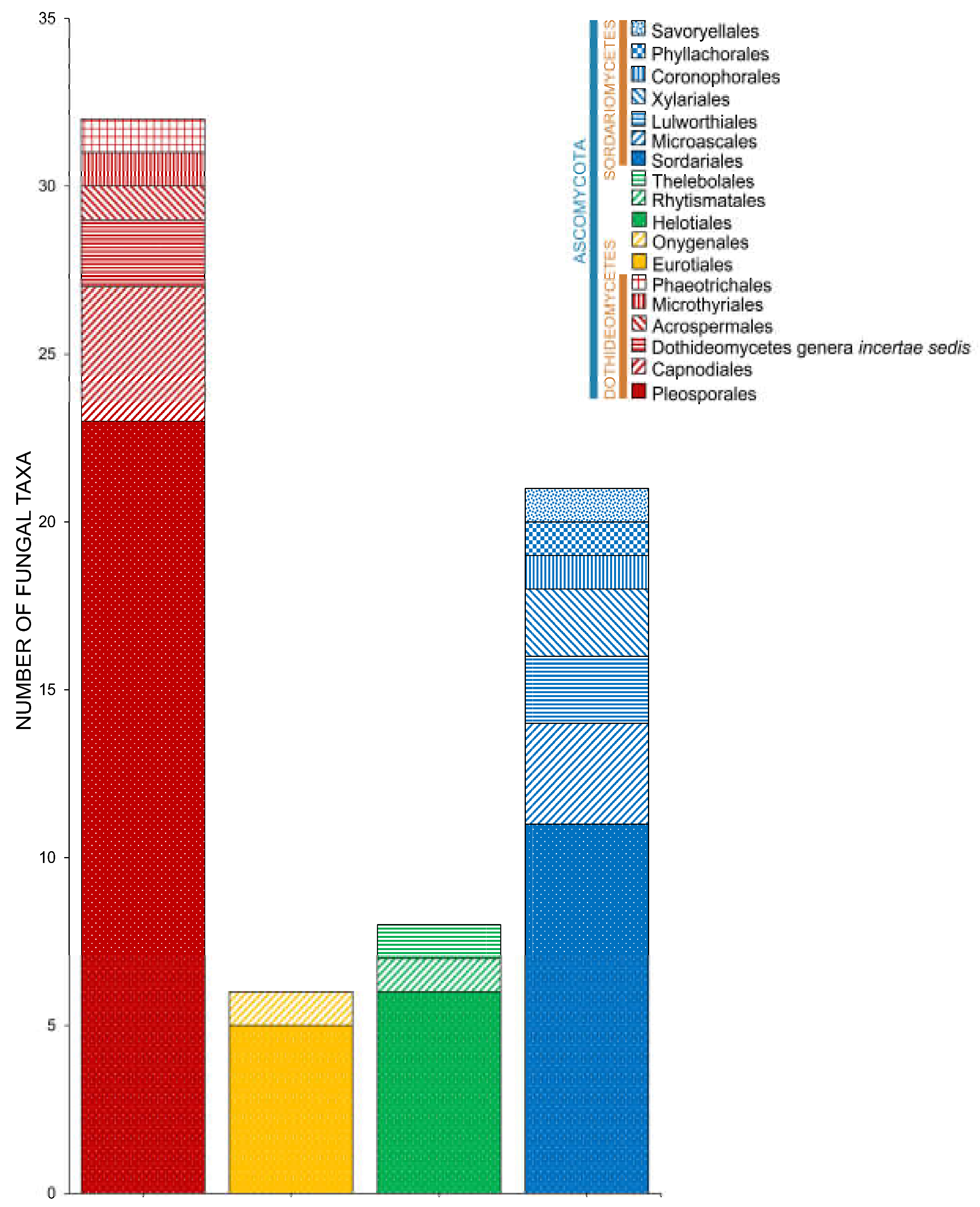
2.2. Class
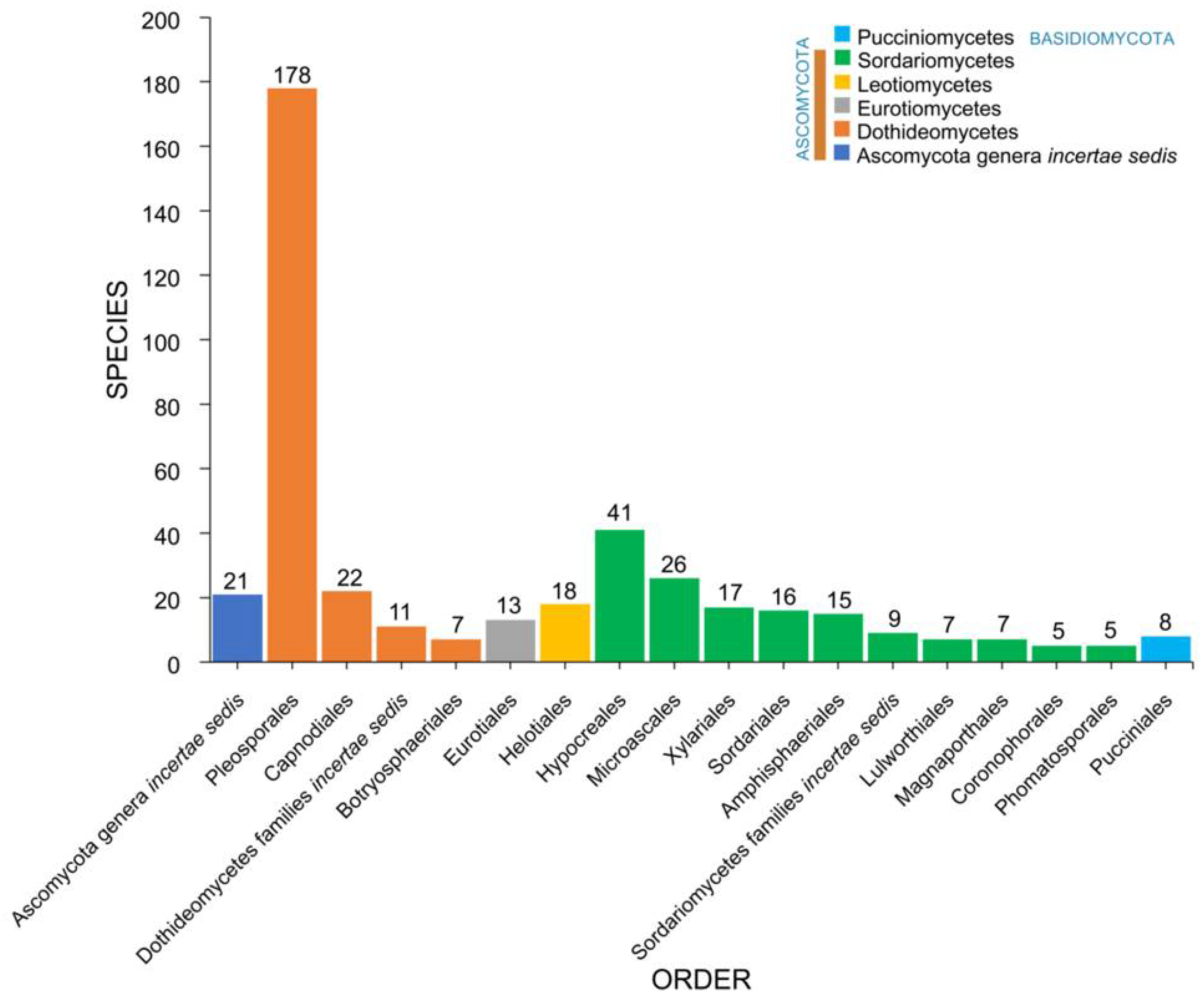
2.3. Orders
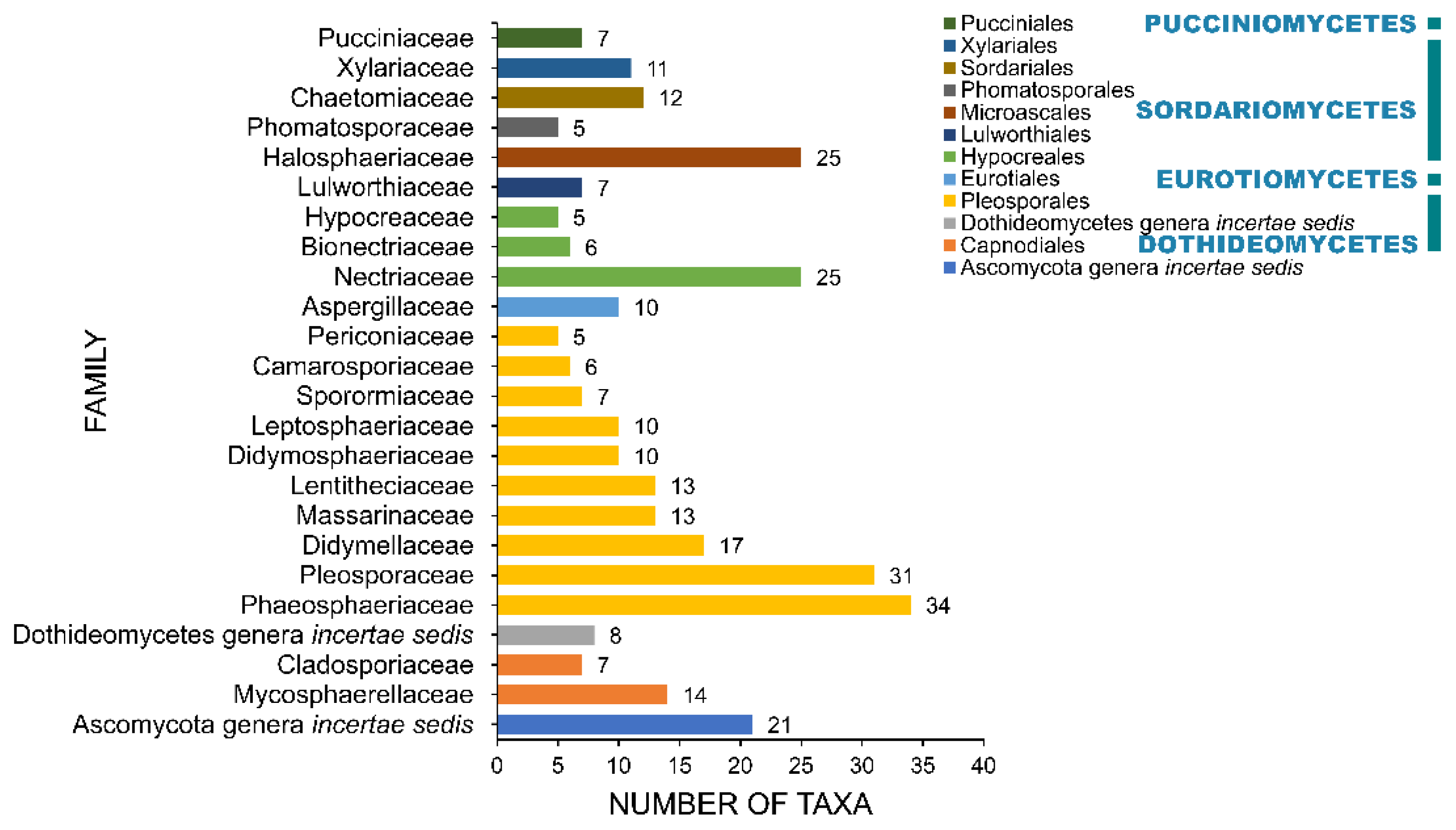
2.4. Families
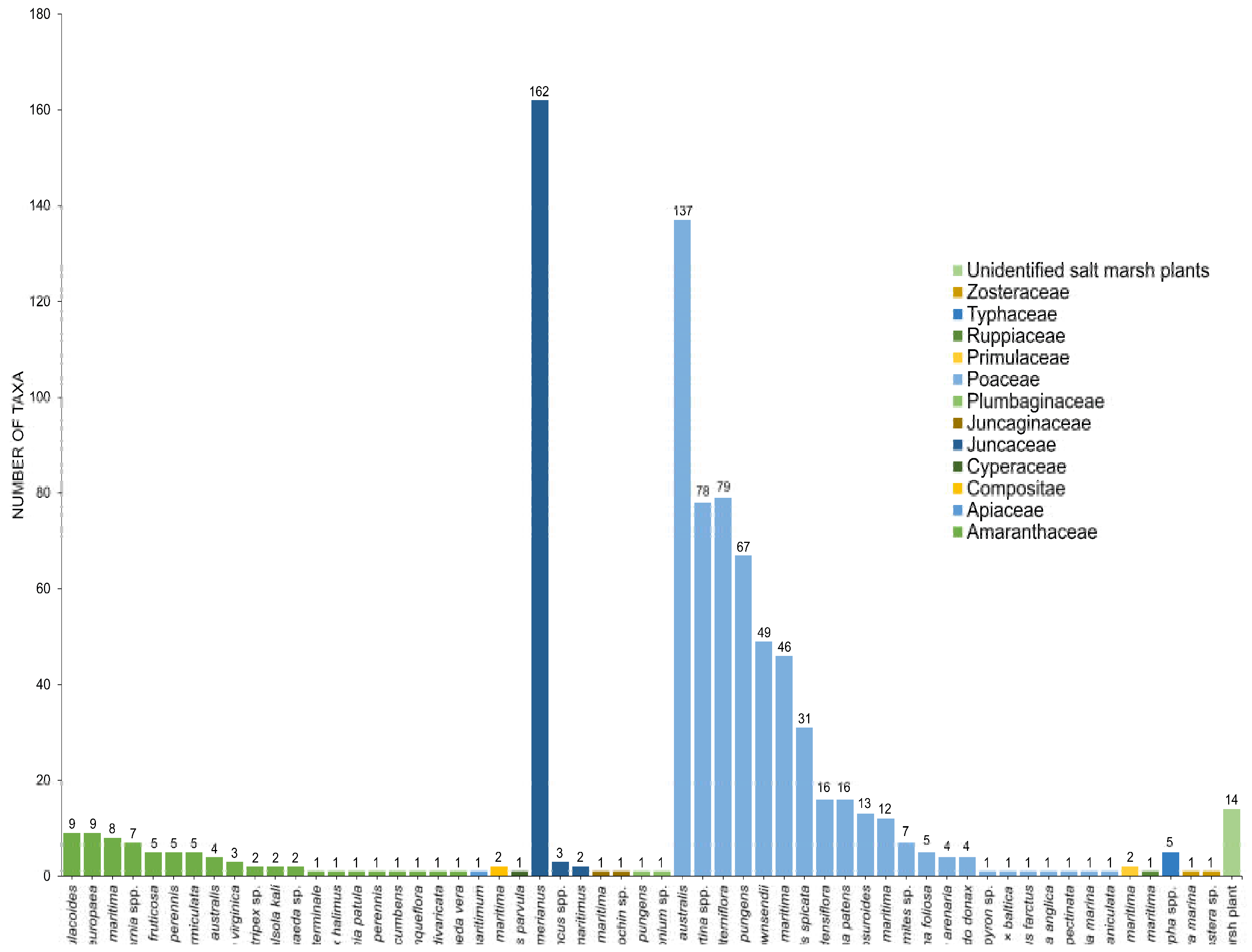
3. Diversity of Fungi in Halophytes
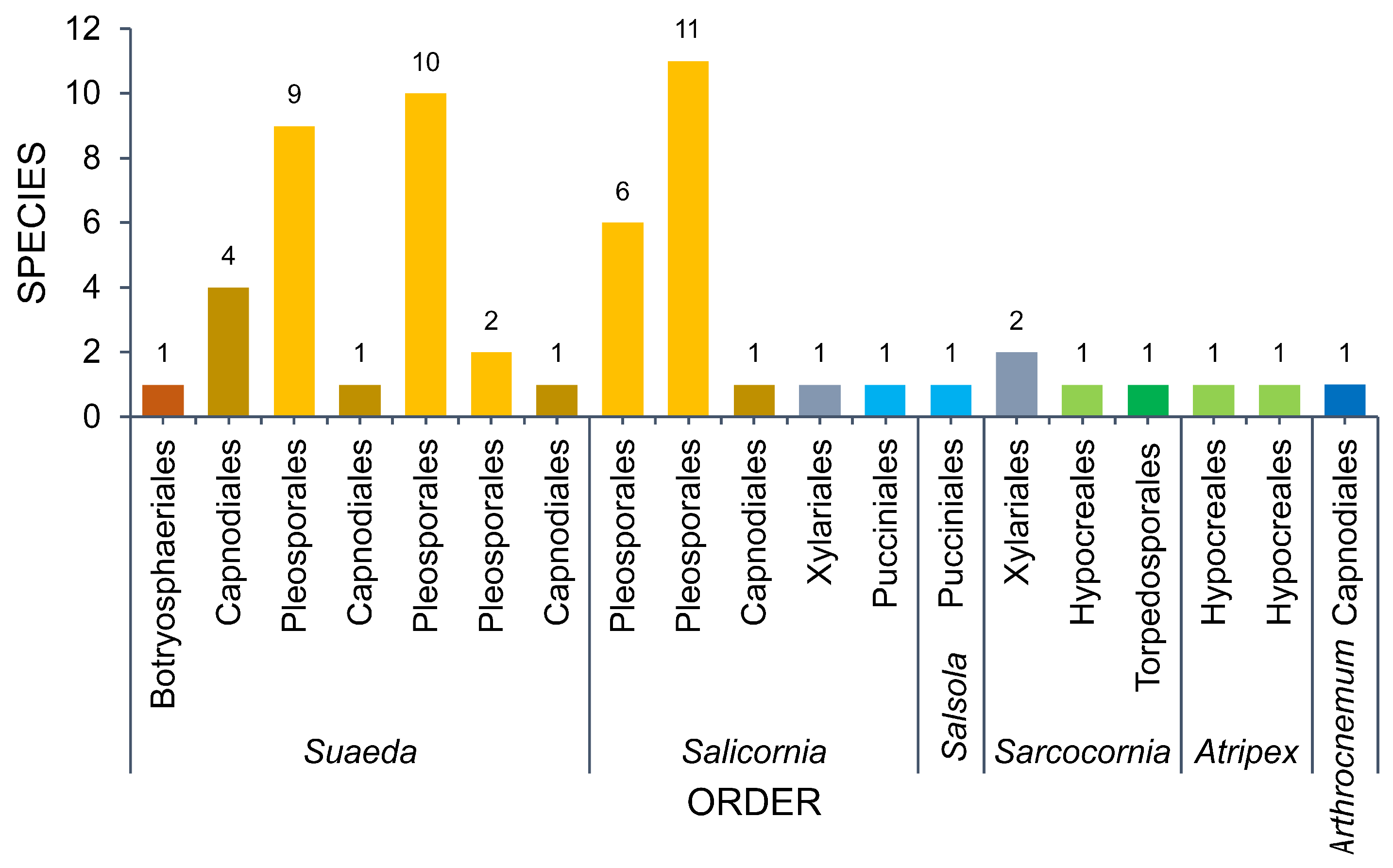
3.1. Amaranthaceae
3.2. Poaceae
3.2.1. Distichlis spicata
3.2.2. Elymus pungens
3.2.3. Puccinellia maritima
3.2.4. Spartina
3.2.5. Phragmites
3.3. Juncaceae
3.4. Other Families
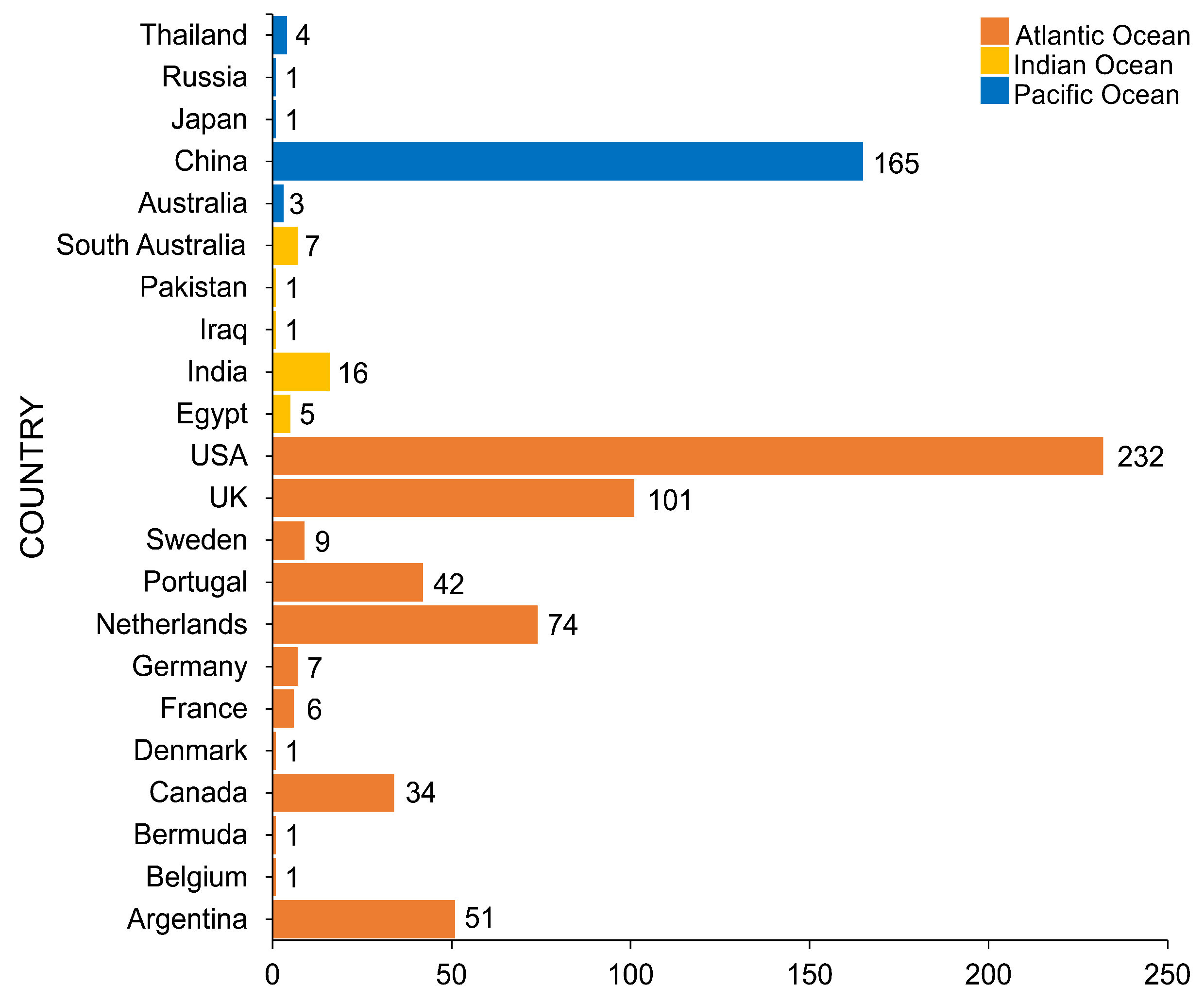
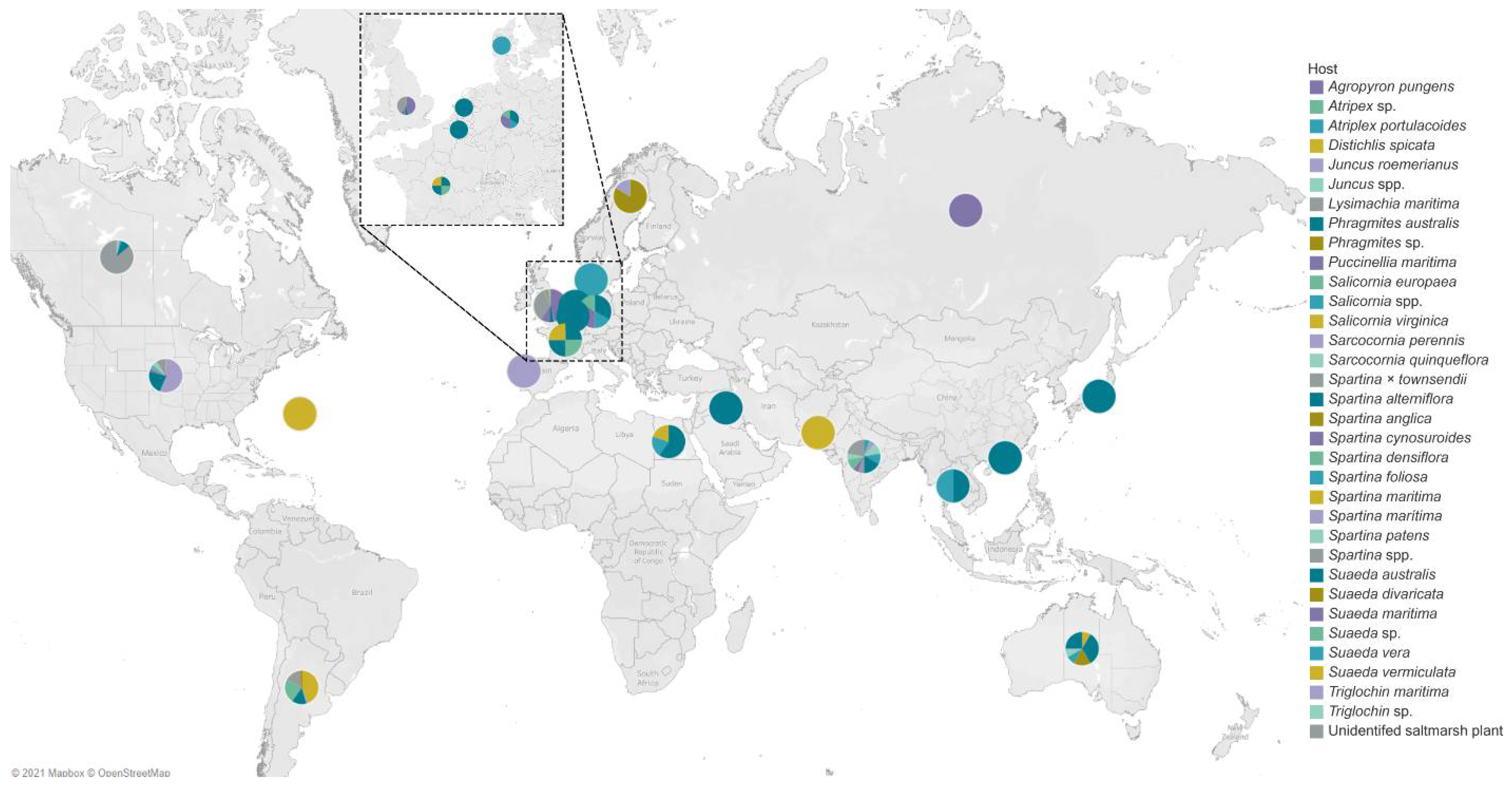
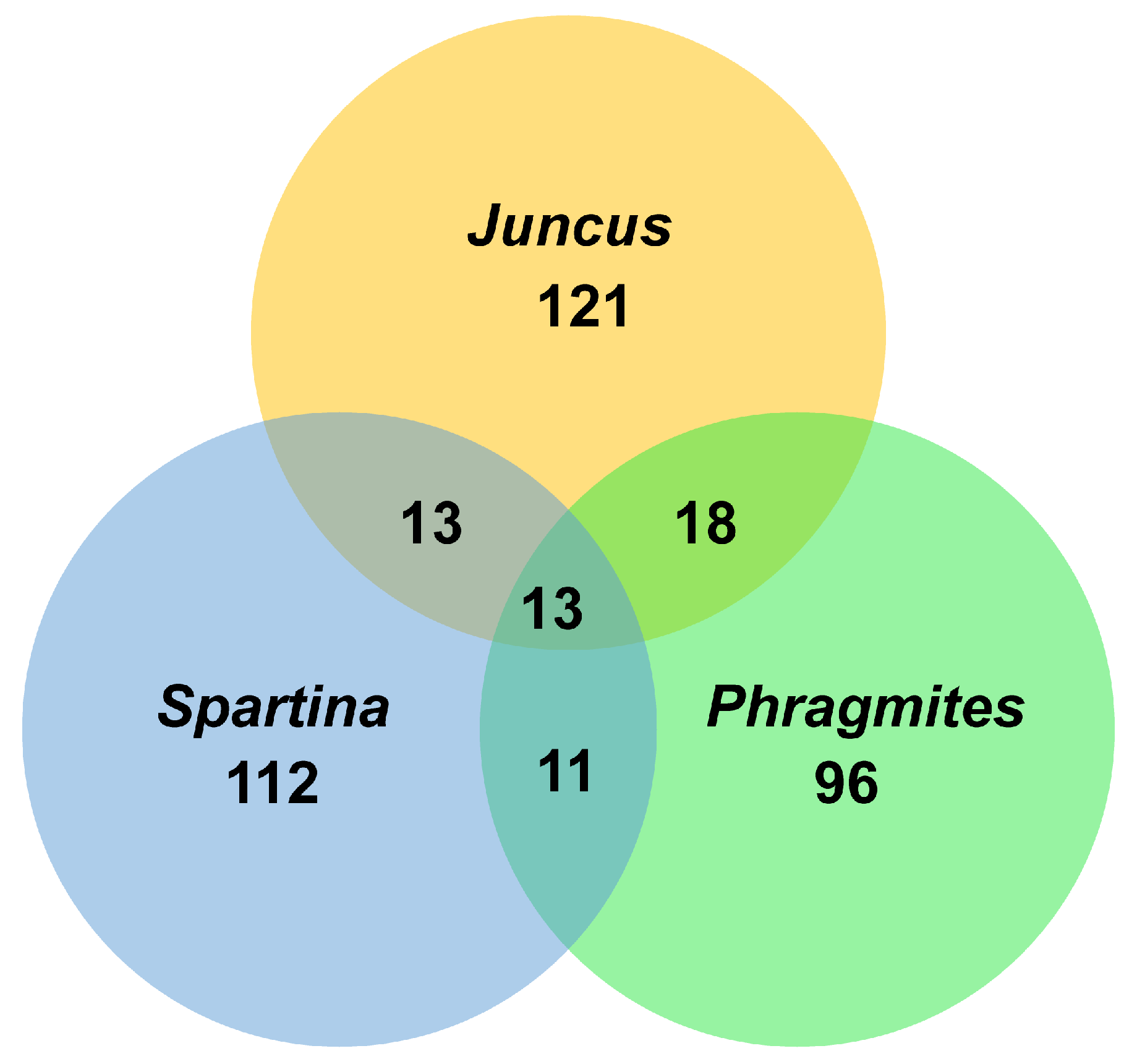
4. Geographical Distribution of Salt Marsh Fungi
United States of America
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertness, M.D. Atlantic Shorelines: Natural History and Ecology; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Öztürk, M.; Altay, V.; Altundağ, E.; Gücel, S. Halophytic plant diversity of unique habitats in Turkey: Salt mine caves of Çankırı and Iğdır. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: New York, NY, USA, 2016; pp. 291–315. [Google Scholar]
- Adam, P. The saltmarsh biota. In Saltmarsh Ecology; Adam, P., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 72–145. [Google Scholar]
- Macdonald, K.B. Plant and animal communities of Pacific North American salt marshes. In Wet Coastal Formations; Chapman, V.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 167–191. [Google Scholar]
- Saenger, P.; Specht, M.M.; Specht, R.L.; Chapman, V.J. Mangal and coastal salt-marsh communities in Australasia. In Wet Coastal Ecosystems; Chapman, V.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 293–345. [Google Scholar]
- Macdonald, K.B. Coastal salt marsh. In Terrestrial Vegetation of California; Barbour, M.G., Major, J., Eds.; Wiley: New York, NY, USA, 1988; pp. 263–294. [Google Scholar]
- Costanza, R.; D’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Mcowen, C.J.; Weatherdon, L.V.; Van Bochove, J.W.; Sullivan, E.; Blyth, S.; Zockler, C.; Stanwell-Smith, D.; Kingston, N.; Martin, C.S.; Spalding, M.; et al. A global map of saltmarshes. Biodivers. Data J. 2017, 5, 11764. [Google Scholar] [CrossRef]
- Silliman, B.R. Salt marshes. Curr. Biol. 2014, 24, R348–R350. [Google Scholar] [CrossRef] [PubMed]
- Teal, J.M. Salt marshes and mud flats. In Encyclopedia of Ocean Sciences; Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 43–48. [Google Scholar]
- Roman, C.T. Salt marsh vegetation. In Encyclopedia of Ocean Sciences; Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 2487–2490. [Google Scholar]
- Garbutt, A.; de Groot, A.; Smit, C.; Pétillon, J. European salt marshes: Ecology and conservation in a changing world. J. Coast. Conserv. 2017, 21, 405–408. [Google Scholar] [CrossRef]
- Davy, A.J. Development and structure of salt marshes: Community patterns in time and space. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 137–156. [Google Scholar]
- Pennings, S.C.; Callaway, R.M. Salt marsh plant zonation: The relative importance of competition and physical factors. Ecology 1992, 73, 681–690. [Google Scholar] [CrossRef]
- Pomeroy, L.R.; Darley, W.M.; Dunn, E.L.; Gallagher, J.L.; Haines, E.B.; Whitney, D.M. Primary production. In The Ecology of Salt Marsh; Pomeroy, L.R., Wiegert, R.G., Eds.; Springer-Verlag: New York, NY, USA, 1981; pp. 39–67. [Google Scholar]
- Howarth, R.W.; Hobbie, J.E. The Regulation of Decomposition and Heterotrophic Microbial Activity in Salt Marsh Soils: A Review; Kennedy, V.S., Ed.; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Long, S.P.; Mason, C.F. Saltmarsh Ecology; Blackie: Glasgow, UK, 1983. [Google Scholar]
- Valiela, I.; Teal, J.M.; Allen, S.D.; Van Etten, R.; Goehringer, D.; Volkmann, S. Decomposition in salt marsh ecosystems: The phases and major factors affecting disappearance of above-ground organic matter. J. Exp. Mar. Biol. Ecol. 1985, 89, 29–54. [Google Scholar] [CrossRef]
- Pomeroy, L.R.; Imberger, J. The physical and chemical environment. In The Ecology of Salt Marsh; Pomeroy, L.R., Weigert, R.G., Eds.; Springer-Verlag: New York, NY, USA, 1981; pp. 21–36. [Google Scholar]
- Gessner, R.V.; Goos, R.D. Fungi from decomposing Spartina alterniflora. Can. J. Bot. 1973, 51, 51–55. [Google Scholar] [CrossRef]
- Gessner, R.V.; Goos, R.D.; Sieburth, J.M.N. The fungal microcosm of the internodes of Spartina alterniflora. Mar. Biol. 1972, 16, 269–273. [Google Scholar] [CrossRef]
- Benner, R.; Newell, S.Y.; Maccubbin, A.E.; Hodson, R.E. Relative contributions of bacteria and fungi to rates of degradation of lignocellulosic detritus in salt-marsh sediments. Appl. Environ. Microbiol. 1984, 48, 36–40. [Google Scholar] [CrossRef]
- Bergbauer, M.; Newell, S.Y. Contribution to lignocellulose degradation and DOC formation from a salt marsh macrophyte by the ascomycete Phaeosphaeria spartinicola. FEMS Microbiol. Ecol. 1992, 86, 34–348. [Google Scholar] [CrossRef]
- Benner, R.; Maccubbin, A.E.; Hodson, R.E. Preparation, characterization, and microbial degradation of specifically radiolabeled [C] lignocelluloses from marine and freshwater macrophytes. Appl. Environ. Microbiol. 1984, 47, 381–389. [Google Scholar] [CrossRef]
- Lyons, J.I.; Alber, M.; Hollibaugh, J.T. Ascomycete fungal communities associated with early decaying leaves of Spartina spp. from central California estuaries. Oecologia 2010, 162, 435–442. [Google Scholar] [CrossRef]
- Maccubbin, A.E.; Hodson, R.E. Mineralization of detrital lignocelluloses by salt marsh sediment microflora. Appl. Environ. Microbiol. 1980, 40, 735–740. [Google Scholar] [CrossRef]
- Newell, S.Y.; Porter, D. Microbial secondary production from salt marsh-grass shoots, and its known and potential fates. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 159–185. [Google Scholar]
- Newell, S.Y.; Fallon, R.D.; Miller, J.D. Decomposition and microbial dynamics for standing, naturally positioned leaves of the salt-marsh grass Spartina alterniflora. Mar. Biol. 1989, 101, 471–481. [Google Scholar] [CrossRef]
- Hernández, E.G.; Baraza, E.; Smit, C.; Berg, M.P.; Salles, J.F. Salt marsh elevation drives root microbial composition of the native invasive grass Elytrigia atherica. Microorganisms 2020, 8, 1619. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, E.; Liu, C.; Jousset, A.; Salles, J.F. Functionality of root-associated bacteria along a salt marsh primary succession. Front. Microbiol. 2017, 8, 2102. [Google Scholar] [CrossRef] [PubMed]
- Calado, M.d.L.; Carvalho, L.; Barata, M.; Pang, K.L. Potential roles of marine fungi in the decomposition process of standing stems and leaves of Spartina maritima. Mycologia 2019, 111, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus and Spartina: New marine species of Anthostomella, with a list of marine fungi known on Spartina. Mycol. Res. 2002, 106, 365–374. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.; Aptroot, A.; Leontyev, D.V.; Ramesh, K.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1–367. [Google Scholar] [CrossRef]
- Calado, M.d.L.; Barata, M. Salt marsh fungi. In Marine Fungi and Fungal-like Organisms; Jones, E.B.G., Pang, K.L., Eds.; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2012; pp. 345–381. [Google Scholar]
- Kohlmeyer, J.; Kohlmeyer, E. Marine Mycology: The Higher Fungi; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Gessner, R.V.; Kohlmeyer, J. Geographical distribution and taxonomy of fungi from salt marsh Spartina. Can. J. Bot. 1976, 54, 2023–2037. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Grasses; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Apinis, A.E.; Chesters, C.G.C. Ascomycetes of some salt marshes and sand dunes. Trans. Br. Mycol. Soc. 1964, 47, 419–435. [Google Scholar] [CrossRef]
- Van Ryckegem, G.; Verbeken, A. Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. I. Leaf sheaths. Fungal Divers. 2005, 19, 157–187. [Google Scholar]
- Van Ryckegem, G.; Verbeken, A. Fungal ecology and succession on Phragmites australis in a brackish tidal marsh. II. Stems. Fungal Divers. 2005, 20, 209–233. [Google Scholar]
- Poon, M.O.K.; Hyde, K.D. Biodiversity of intertidal estuarine fungi on Phragmites at Mai Po Marshes, Hong Kong. Bot. Mar. 1998, 41, 141–155. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 7. Tiarosporella halmyra sp. nov. Mycotaxon 1996, 59, 79–83. [Google Scholar]
- Fell, J.W.; Hunter, I.L. Fungi associated with the decomposition of the Black Rush, Juncus roemerianus, in South Florida. Mycologia 1979, 71, 322–342. [Google Scholar] [CrossRef]
- United State Department of Agriculture Crops Research Division. Index of Plant Diseases in the United States: Agriculture Handbook; USDA: Washington, DC, USA, 1960. [Google Scholar]
- Brunaud, P. Champignons nouvellement obsrevés aux environs de Saintes, Charente-inférieure. J. Hist. Nat. Bord Sud. Oust. Bord. 1888, 7, 4. [Google Scholar]
- Lobik, A.I. Materialen zur Mykoflora des Terskikreises. Morbi Plant. Leningr. 1928, 17, 157–199. [Google Scholar]
- Elíades, L.A.; Voget, C.E.; Arambarri, A.M.; Cabello, M.N. Fungal communities on decaying saltgrass (Distichlis spicata) in Buenos Aires province (Argentina). Sydowia 2007, 59, 227–234. [Google Scholar]
- Miller, J.D.; Whitney, N.J. Fungi of the Bay of Fundy V: Fungi from living species of Spartina Schreber. Proc. Nov. Scotian Inst. Sci. 1983, 33, 75–83. [Google Scholar]
- Goodman, P.J. The possible role of pathogenic fungi in die-back of Spartina townsendii agg. Trans. Br. Mycol. Soc. 1959, 42, 409–415. [Google Scholar] [CrossRef]
- Van Ryckegem, G.; Gessner, M.O.; Verbeken, A. Fungi on leaf blades of Phragmites australis in a brackish tidal marsh: Diversity, succession, and leaf decomposition. Microb. Ecol. 2007, 53, 600–611. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus 12. Two new species of Mycosphaerella and Paraphaeosphaeria (Ascomycotina). Bot. Mar. 1999, 42, 505–511. [Google Scholar] [CrossRef]
- Borse, B.D.; Bhat, D.; Borse, K.; Tuwar, A.; Pawar, N. Marine fungi of India (Monograph); Broadway Book Centre: Panaji, India, 2012. [Google Scholar]
- Kohlmeyer, J.; Kohlmeyer, E. Bermuda marine fungi. Trans. Br. Mycol. Soc. 1977, 68, 207–219. [Google Scholar] [CrossRef]
- Barata, M. Fungi on the halophyte Spartina maritima in salt marshes. In Fungi in Marine Environments; Hyde, K.D., Ed.; Fungal Diversity Press: Hong Kong, China, 2002; pp. 179–193. [Google Scholar]
- Walker, A.K.; Campbell, J. Marine fungal diversity: A comparison of natural and created salt marshes of the north-central Gulf of Mexico. Mycologia 2010, 102, 513–521. [Google Scholar] [CrossRef]
- Buchan, A.; Newell, S.Y.; Moreta, J.I.L.; Moran, M.A. Analysis of internal transcribed spacer (ITS) regions of rRNA genes in fungal communities in a southeastern U.S. salt marsh. Microb. Ecol. 2002, 43, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Newell, S.Y.; Butler, M.; Biers, E.J.; Hollibaugh, J.T.; Moran, M.A. Dynamics of bacterial and fungal communities on decaying salt marsh grass. Appl. Environ. Microbiol. 2003, 69, 6676–6687. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K. Marine Fungi of U.S. GULF of Mexico Barrier Island Beaches: Biodiversity and Sampling Strategy; The University of Southern Mississippi: Hattiesburg, MS, USA, 2012. [Google Scholar]
- Calado, M.d.L.; Carvalho, L.; Pang, K.L.; Barata, M. Diversity and ecological characterization of sporulating higher filamentous marine fungi associated with Spartina maritima (Curtis) Fernald in two Portuguese salt marshes. Microb. Ecol. 2015, 70, 612–633. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus. 11. More new ascomycetes. Can. J. Bot. 1998, 76, 467–477. [Google Scholar] [CrossRef]
- Gessner, R.V.; Goos, R.D. Fungi from Spartina alterniflora in Rhode Island. Mycologia 1973, 65, 1296–1301. [Google Scholar] [CrossRef]
- Hansford, C.G. Australian Fungi. II. New species and revisions. Proc. Linn. Soc. N. S. W. 1954, 79, 97–141. [Google Scholar]
- Barata, M. Marine fungi from Mira River salt marsh in Portugal. Rev. Iberoam. Micol. 2006, 23, 179–184. [Google Scholar] [CrossRef]
- Peña, N.I.; Arambarri, A.M. Hongos marinos lignícolas de la laguna costera de Mar Chiquita (provincia de Buenos Aires, Argentina). L. Ascomycotina y Deuteromycotina sobre Spartina densiflora. Darwiniana 1998, 35, 61–67. [Google Scholar]
- Jones, E.B.G. Marine fungi. I. Trans. Br. Mycol. Soc. 1962, 45, 93–114. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus. 8. New bitunicate ascomycetes. Can. J. Bot. 1996, 74, 1830–1840. [Google Scholar] [CrossRef]
- Johnson, T.W.; Hughes, G.C. Robillarda phragmitis Cunnell in estuarine waters. Trans. Br. Mycol. Soc. 1960, 43, 523–524. [Google Scholar] [CrossRef]
- Seymour, A.B. Host Index of the Fungi of North America; Harvard University Press: Cambridge, MA, USA, 1929. [Google Scholar]
- Saccardo, P.A. Fungi Gallici lecti a cl. viris P. Brunaud, Abb. Letendre, A. Malbranche, J. Therry, vel editi in Mycotheca Gallica C. Roumeguèri. Series II. Michelia 1880, 2, 39–135. [Google Scholar]
- Ahmad, S. Contributions to the fungi of West Pakistan. VI. Biol. Lahore 1967, 13, 15–42. [Google Scholar]
- Johnson, T.W.; Sparrow, F.K. Fungi in Oceans and Estuaries; Cramer: Weinheim, Germany, 1961. [Google Scholar]
- Jones, E.B.G. Marine fungi: II. Ascomycetes and deuteromycetes from submerged wood and drift Spartina. Trans. Br. Mycol. Soc. 1963, 46, 135–144. [Google Scholar] [CrossRef]
- Gessner, R.V. Seasonal occurrence and distribution of fungi associated with Spartina alterniflora from a Rhode Island estuary. Mycologia 1977, 69, 477–491. [Google Scholar] [CrossRef]
- Gessner, R.V. Degradative enzyme production by salt-marsh fungi. Bot. Mar. 1980, 23, 133–139. [Google Scholar] [CrossRef]
- Jaap, O. Weitere Beiträge zur Pilzflora der nordfriesischen Inseln. Schr. Des Nat. Ver. Für Schleswig-Holst. 1907, 14, 15–33. [Google Scholar]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus 9. New obligate and facultative marine ascomycotina. Bot. Mar. 1997, 40, 291–300. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus. New marine and terrestrial ascomycetes. Mycol. Res. 1996, 100, 393–404. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus 2. New dictyosporous Ascomycetes. Bot. Mar. 1995, 38, 165–174. [Google Scholar] [CrossRef]
- Devadatha, B.; Calabon, M.S.; Abeywickrama, P.D.; Hyde, K.D.; Jones, E.B.G. Molecular data reveals a new holomorphic marine fungus, Halobyssothecium estuariae, and the asexual morph of Keissleriella phragmiticola. Mycology 2020, 11, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W. Marine Fungi. I. Leptosphaeria and Pleospora. Mycologia 1956, 48, 495–505. [Google Scholar] [CrossRef]
- Wagner, D. Ecological studies on Leptospheria discors, a graminicolous fungus of salt marshes. Nov. Hedwig. 1969, 18, 383–396. [Google Scholar]
- Webber, E.E. Marine ascomycetes from New England. Bull. Torrey Bot. Club 1970, 97, 119–120. [Google Scholar] [CrossRef]
- Dayarathne, M.C.; Wanasinghe, D.N.; Jones, E.B.G.; Chomnunti, P.; Hyde, K.D. A novel marine genus, Halobyssothecium (Lentitheciaceae) and epitypification of Halobyssothecium obiones comb. nov. Mycol. Prog. 2018, 17, 1161–1171. [Google Scholar] [CrossRef]
- Saccardo, P.A. Sylloge fungorum Omnium Hucusque Cognitorum; R. Friedländer & Sohn.: Berlin, Germany, 1883; Volume 2. [Google Scholar]
- Calabon, M.S.; Jones, E.B.G.; Hyde, K.D.; Boonmee, S.; Tibell, S.; Tibell, L.; Pang, K.L.; Phookamsak, R. Phylogenetic assessment and taxonomic revision of Halobyssothecium and Lentithecium (Lentitheciaceae, Pleosporales). Mycol. Prog. 2021, 20, 701–720. [Google Scholar] [CrossRef]
- Van Ryckegem, G.; Aptroot, A. A new Massarina and a new Wettsteinina (Ascomycota) from freshwater and tidal reeds. Nov. Hedwig. 2001, 73, 161–166. [Google Scholar] [CrossRef]
- Tibell, S.; Tibell, L.; Pang, K.L.; Calabon, M.S.; Jones, E.B.G. Marine fungi of the Baltic Sea. Mycology 2020, 11, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]
- Ellis, J.B.; Everhart, B.M. New Fungi. J. Mycol. 1885, 1, 42–44. [Google Scholar] [CrossRef]
- Ellis, J.B.; Everhart, B.M. The North American Pyrenomycetes. A Contribution to Mycologic Botany; Ellis & Everhart: Newfield, NJ, USA, 1892. [Google Scholar]
- Lucas, M.T. Culture studies on portuguese species of Leptosphaeria I. Trans. Br. Mycol. Soc. 1963, 46, 361–367. [Google Scholar] [CrossRef]
- Webber, E.E. Fungi from a Massachusetts salt marsh. Trans. Am. Microsc. Soc. 1966, 85, 556–558. [Google Scholar] [CrossRef]
- Dennis, R.W.G. British Ascomycetes; J. Cramer: Lehre, Germany, 1968. [Google Scholar]
- Poli, A.; Vizzini, A.; Prigione, V.; Varese, G.C. Basidiomycota isolated from the Mediterranean Sea—Phylogeny and putative ecological roles. Fungal Ecol. 2018. [Google Scholar] [CrossRef]
- Hansford, C.G. Australian fungi IV. New species and revisions (cont’d). Proc. Linn. Soc. N. S. W. 1957, 82, 209–229. [Google Scholar]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; et al. Fungal diversity notes 367–490: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus. 4. New marine ascomycetes. Mycologia 1995, 87, 532–542. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 14. Three new coelomycetes, including Floricola, anam.-gen. nov. Bot. Mar. 2000, 43, 385–392. [Google Scholar] [CrossRef]
- Sydow, H. Mycotheca germanica. Fasc. XX–XXI. Ann. Mycol. 1911, 9, 554–558. [Google Scholar]
- Dayarathne, M.; Jones, E.; Maharachchikumbura, S.; Devadatha, B.; Sarma, V.; Khongphinitbunjong, K.; Chomnunti, P.; Hyde, K. Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 2020, 11, 1–188. [Google Scholar] [CrossRef]
- Hyde, K.D.; Chaiwan, N.; Norphanphoun, C.; Boonmee, S.; Camporesi, E.; Chethana, K.W.T.; Dayarathne, M.C.; de Silva, N.I.; Dissanayake, A.J.; Ekanayaka, A.H.; et al. Mycosphere notes 169–224. Mycosphere 2018, 9, 271–430. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Hyde, K.D.; Jeewon, R.; Crous, P.W.; Wijayawardene, N.N.; Jones, E.B.G.; Bhat, D.J.; Phillips, A.J.L.; Groenewald, J.Z.; Dayarathne, M.C.; et al. Phylogenetic revision of Camarosporium (Pleosporineae, Dothideomycetes) and allied genera. Stud. Mycol. 2017, 87, 207–256. [Google Scholar] [CrossRef]
- Grove, W.B. British Stem- and Leaf-Fungi Vol. II; Cambridge University Press: Cambridge, UK, 1937. [Google Scholar]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Atrotorquata and Loratospora: New ascomycete genera on Juncus roemerianus. Syst. Ascomycetum 1993, 12, 7–22. [Google Scholar]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Tsui, C.K.M. Fungi on Juncus roemerianus. 17. New ascomycetes and the hyphomycete genus Kolletes gen. nov. Bot. Mar. 2005, 48, 306–317. [Google Scholar] [CrossRef]
- Yusoff, M.; Moss, S.T.; Jones, E.B.G. Ascospore ultrastructure of Pleospora gaudefroyi (Pleosporaceae, Loculoascomycetes, Ascomycotina). Can. J. Bot. 1994, 72, 1–6. [Google Scholar] [CrossRef]
- Webster, J.; Lucas, M.T. Observations on British species of Pleospora. II. Trans. Br. Mycol. Soc. 1961, 44, 417–436. [Google Scholar] [CrossRef]
- Sutton, B.C.; Pirozynski, K.A. Notes on British microfungi. I. Trans. Br. Mycol. Soc. 1963, 46, 505–522. [Google Scholar] [CrossRef]
- Spegazzini, C. Mycetes Argentinenses. Series IV. An. del Mus. Nac. Hist. Nat. Buenos Aires. Ser. 3 1909, 12, 257–458. [Google Scholar]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 6. Glomerobolus gen. nov., the first ballistic member of Agonomycetales. Mycologia 1996, 88, 328–337. [Google Scholar] [CrossRef]
- Cantrell, S.A.; Hanlin, R.T.; Newell, S.Y. A new species of Lachnum on Spartina alterniflora. Mycotaxon 1996, 57, 479–485. [Google Scholar]
- Ellis, J.B.; Everhart, B.M. New species of fungi from various localities. J. Mycol. 1888, 4, 121–124. [Google Scholar] [CrossRef][Green Version]
- Kohlmeyer, J.; Baral, H.-O.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 10. A new Orbilia with ingoldian anamorph. Mycologia 1998, 90, 303–309. [Google Scholar] [CrossRef]
- Seaver, F.J. The North American Cup-Fungi (Inoperculates); Lancaster Press Inc.: Lancaster, PA, USA, 1951. [Google Scholar]
- Wong, M.K.M.; Goh, T.K.; Hyde, K.D. A new species of Phragmitensis (ascomycetes) from senescent culms of Phragmites australis. Fungal Divers. 1999, 2, 175–180. [Google Scholar]
- Kohlmeyer, J.; Volkmann Kohlmeyer, B.; Eriksson, O.E. Fungi on Juncus roemerianus. 3. New ascomycetes. Bot. Mar. 1995, 38, 175–186. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 16. More new coelomycetes, including Tetranacriella, gen. nov. Bot. Mar. 2001, 44, 147–156. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Two new genera of Ascomycotina from saltmarsh Juncus. Syst. Ascomycetum 1993, 11, 95–106. [Google Scholar]
- Loveless, A.R.; Peach, J.M. Evidence from ascospores for host restriction in Claviceps purpurea. Trans. Br. Mycol. Soc. 1986, 86, 603–610. [Google Scholar] [CrossRef]
- Loveless, A.R. Conidial evidence for host restriction in Claviceps purpurea. Trans. Br. Mycol. Soc. 1971, 56, 419–434. [Google Scholar] [CrossRef]
- Sprague, R. Septoria disease of Gramineae in western United States. Oregon State Monogr. Stud. Bot. 1944, 6, 1–151. [Google Scholar]
- Spegazzini, C. Fungi Argentini additis nonnullis brasiliensibus montevidensibusque. An. la Soc. Cient. Argent. 1882, 13, 60–64. [Google Scholar]
- Peach, J.M.; Loveless, A.R. A comparison of two methods of inoculating Triticum aestivum with spore suspensions of Claviceps purpurea. Trans. Br. Mycol. Soc. 1975, 64, 328–331. [Google Scholar] [CrossRef]
- Eleuterius, L.N.; Meyers, S.P. Claviceps purpurea on Spartina in coastal marshes. Mycologia 1974, 66, 978–986. [Google Scholar] [CrossRef]
- Sprague, R. Diseases of Cereals and Grasses in North America: Fungi Except Smuts and Rusts; Ronald Press Company: New York, NY, USA, 1950. [Google Scholar]
- Mantle, P.G. Development of alkaloid production in vitro by a strain of Claviceps purpurea from Spartina townsendii. Trans. Br. Mycol. Soc. 1969, 52, 381–392. [Google Scholar] [CrossRef]
- Moberley, D.G. Taxonomy and distribution of the genus Spartina. Iowa State J. Sci. 1956, 30, 471–574. [Google Scholar]
- Saccardo, P.A. Sylloge Fungorum Omnium Hucusque Cognitorum; R. Friedländer & Sohn.: Berlin, Germany, 1886; Volume 4. [Google Scholar]
- Abdullah, S.K.; Abdulkadder, M.A.; Goos, R.D. Basramyces marinus nom.nov. (hyphomycete) from southern marshes of Iraq. Int. J. Mycol. Lichenol. 1989, 4, 181–186. [Google Scholar]
- Abdel-Wahab, M.A.; Pang, K.L.; Nagahama, T.; Abdel-Aziz, F.A.; Jones, E.B.G. Phylogenetic evaluation of anamorphic species of Cirrenalia and Cumulospora with the description of eight new genera and four new species. Mycol. Prog. 2010, 9, 537–558. [Google Scholar] [CrossRef]
- Johnson, T.W. Marine Fungi. IV. Lulworthia and Ceriosporopsis. Mycologia 1958, 50, 151–163. [Google Scholar] [CrossRef]
- Mounce, I.; Diehl, W.W. A new Ophiobolus on eelgrass. Can. J. Res. 1934, 11, 242–246. [Google Scholar] [CrossRef]
- Johnson, T.W. Marine fungi. II. Ascomycetes and Deuteromycetes from submerged wood. Mycologia 1956, 48, 841–851. [Google Scholar] [CrossRef]
- Lloyd, L.S.; Wilson, I.M. Development of the perithecium in Lulworthia medusa (Ell. & Ev.) Cribb & Cribb, a saprophyte on Spartina townsendii. Trans. Br. Mycol. Soc. 1962, 45, 359–372. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 1. Trichocladium medullare sp. nov. Mycotaxon 1995, 53, 349–353. [Google Scholar]
- Volkmann-Kohlmeyer, B.; Kohlmeyer, J. A new Aniptodera (Ascomycotina) from saltmarsh Juncus. Bot. Mar. 1994, 37, 109–114. [Google Scholar] [CrossRef]
- Gessner, R.V. Spartina alterniflora seed fungi. Can. J. Bot. 1978, 56, 2942–2947. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Kohlmeyer, E. Icones Fungorum Maris. Fasc. 1–9; J. Cramer: Weinheim/Lehre, Germany, 1967. [Google Scholar]
- Jones, E.B.G. Haligena spartinae sp. nov., a pyrenomycete on Spartina townsendii. Trans. Br. Mycol. Soc. 1962, 45, 245–248. [Google Scholar] [CrossRef]
- Liu, J.K.; Hyde, K.D.; Jones, E.B.G.; Ariyawansa, H.A.; Bhat, D.J.; Boonmee, S.; Maharachchikumbura, S.S.N.; McKenzie, E.H.C.; Phookamsak, R.; Phukhamsakda, C.; et al. Fungal diversity notes 1–110: Taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 2015, 72, 1–197. [Google Scholar] [CrossRef]
- Orton, C.R. Graminicolous species of Phyllachora in North America. Mycologia 1944, 36, 18–53. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Octopodotus stupendus gen. & sp. nov. and Phyllachora paludicola sp. nov., two marine fungi from Spartina alterniflora. Mycologia 2003, 95, 117–123. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Aropsiclus nom. nov. (Ascomycotina) to replace Sulcospora Kohlm. & Volk.-Kohlm. Syst. Ascomycetum 1994, 13, 24. [Google Scholar]
- Spooner, B.M. New records and species of British microfungi. Trans. Br. Mycol. Soc. 1981, 76, 265–301. [Google Scholar] [CrossRef]
- Dayarathne, M.C.; Wanasinghe, D.N.; Devadatha, B.; Abeywickrama, P.; Gareth Jones, E.B.; Chomnunti, P.; Sarma, V.V.; Hyde, K.D.; Lumyong, S.; Mckenzie, E.H.C. Modern taxonomic approaches to identifying diatrypaceous fungi from marine habitats, with a novel genus Halocryptovalsa Dayarathne & K.D.Hyde, gen. Nov. Cryptogam. Mycol. 2020, 41, 21–67. [Google Scholar] [CrossRef]
- Johnson, T.W.J.; Gold, H.S. Didymosamarospora, a new genus of fungi from fresh and marine waters. J. Elisha Mitchell Sci. Soc. 1957, 73, 103–108. [Google Scholar]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus: New coelomycetes with notes on Dwayaangam junci. Mycol. Res. 2001, 105, 500–505. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi on Juncus roemerianus. 13. Hyphopolynema juncatile sp. nov. Mycotaxon 1999, 70, 489–495. [Google Scholar]
- Kohlmeyer, J.; Kohlmeyer, E. Marine fungi from tropical America and Africa. Mycologia 1971, 63, 831–861. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Kohlmeyer, E. New marine fungi from mangroves and trees along eroding shorelines. Nov. Hedwig. 1965, 9, 89–104. [Google Scholar]
- Ellis, J.B.; Everhart, B.M. New species of North American fungi from various localities. Proc. Acad. Nat. Sci. Phila. 1893, 45, 128–172. [Google Scholar] [CrossRef]
- Cummins, G.B. The Rust Fungi of Cereals, Grasses and Bamboos; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1971. [Google Scholar]
- Orton, C.R. Manual of the Rusts in United States and Canada; Purdue Research Foundation: Lafayette, IN, USA, 1934. [Google Scholar]
- von Thümen, F. Fungi Egyptiaci, Ser. III. Flora (Regensbg.) 1880, 63, 477–479. [Google Scholar]
- McAlpine, D. The Smuts of Australia, Their Structure, Life History, Treatment, and Classification; Department of Agriculture of Victoria, Melbourn, Australia: Melbourne, VIC, Australia, 1910. [Google Scholar]
- Bauer, R.; Lutz, M.; Piatek, M.; Vánky, K.; Oberwinkler, F. Flamingomyces and Parvulago, new genera of marine smut fungi (Ustilaginomycotina). Mycol. Res. 2007, 111, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.W.G. Fungi of Ammophila arenaria in Europe. Rev. Biol. 1983, 12, 15–48. [Google Scholar]
- Treigienė, A. Fungi associated with Ammophila arenaria in Lithuania and taxonomical notes on some species. Bot. Lith. 2011, 17, 47–53. [Google Scholar]
- Rodríguez-Echeverría, S.; Hol, W.H.G.; Freitas, H.; Eason, W.R.; Cook, R. Arbuscular mycorrhizal fungi of Ammophila arenaria (L.) Link: Spore abundance and root colonisation in six locations of the European coast. Eur. J. Soil Biol. 2008, 44, 30–36. [Google Scholar] [CrossRef]
- Alva, P.; McKenzie, E.H.C.; Pointing, S.B.; Pena-Murala, R.; Hyde, K.D. Do sea grasses harbour endophytes? In Fungi in Marine Environments; Hyde, K.D., Ed.; Fungal Diversity Press: Hong Kong, China, 2002; pp. 167–178. [Google Scholar]
- Wagner, D.T. Developmental morphology of Leptosphaeria discors (Saccardo and Ellis) Saccardo and Ellis. Nov. Hedwig. 1965, 9, 45–61. [Google Scholar]
- Meyers, S.P.; Ahearn, D.G.; Alexander, S.K.; Cook, W.L. Pichia spartinae, a dominant yeast of the Spartina salt marsh. Dev. Ind. Microbiol. 1975, 16, 261–267. [Google Scholar]
- Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An online resource for marine fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Mohamed, D.J.; Martiny, J.B.H. Patterns of fungal diversity and composition along a salinity gradient. ISME J. 2011, 5, 379–388. [Google Scholar] [CrossRef]
- Stoeck, T.; Epstein, S. Novel eukaryotic lineages inferred from small-subunit rRNA analyses of oxygen-depleted marine environments. Appl. Environ. Microbiol. 2003, 69, 2657–2663. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Pylro, V.S.; Baldrian, P.; Van Elsas, J.D.; Salles, J.F. Ecological succession reveals potential signatures of marine-terrestrial transition in salt marsh fungal communities. ISME J. 2016, 10, 1984–1997. [Google Scholar] [CrossRef]
- D’entremont, T.W. Saltmarsh Sediment Fungal Communities and Arbuscular Mycorrhizal Fungi in Sporobolus Pumilus (Roth) (Poaceae) (Spartina Patens) of the Minas Basin, Nova Scotia; Identification, Abundance and Role in Restoration. Ph.D. Thesis, Acadia University, Wolfville, NS, Canada, 2019. [Google Scholar]
- Khalmuratova, I.; Kim, H.; Nam, Y.J.; Oh, Y.; Jeong, M.J.; Choi, H.R.; You, Y.H.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; et al. Diversity and plant growth promoting capacity of endophytic fungi associated with halophytic plants from the west coast of Korea. Mycobiology 2015, 43, 373–383. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Yoon, H.; Kang, S.M.; Shin, J.H.; Choo, Y.S.; Lee, I.J.; Lee, J.M.; Kim, J.G. Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in Suncheon Bay. J. Microbiol. Biotechnol. 2012, 22, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Khalmuratova, I.; Choi, D.H.; Woo, J.R.; Jeong, M.J.; Oh, Y.; Kim, Y.G.; Lee, I.J.; Choo, Y.S.; Kim, J.G. Diversity and plant growth-promoting effects of fungal endophytes isolated from salt-tolerant plants. J. Microbiol. Biotechnol. 2020, 30, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Kandalepas, D.; Blum, M.J.; Van Bael, S.A. Shifts in symbiotic endophyte communities of a foundational salt marsh grass following oil exposure from the deepwater horizon oil spill. PLoS ONE 2015, 10, e0122378. [Google Scholar] [CrossRef] [PubMed]
- Maciá-Vicente, J.G.; Jansson, H.B.; Abdullah, S.K.; Descals, E.; Salinas, J.; Lopez-Llorca, L.V. Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol. Ecol. 2008, 64, 90–105. [Google Scholar] [CrossRef]
- You, Y.-H.; Yoon, H.-J.; Woo, J.-R.; Seo, Y.-G.; Kim, M.-A.; Lee, G.-M.; Kim, J.-G. Diversity of endophytic fungi from the roots of halophytes growing in Go-chang salt marsh. Korean J. Mycol. 2012, 40, 86–92. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Choi, D.H.; Yoon, H.J.; Yoon, T.M.; Kim, J.G. Diversity and plant growth promotion of fungal endophytes in five halophytes from the Buan salt marsh. J. Microbiol. Biotechnol. 2021, 31, 408–418. [Google Scholar] [CrossRef]
- Kalyanasundaram, I.; Nagamuthu, J.; Muthukumaraswamy, S. Antimicrobial activity of endophytic fungi isolated and identified from salt marsh plant in Vellar Estuary. J. Microbiol. Biotechnol. 2015, 7, 13–20. [Google Scholar] [CrossRef]
- Elmer, W.H.; Marra, R.E. New species of Fusarium associated with dieback of Spartina alterniflora in Atlantic salt marshes. Mycologia 2011, 103, 806–819. [Google Scholar] [CrossRef]
- Alber, M.; Swenson, E.M.; Adamowicz, S.C.; Mendelssohn, I.A. Salt marsh dieback: An overview of recent events in the US. Estuar. Coast. Shelf Sci. 2008, 80, 1–11. [Google Scholar] [CrossRef]
- Elmer, W.H. Pathogenic microfungi associated with Spartina in salt marshes. In Biology of Microfungi; Li, D.-W., Ed.; Springer: New York, NY, USA; Cham: Switzerland, 2016; pp. 615–630. [Google Scholar]
- Govers, L.L.; in’T Veld, W.A.M.; Meffert, J.P.; Bouma, T.J.; van Rijswick, P.C.J.; Heusinkveld, J.H.T.; Orth, R.J.; van Katwijk, M.M.; van der Heide, T. Marine Phytophthora species can hamper conservation and restoration of vegetated coastal ecosystems. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160812. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.J.; DiTomaso, J.M.; Gordon, T.R.; Aegerter, B.J.; Ayres, D.R. Salt marsh Claviceps purpurea in native and invaded Spartina marshes in Northern California. Plant Dis. 2007, 91, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Raybould, A.F.; Gray, A.J.; Clarke, R.T. The long-term epidemic of Claviceps purpurea on Spartina anglica in Poole Harbour: Pattern of infection, effects on seed production and the role of Fusarium Heterosporum. New Phytol. 1998, 138, 497–505. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Chen, J.; Dissanayake, A.J.; Doilom, M.; Hongsanan, S.; Jayawardena, R.S.; Jeewon, R.; Perera, R.H.; Thongbai, B.; et al. Thailand’s amazing diversity: Up to 96% of fungi in northern Thailand may be novel. Fungal Divers. 2018, 93, 215–239. [Google Scholar] [CrossRef]
- Devadatha, B.; Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Hyde, K.D.; Sakayaroj, J.; Bahkali, A.H.; Calabon, M.S.; Sarma, V.V.; Sutreong, S.; et al. Occurrence and geographical distribution of mangrove fungi. Fungal Divers. 2021, 106, 137–227. [Google Scholar] [CrossRef]
- Dong, W.; Wang, B.; Hyde, K.D.; McKenzie, E.H.C.; Raja, H.A.; Tanaka, K.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Doilom, M.; Phookamsak, R.; et al. Freshwater Dothideomycetes. Fungal Divers. 2020, 105, 319–575. [Google Scholar] [CrossRef]
- Luo, Z.L.; Hyde, K.D.; Liu, J.K.; Maharachchikumbura, S.S.N.; Jeewon, R.; Bao, D.F.; Bhat, D.J.; Lin, C.G.; Li, W.L.; Yang, J.; et al. Freshwater Sordariomycetes. Fungal Divers. 2019, 99, 451–660. [Google Scholar] [CrossRef]
- Hyde, K.D.; Chethana, K.W.T.; Jayawardena, R.S.; Luangharn, T.; Calabon, M.S.; Jones, E.B.G.; Hongsanan, S.; Lumyong, S. The rise of mycology in Asia. Sci. Asia 2020, 46, 1–11. [Google Scholar] [CrossRef]
- Calabon, M.S.; Jones, E.B.G.; Boonmee, S.; Doilom, M.; Lumyong, S.; Hyde, K.D. Five novel freshwater ascomycetes indicate high undiscovered diversity in lotic habitats in Thailand. J. Fungi 2021, 7, 1–27. [Google Scholar] [CrossRef]
- Saintilan, N. Biogeography of Australian saltmarsh plants. Austral Ecol. 2009, 34, 929–937. [Google Scholar] [CrossRef]
- Spencer, D.M.; Hickling, V.; Spencer, J.F.T. Yeasts from ponds, streams and salt marsh on the Gower Peninsula, Wales. In Advances in Biotechnology. Proceedings of the Fifth International Yeast Symposium Held in London, Canada, July 20–25; Stewart, G., Russell, I., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 515–519. [Google Scholar]
- Kurtzman, C.P. Saturnospora ahearnii, a new salt marsh yeast from Louisiana. Antonie Van Leeuwenhoek 1991, 60, 31–34. [Google Scholar] [CrossRef] [PubMed]


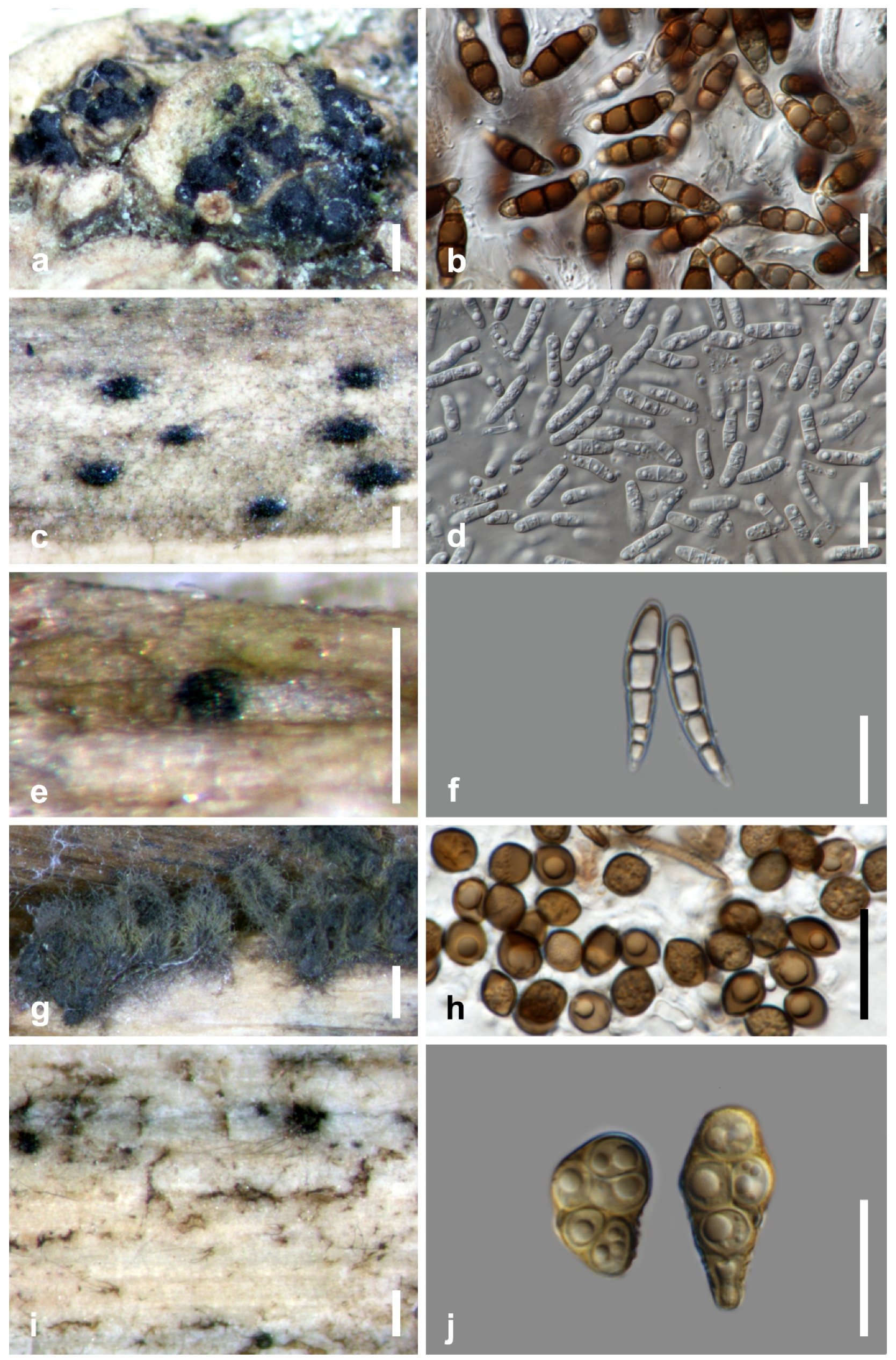

| Taxon | Host Part | Life Mode | Hosts | Distribution | References |
|---|---|---|---|---|---|
| ASCOMYCOTA | |||||
| DOTHIDEOMYCETES | |||||
| Acrospermales | |||||
| Acrospermaceae | |||||
| Acrospermum graminum Lib. | – | – | Elymus pungens | UK | [38] |
| Asterinales | |||||
| Morenoinaceae | |||||
| Morenoina phragmitis J.P. Ellis | Living/decomposing leaf sheaths and stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Botryosphaeriales | |||||
| Botryosphaeriaceae | |||||
| Botryosphaeria festucae (Lib.) Arx and E. Müll. | Living/decomposing leaf sheaths and stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Macrophomina sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Tiarosporella halmyra Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [42] |
| Phyllostictaceae | |||||
| Guignardia spp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Phyllosticta sp. | – | Pathogenic | Spartina cynosuroides | USA: Maryland | [44] |
| Phyllosticta spartinae Brunaud | – | – | Spartina maritima | France | [45] |
| Phyllosticta suaedae Lobik | Leaves | – | Suaeda maritima | Russia | [46] |
| Capnodiales | |||||
| Cladosporiaceae | |||||
| Cladosporium algarum Cooke and Massee | – | – | Spergularia marina | – | [35] |
| – | – | Suaeda maritima | – | [35] | |
| Cladosporium allicinum (Fr.) Bensch, U. Braun and Crous | – | – | Elymus pungens | UK | [38] |
| Cladosporium cladosporioides (Fresen.) G.A. de Vries | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Leaves and roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| Cladosporium herbarum (Pers.) Link | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Stem | Saprobic | Spartina townsendii | UK: England | [49] | |
| Leaves, stems, and roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| Cladosporium macrocarpum Preuss | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Cladosporium sphaerospermum Penz. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Living/decomposing leaf sheaths and blades | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,41,50] | |
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| – | Saprobic | Spartina sp. | Canada | [36] | |
| Capnodiales genera incertae sedis | |||||
| Mucomycosphaerella eurypotami (Kohlm., Volkm.-Kohlm. and O.E. Erikss.) Quaedvl. and Crous | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [51] |
| Mycosphaerellaceae | |||||
| Fulvia fulva (Cooke) Cif. | Leaves and stems | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Micronectriella agropyri Apinis and Chesters | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsedii | UK | [38] | |
| Mycosphaerella lineolata (Roberge ex Desm.) J. Schröt. | Living/decomposing leaf sheaths and stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| – | – | Elymus pungens | UK | [38] | |
| Mycosphaerella salicorniae (Auersw.) Lindau | – | – | Arthrocnemum subterminale | – | [35] |
| – | – | Limonium sp. | – | [35] | |
| – | – | Sarcocornia perennis | – | [35] | |
| – | – | Salicornia fruticosa | – | [35] | |
| – | – | Salicornia procumbens | – | [35] | |
| – | – | Salicornia europaea | – | [35] | |
| – | – | Salicornia perennis | – | [35] | |
| – | – | Sarcocornia fruticosa | – | [35] | |
| Drying stalks and inflorescence | Saprobic | Salicornia sp. | India | [52] | |
| Dried inflorescences | Saprobic | Salicornia virginica | Bermuda | [35,53] | |
| – | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon | [54] | |
| – | – | Suaeda vermiculata | – | [35] | |
| Drying stalks and inflorescence | Saprobic | Suaeda sp. | India | [52] | |
| Mycosphaerella spp. | – | – | Elymus pungens | UK | [38] |
| Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida, Mississippi | [43,55] | |
| Decaying leaves, leaf blades | Saprobic | Spartina alterniflora | Argentina: Buenos Aires; USA: Alabama, California, Georgia, Mississippi | [25,35,36,55,56,57,58] | |
| – | – | Spartina cf. densiflora | USA: California | [25,35] | |
| – | – | Spartina cf. pectinata | – | [35] | |
| – | – | Spartina sp. | Argentina: Buenos Aires; Canada | [35,36] | |
| Decaying leaf blades | Saprobic | Spartina foliosa | USA: California | [25] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Centro | [54,59] | |
| Mycosphaerella staticicola (Pat.) Dias | – | – | Armeria pungens | – | [35] |
| Mycosphaerella suaedae-australis Hansf. | – | – | Suaeda australis | – | [35] |
| Rivilata ius Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Tips of senescent, very old, and brittle leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [60] |
| Septoria spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| Upper leaves, inflorescence, seeds | Saprobic | Spartina alterniflora | USA: Rhode Island | [61] | |
| Septoria suaedae-australis Hansf. | Dead stems | Saprobic | Suaeda australis | South Australia | [62] |
| Sphaerulina albispiculata Tubaki | Sheath | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon | [54] |
| Stem | Saprobic | Spartina marítima | Portugal: Alentejo | [63] | |
| Sphaerulina orae-maris Linder | – | – | Ammophila arenaria | – | [35] |
| Rhizome and root | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59,63] | |
| Sphaerulina pedicellata T.W. Johnson | – | Saprobic | Spartina townsendii | – | [65] |
| Attached culms, stems | Saprobic, parasitic | Spartina alterniflora | USA: Rhode Island | [20,61] | |
| Sphaerulina sp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Dothideales | |||||
| Saccotheciaceae | |||||
| Aureobasidium sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Pseudoseptoria donacis (Pass.) B. Sutton | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Selenophoma sp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Dothideaceae | |||||
| Scirrhia annulata Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms and leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [66] |
| Dothideomycetes families incertae sedis | |||||
| Eriomycetaceae | |||||
| Heleiosa barbatula Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [66] |
| Pseudorobillardaceae | |||||
| Pseudorobillarda phragmitis (Cunnell) M. Morelet | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41,67] |
| Pseudorobillarda sp. | Dead stems | Saprobic | Spartina alterniflora | Canada | [36] |
| Dothideomycetes genera incertae sedis | |||||
| Bactrodesmium atrum M.B. Ellis Lautitia danica (Berl.) S. Schatz | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| – | – | Elymus pungens | UK | [38] | |
| – | – | Puccinellia maritima | UK | [38] | |
| Monodictys austrina Tubaki | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Monodictys castaneae (Wallr.) S. Hughes | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Neottiosporina australiensis B. Sutton and Alcorn | Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] |
| Neottiosporina sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Otthia sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Trichometasphaeria setulosa. (Sacc. and Roum.) Apinis and Chesters ined. | – | – | Elymus pungens | UK | [38] |
| Trichometasphaeria sp. | – | – | Elymus pungens | UK | [38] |
| Microthyriales | |||||
| Microthyriaceae | |||||
| Microthyrium microscopicum Desm. | – | – | Spartina patens | – | [68] |
| Microthyrium gramineum Sacc., E. Bommer and M. Rousseau | – | – | Elymus pungens | UK | [38] |
| Muyocopronales | |||||
| Muyocopronaceae | |||||
| Ellisiodothis inquinans (Ellis and Everh.) Theiss. | – | Saprobic | Spartina alterniflora | Argentina: Buenos Aires | [36] |
| Mytilinidiales | |||||
| Mytilinidiaceae | |||||
| Septonema secedens Corda | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Phaeotrichales | |||||
| Phaeotrichaceae | |||||
| Trichodelitschia bisporula (P. Crouan and H. Crouan) E. Müll. and Arx | – | – | Elymus pungens | UK | [38] |
| Spartina townsendii | UK | [38] | |||
| Pleosporales | |||||
| Amniculicolaceae | |||||
| Neomassariosphaeria typhicola (P. Karst.) Y. Zhang ter, J. Fourn. and K.D. Hyde | – | – | Juncus roemerianus | – | [35] |
| Decaying herbaceous stems | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | Saprobic | Spartina spp. | Argentina: Buenos Aires | [32,35,36] | |
| – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [58] | |
| Camarosporiaceae | |||||
| Camarosporium feurichii Henn. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Camarosporium palliatum Kohlm. and E. Kohlm. | – | – | Sarcocornia perennis | – | [35] |
| – | – | Salicornia sp. | – | [35] | |
| – | – | Salicornia virginica | – | [35] | |
| – | Saprobic or perthophytic | Salt marsh plants | India: Maharashtra | [52] | |
| – | – | Suaeda vermiculata | [35] | ||
| Camarosporium roumeguerei Sacc. | – | – | Atripex halimus | [35] | |
| – | – | Atripex sp. | [35] | ||
| – | – | Distichlis spicata | [35] | ||
| Twigs | – | Salicornia europaea | France | [35,69] | |
| – | – | Sarcocornia fruticosa | [35] | ||
| – | – | Salicornia sp. | [35] | ||
| – | Saprobic or perthophytic | Salt marsh plants | India: Gujarat, Maharashtra, Tamil Nadu, Andhara Pradesh, West Bengal | [52] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve, Centro | [59] | |
| – | – | Suaeda maritima | – | [35] | |
| Camarosporium salicorniae Hansf. | Twigs | – | Sarcocornia quinqueflora | South Australia | [62] |
| Camarosporium spp. | Living/decomposing leaf sheaths and stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Camarosporium suaedae-fruticosae S. Ahmad | Dead branches | Saprobic | Suaeda vermiculata | Pakistan | [70] |
| Coniothyriaceae | |||||
| Coniothyrium obiones Jaap | – | – | Atriplex portulacoides | – | [35] |
| – | Saprobic | Salt marsh plants | India: Orissa | [52] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve | [59] | |
| Coniothyrium spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Cyclothyriellaceae | |||||
| Massariosphaeria erucacea Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms and leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [66] |
| Massariosphaeria scirpina (G. Winter) Leuchtm. | – | Saprobic | Spartina sp. | USA: Florida, North Carolina | [71] |
| Massariosphaeria sp. | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Dictyosporiaceae | |||||
| Dictyosporium oblongum (Fuckel) S. Hughes | Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] |
| Dictyosporium pelagicum (Linder) G.C. Hughes ex E.B.G. Jones | Decomposing culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [35,61] |
| – | – | Spartina spp. | – | [32] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Algarve, Centro | [54,59,63] | |
| Jalapriya toruloides (Corda) M.J. D’souza, Hong Y. Su, Z.L. Luo and K.D. Hyde | Stems | Saprobic | Spartina sp. | UK | [72] |
| Didymellaceae | |||||
| Ascochyta cf. arundinariae Tassi | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Ascochyta leptospora (Trail) Hara | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Ascochyta salicorniae-patulae (Trotter) Melnik | – | Saprobic, parasitic | Salicornia spp. | Canada, Denmark, Germany, India, UK, USA | [52] |
| Ascochyta spp. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Sheath | Saprobic | Spartina marítima | Portugal: Alentejo | [54] | |
| Chaetasbolisia sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Didymella glacialis Rehm | Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] |
| Didymella glomerata (Corda) Qian Chen and L. Cai | Rhizome and basal area | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] |
| Didymella spp. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| – | Pathogenic | Spartina cynosuroides | USA: Louisiana | [44] | |
| Epicoccum nigrum Link | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Inflorescence, upper leaves, seeds | Saprobic, parasitic | Spartina alterniflora | USA: Rhode Island, Connecticut, Virginia, Florida, North Carolina | [36,61,73,74] | |
| Epicoccum sp. | – | – | Spartina alterniflora | – | [35] |
| Microsphaeropsis spp. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,41,50] |
| Phoma herbarum Westend. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Phoma leveillei Boerema and G.J. Bollen | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Phoma suaedae Jaap | Twigs, leaves, stems | Saprobic | Suaeda maritima, Suaeda sp. | Germany; India | [75] |
| – | – | Suaeda maritima | – | [35] | |
| Phoma spp. | – | – | Crithmum maritimum | – | [35] |
| – | – | Atriplex portulacoides | – | [35] | |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | China: Hong Kong; Netherlands: Zeeland | [39,40,41,50] | |
| – | – | Salicornia europaea | – | [35] | |
| – | – | Spartina alterniflora | USA: North Carolina, Rhode Island | [20,35,36,61,73,74] | |
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| – | Saprobic | Spartina sp. | Argentina: Buenos Aires; Canada; USA: Maine, South Carolina | [36,71] | |
| – | – | Spartina townsendii | UK: England | [35,49,65] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Algarve, Centro | [54,59,63] | |
| Paraboeremia putaminum (Speg.) Qian Chen and L. Cai | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Stagonosporopsis salicorniae (Magnus) Died. | – | – | Salicornia europaea | – | [35] |
| – | – | Salicornia patula | – | [35] | |
| Didymosphaeriaceae | |||||
| Didymosphaeria lignomaris Strongman and J.D. Mill. | Basal area of the sheath | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] |
| – | – | Spartina spp. | – | [32] | |
| Julella herbatilis Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [76] |
| Paraphaeosphaeria apicicola Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [51] |
| Paraphaeosphaeria pilleata Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [77] |
| Paraphaeosphaeria michotii (Westend.) O.E. Erikss. | – | – | Elymus pungens | UK | [38] |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| Pseudopithomyces atro-olivaceus (Cooke and Harkn.) G. Guevara, K.C. Cunha and Gené | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Pseudopithomyces chartarum (Berk. and M.A. Curtis) Jun F. Li, Ariyaw. and K.D. Hyde | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Pseudopithomyces maydicus (Sacc.) Jun F. Li, Ariyaw. and K.D. Hyde | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Spegazzinia tessarthra (Berk. and M.A. Curtis) Sacc. | Living leaves | Juncus roemerianus | USA: Florida | [43] | |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Tremateia halophila Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower and middle parts of senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [78] |
| – | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon | [54] | |
| Lentitheciaceae | |||||
| Halobyssothecium estuariae B. Devadatha, Calabon, K.D. Hyde and E.B.G. Jones | Dead culm | Saprobic | Phragmites australis | UK: Pembrokeshire | [79] |
| Halobyssothecium obiones (P. Crouan and H. Crouan) Dayarathne, E.B.G. Jones and K.D. Hyde | Drift stems, attached and dead culms | Saprobic | Spartina alterniflora | India: Maharashtra, Tamil Nadu, Andhara Pradesh; USA: Maine, Rhode Island, Connecticut, Massachusetts, New Jersey, Maryland, Virginia, North Carolina, South Carolina, Florida, Mississippi, Texas | [20,35,52,61,71,74,80,81,82] |
| – | – | Spartina cynosuroides | – | [35] | |
| Pod and rhizome | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| Culms | Saprobic | Spartina sp. | UK: England, Hampshire | [79,83] | |
| Stem | Saprobic | Spartina townsendii | UK: Hampshire, Wales | [49,65] | |
| – | Saprobic | Spartina spp. | USA: New Jersey, South Carolina; Mississippi, Argentina: Buenos Aires | [32,35,36,58,84] | |
| Stem, leaf sheaths, and blades | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59,63] | |
| – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [55,58] | |
| – | – | Elymus pungens | – | [35] | |
| – | – | Atriplex portulacoides | – | [35] | |
| – | – | Spartina townsendii | – | [35] | |
| Halobyssothecium phragmitis M.S. Calabon, E.B.G. Jones, S. Tibell and K.D. Hyde | Dead culm and stem | Saprobic | Phragmites sp. | Sweden: Gotland | [85] |
| Halobyssothecium versicolor M.S. Calabon, E.B.G. Jones and K.D. Hyde | Dead stem | Saprobic | Atriplex portulacoides | UK: Hampshire | [85] |
| Keissleriella culmifida (P. Karst.) S.K. Bose | – | – | Elymus pungens | UK | [38] |
| Keissleriella linearis E. Müll. ex Dennis | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Dead culm | Saprobic | Phragmites sp. | Sweden: Gotland | [85] | |
| Keissleriella phragmiticola Wanas., E.B.G. Jones and K.D. Hyde | Culms | Saprobic | Phragmites australis | UK: Wales | [79] |
| Keissleriella rara Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [77] |
| Keissleriella spp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Lentithecium fluviatile (Aptroot and Van Ryck.) K.D. Hyde, J. Fourn. and Ying Zhang | Dead leaf sheaths | Saprobic | Phragmites australis | Belgium: East Flanders | [86] |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] | |
| Setoseptoria arundinacea (Sowerby) Kaz. Tanaka and K. Hiray. | – | – | Elymus pungens | UK | [38] |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] | |
| – | Saprobic | Spartina sp. | USA: North Carolina, Florida | [71] | |
| Setoseptoria phragmitis Quaedvl., Verkley and Crous | Culm | Saprobic | Phragmites sp. | Sweden: Södermanland | [87] |
| Towyspora aestuari Wanas., E.B.G. Jones and K.D. Hyde | – | – | Phragmites australis | UK: Wales | [88] |
| Leptosphaeriaceae | |||||
| Leptosphaeria albopunctata (Westend.) Sacc. | – | – | Juncus maritimus | – | [35] |
| – | – | Phragmites australis | – | [35] | |
| Attached culms | - | Spartina alterniflora | USA: Rhode Island | [35,36,61,71,73,80] | |
| – | – | Spartina spp. | Canada: Bay of Fundy; USA: New Jersey, South Carolina; Argentina: Buenos Aires | [35,36,48,89,90] | |
| Stem | Saprobic | Spartina townsendii | UK: Wales | [35,65] | |
| Leptosphaeria australiensis (Cribb and J.W. Cribb) G.C. Hughes | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Pod | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | – | Spartina spp. | – | [32] | |
| Leptosphaeria culmifraga (Fr.) Ces. and De Not. | – | – | Elymus pungens | UK | [38] |
| Leptosphaeria littoralis Sacc. | – | – | Elymus pungens | UK | [38] |
| Leptosphaeria marina Ellis and Everh. | – | – | Juncus roemerianus | [35] | |
| – | Saprobic | Spartina alterniflora | USA: Maine, Rhode Island, Connecticut, New Jersey, Delaware, Virginia, North Carolina, South Carolina | [35,36,71,73,80] | |
| – | Saprobic | Spartina spp. | Canada; USA: New Jersey | [32,35,36,65,89,90,91] | |
| – | – | Spartina townsendii | UK | [35,38] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve | [31,59] | |
| Leptosphaeria orae-maris Linder | – | – | Arundo donax | – | [35] |
| – | Saprobic | Lysimachia maritima | USA: Massachusetts | [35,92] | |
| – | Saprobic | Spartina alterniflora | USA: Massachusetts, Rhode Island, North Carolina, Florida, Texas | [36,71,80,92] | |
| Rhizome | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | – | Spartina spp. | – | [32] | |
| – | Saprobic | Spartina townsendii | UK | [35,65,93] | |
| Leptosphaeria pelagica E.B.G. Jones | – | – | Elymus pungens | UK | [35,38] |
| – | – | Puccinellia maritima | UK | [38] | |
| Decaying herbaceous stems, dead culms, decaying leaves | Saprobic | Spartina alterniflora | USA: Connecticut, Mississippi, Rhode Island; India: Goa, Karanataka | [20,36,52,55,73,94] | |
| – | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| – | – | Spartina townsendii | UK | [38] | |
| – | – | Spartina spp. | UK | [32,65] | |
| Sheath | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon | [54] | |
| Stem | Saprobic | Spartina marítima | Portugal: Alentejo | [63] | |
| Leptosphaeria peruvianae Speg. | Decaying stems | Saprobic | Sarcocornia perennis | Argentina: Buenos Aires; in temperate marine waters | [52] |
| Leptosphaeria spp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Mississippi | [55] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| – | – | Spartina alterniflora | USA: Rhode Island | [74] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Centro | [59] | |
| Leptosphaeria suaedae Hansf. | Dead twigs | Saprobic | Suaeda australis | South Australia | [95] |
| Lindgomycetaceae | |||||
| Arundellina typhae Wanas., E.B.G. Jones and K.D. Hyde | Dead stem | Saprobic | Typha sp. | UK: England | [96] |
| Lophiostomataceae | |||||
| Lophiostoma semiliberum (Desm.) Ces. and De Not. | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Lophiostoma sp. | – | – | Elymus pungens | UK | [38] |
| Sigarispora arundinis (Pers.) Thambug., Qing Tian, Kaz. Tanaka and K.D. Hyde | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Massarinaceae | |||||
| Helminthosporium sp. | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] |
| Massarina carolinensis Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [77] |
| Massarina igniaria (C. Booth) Aptroot | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Massarina phragmiticola Poon and K.D. Hyde | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Massarina ricifera Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower parts of senescent culms, decaying leaves | Saprobic | Juncus roemerianus | USA: Alabama, Mississippi, North Carolina | [55,58,97] |
| Massarina spp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| Stagonospora abundata Kohlm. and Volkm.-Kohlm. | Senescent leaves and bracts | Saprobic | Juncus roemerianus | USA: Florida, Georgia, North Carolina | [98] |
| Stagonospora cylindrica Gunnell | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Stagonospora elegans (Berk.) Sacc. and Traverso | Living/decomposing leaf sheaths, stems, culms | Saprobic | Phragmites australis | Australis; Netherlands: Zeeland | [39,40,95] |
| Stagonospora epicalamia (Cooke) Sacc. | – | – | Phragmites australis | Australia | [95] |
| Stagonospora haliclysta Kohlm. | Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve | [59] |
| Stagonospora spp. | Living and senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | China: Hong Kong; Netherlands: Zeeland | [39,40,41,50] | |
| Senescent and dead leaves/inflorescence, living and dead seeds, decaying leaf blades | Saprobic, pathogenic | Spartina alterniflora | Canada; USA: Maine, Rhode Island, Georgia, Connecticut, New Jersey, Virginia, Florida, North Carolina; Argentina: Buenos Aires | [35,36,56,73,74] | |
| – | Pathogenic | Spartina cynosuroides | USA: Maryland | [44] | |
| – | Saprobic | Spartina patens | USA: Rhode Island | [35,36] | |
| – | Saprobic | Spartina spp. | Canada | [35,36] | |
| Leaf sheaths and blades, stem, limb | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59] | |
| Stagonospora suaedae Syd. and P. Syd. | Leaves | – | Suaeda marítima | Germany | [99] |
| Melanommataceae | |||||
| Aposphaeria spp. | Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Bicrouania maritima (P. Crouan and H. Crouan) Kohlm. and Volkm.-Kohlm. | Dead stems | Saprobic | Atriplex portulacoides | India | [35,52] |
| Morosphaeriaceae | |||||
| Helicascus kanaloanus Kohlm. | – | – | Spartina spp. | – | [32] |
| Neocamarosporiaceae | |||||
| Neocamarosporium artemisiae Dayarathne and E.B.G. Jones | – | Saprobic | Artemisia maritima | Sweden: Bohuslän | [100] |
| Neocamarosporium maritimae Dayarathne and E.B.G. Jones | – | Saprobic | Artemisia maritima | Sweden: Bohuslän | [100] |
| Neocamarosporium obiones (Jaap) Wanas. and K.D. Hyde | – | – | Atriplex portulacoides | – | [35] |
| Neocamarosporium phragmitis D.N. Wanasinghe, E.B.G. Jones and K.D. Hyde | Decaying culms | Saprobic | Phragmites australis | UK | [101] |
| Neocamarosporium salicorniicola Dayar., E.B.G. Jones and K.D. Hyde | Dead stems | Saprobic | Salicornia sp. | Thailand | [102] |
| Periconiaceae | |||||
| Periconia cookei E.W. Mason and M.B. Ellis | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] | |
| Periconia digitata (Cooke) Sacc. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Periconia digitata (Cooke) Sacc. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Periconia echinochloae (Bat.) M.B. Ellis | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Periconia minutissima Corda | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| Periconia sp. | – | Saprobic | Unidentifed saltmarsh plants | USA: Mississippi | [58] |
| Phaeosphaeriaceae | |||||
| Amarenomyces ammophilae (Lasch) O.E. Erikss. | – | – | Ammophila arenaria | – | [35] |
| – | – | × Ammocalamagrostis baltica | – | [35] | |
| – | – | Uniola paniculata | – | [35] | |
| Amphisphaeria culmicola Sacc. | Stem | Spartina townsendii | UK: England | [49] | |
| Camarosporioides phragmitis W.J. Li and K.D. Hyde | Dead stem | Saprobic | Phragmites australis | Germany | [96] |
| Hendersonia culmiseda Sacc. | Living/decomposing leaf blades | Saprobic | Phragmites australis | Netherlands: Zeeland | [50] |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| – | – | Spartina townsendii | UK | [103] | |
| Hendersonia spp. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland; USA: Florida | [39,43,50] |
| Loratospora aestuarii Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [104] |
| Loratospora aestuarii Kohlm. and Volkm.-Kohlm. | – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [58] |
| Ophiobolus littoralis (P. Crouan and H. Crouan) Sacc. | – | – | Elymus pungens | UK | [38] |
| Phaeoseptoria sp. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Phaeosphaeria anchiala Kohlm., Volkm.-Kohlm. and C.K.M. Tsui | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida, Georgia, Maryland, North Carolina, Virginia | [105] |
| Phaeosphaeria caricinella (P. Karst.) O.E. Erikss. | – | – | Spartina sp. | USA: Florida, North Carolina | [71] |
| Phaeosphaeria culmorum (Auersw.) Leuchtm. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Phaeosphaeria eustoma (Fuckel) L. Holm | Living/decomposing leaf blades and sheaths, stems, culms | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50,95] |
| Phaeosphaeria fuckelii (Niessl) L. Holm | – | – | Elymus pungens | UK | [38] |
| Phaeosphaeria gessneri Shoemaker and C.E. Babc. | – | – | Spartina spp. | – | [32] |
| Phaeosphaeria halima (T.W. Johnson) Shoemaker and C.E. Babc. | Dead culms; Decaying leaves, leaf blades | Saprobic | Spartina alterniflora | India: Kerala; USA: California, Georgia, Mississippi, Vancouver, North Carolina | [25,35,52,55,56,57,58,71,80] |
| Decaying leaf blades | Saprobic | Spartina densiflora | USA: California | [25] | |
| Spartina spp. | [32] | ||||
| Decaying leaves | Saprobic | Spartina foliosa | USA: California | [25] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve, Centro | [31] | |
| Phaeosphaeria herpotrichoides (De Not.) L. Holm | – | – | Spartina patens | USA: North Carolina, Florida | [71] |
| Phaeosphaeria juncina (Auersw.) L. Holm | – | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Phaeosphaeria luctuosa (Niessl ex Sacc.) Y. Otani and Mikawa | Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| – | – | Elymus pungens | UK | [38] | |
| Phaeosphaeria macrosporidium (E.B.G. Jones) Shoemaker and C.E. Babc. | Decaying stems | Saprobic | Spartina sp | UK: Wales, England | [65] |
| Stem | Saprobic | Spartina marítima | Portugal: Lisbon | [54,63] | |
| Phaeosphaeria microscopica (P. Karst.) O.E. Erikss. | – | – | Elymus pungens | UK | [38] |
| Phaeosphaeria neomaritima (R.V. Gessner and Kohlm.) Shoemaker and C.E. Babc. | – | – | Juncus maritimus | – | [35] |
| – | – | Juncus roemerianus | – | [35] | |
| – | Saprobic | Juncus sp. | Canada; India: Maharashtra, Karnataka; USA: Virginia, North Carolina | [36,52,71,80] | |
| – | – | Spartina alterniflora | – | [35] | |
| – | Saprobic | Spartina spp. | Canada; USA: North Carolina, Virginia | [32,71,80] | |
| – | – | Spartina townsendii | UK | [35,93] | |
| Stem | Saprobic | Spartina marítima | Portugal: Alentejo | [63] | |
| Phaeosphaeria nigrans (Roberge ex Desm.) L. Holm | – | – | Elymus pungens | UK | [38] |
| Phaeosphaeria olivacea Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina, Mississippi | [58,76] |
| Phaeosphaeria pontiformis (Fuckel) Leuchtm. | – | – | Elymus pungens | UK | [38] |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] | |
| Phaeosphaeria roemeriani Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Mississippi, North Carolina | [55,58,60] |
| Phaeosphaeria spartinae (Ellis and Everh.) Shoemaker and C.E. Babc. | – | Saprobic | Spartina spp. | India: Kerala | [32,52] |
| Decaying herbaceous stems and pod | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | Saprobic | Spartina marítima | Portugal: Lisbon | [54] | |
| Phaeosphaeria spartinicola Leuchtm. | – | Saprobic | Juncus sp. | India | [52] |
| Dead leaves, decaying leaf blades | Saprobic | Spartina alterniflora | Mexico; USA: Alabama, California, Georgia, Mississippi; Canada: Nova Scotia, New Brunswick | [25,36,55,56,57,58] | |
| Pod, leaf blades | Saprobic | Spartina densiflora | Argentina: Buenos Aires; USA: California | [25,64] | |
| – | – | Spartina spp. | – | [32] | |
| Leaf blades | Saprobic | Spartina foliosa | USA: California | [25] | |
| Leaf sheaths and blades, stem, limb | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59,63] | |
| Phaeosphaeria spp. | Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] |
| – | Saprobic | Spartina alterniflora | USA: Rhode Island | [74] | |
| Sclerostagonospora sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Septoriella phragmitis Oudem. | Living/decomposing leaf sheaths and stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Septoriella spp. | Decaying stems and leaf sheaths and blades, stems | Saprobic | Phragmites australis | China: Hong Kong; Netherlands: Zeeland | [39,40,41,50] |
| Septoriella thalassica (Speg.) Nag Raj | – | – | Distichlis spicata | – | [35] |
| Distichlis spicata | [35] | ||||
| Septoriella unigalerita Kohlm. and Volkm.-Kohlm. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [98] |
| Septoriella vagans (Niessl) Y. Marín and Crous | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| – | Saprobic | Spartina alterniflora | USA: Rhode Island | [74] | |
| Pleomassariaceae | |||||
| Splanchnonema sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Pleosporaceae | |||||
| Alternaria alternata (Fr.) Keissl. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,41,50] | |
| – | Saprobic | Spartina alterniflora | USA: North Carolina | [74] | |
| Leaves, stems, and roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| Alternaria infectoria E.G. Simmons | – | – | Elymus pungens | UK | [38] |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| Alternaria longissima Deighton and MacGarvie | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Alternaria maritima G.K. Sutherl. | Stem | Saprobic, pathogenic | Spartina townsendii | UK: England | [49] |
| Alternaria spp. | – | – | Atriplex portulacoides | – | [35] |
| – | – | Juncus roemerianus | – | [35] | |
| – | – | Salsola kali | – | [35] | |
| Inflorescence and upper leaves | Saprobic, parasitic | Spartina alterniflora | USA: Rhode Island | [35,61] | |
| Culms | Saprobic | Spartina sp. | Thailand | This study | |
| – | – | Spartina townsendii | – | [35] | |
| Bipolaris cynodontis (Marignoni) Shoemaker | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Curvularia hawaiiensis (Bugnic. ex M.B. Ellis) Manamgoda, L. Cai and K.D. Hyde | Living and senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Curvularia protuberata R.R. Nelson and Hodges | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Curvularia spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| – | Saprobic | Spartina altrerniflora | USA: North Carolina | [74] | |
| Curvularia tuberculata B.L. Jain | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decorospora gaudefroyi (Pat.) Inderb., Kohlm. and Volkm.-Kohlm. | Stems | Saprobic | Atriplex sp. | UK: Portsmouth | [106] |
| – | – | Atriplex portulacoides | – | [35] | |
| – | – | Sarcocornia perennis | – | [35] | |
| – | – | Sarcoconia fructicosa | – | [35] | |
| – | – | Salicornia europaea | – | [35] | |
| – | – | Salicornia sp. | – | [35] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve | [59] | |
| – | – | Suaeda maritima | – | [35] | |
| Drechslera sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Exserohilum rostratum (Drechsler) K.J. Leonard and Suggs | – | – | Distichlis spicata | – | [35] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Senescent and dead leaves | Saprobic | Spartina alterniflora | USA: Rhode Island, North Carolina, Florida | [35,36,73] | |
| – | – | Spartina spp. | – | [32] | |
| Paradendryphiella arenariae (Nicot) Woudenb. and Crous | Decomposing culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [35,61] |
| – | – | Spartina spp. | – | [32] | |
| Paradendryphiella salina (G.K. Sutherl.) Woudenb. and Crous | – | – | Atriplex portulacoides | – | [35] |
| Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| – | – | Puccinellia maritima | – | [35] | |
| – | – | Salicornia europaea | – | [35] | |
| Decomposing culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [35,61] | |
| – | – | Spartina spp. | – | [32] | |
| – | – | Spartina townsendii | – | [35] | |
| Leaves and stems | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| – | – | Suaeda maritima | – | [35] | |
| Pleospora abscondita Sacc. and Roum. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Pleospora pelagica T.W. Johnson | Decomposing culms; decaying leaf blades | Saprobic | Spartina alterniflora | India: Maharashtra, Kerala; USA: Georgia, Rhode Island, North Carolina, Florida | [35,36,52,56,71,73,74,80] |
| Decaying leaf blades | Saprobic | Spartina densiflora | USA: California | [25] | |
| Saprobic | Spartina spp. | USA: South Carolina | [32,36] | ||
| Typha sp. | [35] | ||||
| Pleospora pelvetiae G.K. Sutherl. | – | Saprobic | Unidentifed saltmarsh plants | USA: Mississippi | [58] |
| Pleospora spp. | – | – | Salicornia virginica | – | [35] |
| Dead leaves/culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [61] | |
| Pleospora spartinae (J. Webster and M.T. Lucas) Apinis and Chesters | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] | |
| Stem | Saprobic | Spartina spp. | Canada: Bay of Fundy | [32,48] | |
| – | – | Spartina townsendii | UK | [35,38,107] | |
| Pleospora straminis Sacc. and Speg. | – | – | Elymus pungens | UK | [38] |
| Pleospora vagans Niessl var. vagans | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Dead culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [73] | |
| Pyrenophora tritici-repentis (Died.) Drechsler | – | – | Elymus pungens | UK | [38] |
| Stemphylium botryosum Wallr. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Stemphylium lycopersici (Enjoji) W. Yamam. | Living leaves | – | Juncus roemerianus | USA: Florida | [43] |
| Stemphylium maritimum T.W. Johnson | – | Saprobic | Spartina sp. | UK | [65] |
| Stemphylium spp. | – | – | Salsola kali | – | [35] |
| Leaves | Saprobic | Spartina spp. | Canada: Bay of Fundy | [35,48] | |
| Stemphylium vesicarium (Wallr.) E.G. Simmons | – | – | Elymus pungens | UK | [38] |
| Living, senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| – | Saprobic | Lysimachia maritima | USA: Massachusetts | [92] | |
| – | Saprobic | Spartina alterniflora | USA: Rhode Island | [61] | |
| Glumes, rachis | – | Spartina townsendii | UK: England | [38,49] | |
| Spartina sp. | UK | [65] | |||
| Stemphylium triglochinicola B. Sutton and Piroz. | – | – | Triglochin maritima | Sweden: Västergötland | [35,87] |
| Dead leaves and inflorescences | Saprobic | Triglochin sp. | India: Kerala; UK | [52,108] | |
| Typhicola typharum (Desm.) Crous | Senescent and dead leaves | Saprobic, pathogenic | Spartina alterniflora | Canada; USA: Maine, Rhode Island, Connecticut, New Jersey, Virginia, North Carolina, Florida | [35,36,61,73,74] |
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| – | Spartina townsendii | UK | [38] | ||
| – | Saprobic | Spartina spp. | Argentina: Buenos Aires; Canada; USA: Maine | [35,36] | |
| Stems | Saprobic | Spartina townsendii | UK: England | [35,49,65] | |
| Pleosporales genera incertae sedis | |||||
| Phialophorophoma litoralis Linder | Stem and sheath | Saprobic | Spartina marítima | Portugal: Alentejo, Lisbon | [54,63] |
| Phialophorophoma spp. | Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Pyrenochaeta sp. | Living leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Scolecobasidium humicola G.L. Barron and L.V. Busch | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Roussoellaceae | |||||
| Cytoplea sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Sporormiaceae | |||||
| Preussia funiculata (Preuss) Fuckel | – | – | Spartina townsendii | UK | [38] |
| Preussia terricola Cain | – | – | Elymus pungens | UK | [38] |
| Sporormia longipes Massee and E.S. Salmon | – | – | Elymus pungens | UK | [38] |
| Sporormia sp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Sporormiella intermedia (Auersw.) S.I. Ahmed and Cain ex Kobayasi | – | – | Elymus pungens | UK | [38] |
| Sporormiella lageniformis (Fuckel) S.I. Ahmed and Cain | – | – | Spartina townsendii | UK | [38] |
| Sporormiella minima (Auersw.) S.I. Ahmed and Cain | – | – | Elymus pungens | UK | [38] |
| – | – | Spartina townsendii | UK | [38] | |
| Teichosporaceae | |||||
| Teichospora striata (Kohlm. and Volkm.-Kohlm.) Jaklitsch and Voglmayr | Senescent leaves and inflorescences | Saprobic | Juncus roemerianus | USA: North Carolina, Virginia | [98] |
| Teichospora suaedae Speg. | Dead branches | Saprobic | Suaeda divaricata | Argentina: Mendoza | [109] |
| Testudinaceae | |||||
| Verruculina enalia (Kohlm.) Kohlm. and Volkm.-Kohlm. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Tetraplosphaeriaceae | |||||
| Tetraploa aristata Berk. and Broome | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Torulaceae | |||||
| Torula herbarum (Pers.) Link | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Trematosphaeriaceae | |||||
| Halomassarina thalassiae (Kohlm. and Volkm.-Kohlm.) Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. and C.L. Schoch | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| EUROTIOMYCETES | |||||
| Chaetothyriales | |||||
| Herpotrichiellaceae | |||||
| Rhinocladiella spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Veronaea sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Eurotiales | |||||
| Aspergillaceae | |||||
| Aspergillus fumigatus Fresen. | – | – | Elymus pungens | UK | [38] |
| Aspergillus nidulans (Eidam) G. Winter | – | – | Elymus pungens | UK | [38] |
| – | – | Spartina townsendii | UK | [38] | |
| Aspergillus niger Tiegh. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Aspergillus spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| – | – | Spartina townsendii | UK: England | [49] | |
| Monascus purpureus Went | – | – | Elymus pungens | UK | [38] |
| Penicillium aurantiogriseum Dierckx | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Penicillium brevicompactum Dierckx | Roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Penicillium chrysogenum Thom | Roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Penicillium lividum Westling | Leaves and stems | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Penicillium spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| – | – | Spartina townsendii | UK: England | [49] | |
| Thermoascaceae | |||||
| Thermoascus crustaceus (Apinis and Chesters) Stolk | – | – | Elymus pungens | UK | [38] |
| Paecilomyces spp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| – | Saprobic | Salt marsh plants | India: Goa | [52] | |
| Trichocomaceae | |||||
| Thermomyces dupontii (Griffon and Maubl.) Houbraken and Samson | – | – | Elymus pungens | UK | [38] |
| Onygenales | |||||
| Onygenaceae | |||||
| Amauroascus albicans (Apinis) Arx | – | – | Elymus pungens | UK | [38] |
| Amauroascus albicans (Apinis) Arx | – | – | Spartina townsendii | UK | [38] |
| LECANOROMYCETES | |||||
| Ostropales | |||||
| Stictidaceae | |||||
| Glomerobolus gelineus Kohlm. and Volkm.-Kohlm. Stictis sp. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [110] |
| Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] | |
| LEOTIOMYCETES | |||||
| Helotiales | |||||
| Amorphothecaceae | |||||
| Amorphotheca resinae Parbery | Roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Calloriaceae | |||||
| Cistella fugiens (W. Phillips) Matheis | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Helotiaceae | |||||
| Cyathicula culmicola (Desm.) De Not. | – | – | Elymus pungens | UK | [38] |
| Helotium sp. | – | – | Elymus pungens | UK | [38] |
| Lachnaceae | |||||
| Brunnipila palearum (Desm.) Baral | – | – | Elymus pungens | UK | [38] |
| – | – | Spartina townsendii | UK | [38] | |
| Lachnum controversum (Cooke) Rehm | – | – | Elymus pungens | UK | [38] |
| Lachnum spartinae S.A. Cantrell | Decaying leaf sheaths | Saprobic | Spartina alterniflora | USA: Georgia | [56,111] |
| – | – | Spartina spp. | – | [32] | |
| Mollisiaceae | |||||
| Belonopsis atriella (Cooke) Lindau | – | – | Spartina cynosuroides | USA: Louisiana | [68,90,112] |
| Mollisia hydrophila (P. Karst.) Sacc. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Mollisia palustris (P. Karst.) P. Karst. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Trichobelonium kneiffii (Wallr.) J. Schröt. | Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Ploettnerulaceae | |||||
| Cadophora melinii Nannf. | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Sclerotiniaceae | |||||
| Botrytis cinerea Pers. | Stem | Spartina townsendii | UK: England | [49] | |
| Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| Monilia sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Solenopeziaceae | |||||
| Halenospora varia (Anastasiou) E.B.G. Jones | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Basal area of the sheath | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] | |
| – | – | Spartina spp. | – | [32] | |
| Helotiales genera incertae sedis | |||||
| Cejpia hystrix (De Not.) Baral | – | – | Elymus pungens | UK | [38] |
| Dactylaria sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Crocicreas gramineum (Fr.) Fr. | – | – | Elymus pungens | UK | [38] |
| Leotiales | |||||
| Leotiales genera incertae sedis | |||||
| Flagellospora sp. | Living leaves | – | Juncus roemerianus | USA: Florida | [43] |
| Rhytismatales | |||||
| Rhytismataceae | |||||
| Lophodermium arundinaceum (Schrad.) Chevall. | – | – | Elymus pungens | UK | [38] |
| Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] | |
| Thelebolales | |||||
| Thelebolaceae | |||||
| Thelebolus crustaceus (Fuckel) Kimbr. | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsendii | UK | [38] | |
| ORBILIOMYCETES | |||||
| Orbiliales | |||||
| Orbiliaceae | |||||
| Arthrobotrys conoides Drechsler | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Arthrobotrys sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Orbilia junci Kohlm., Baral and Volkm.-Kohlm. | Tips of senescent leaves | – | Juncus roemerianus | USA: North Carolina | [113] |
| PEZIZOMYCETES | |||||
| Pezizales | |||||
| Pezizaceae | |||||
| Belonium heteromorphum (Ellis and Everh.) Seaver | – | – | Spartina cynosuroides | USA: Louisiana | [68,114] |
| SACCHAROMYCETES | |||||
| Saccharomycetales | |||||
| Debaryomycetaceae | |||||
| Debaryomyces hansenii (Zopf) Lodder and Kreger-van Rij | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Louisiana | [56] |
| Scheffersomyces spartinae (Ahearn, Yarrow and Meyers) Kurtzman and M. Suzuki | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Louisiana | [56] |
| Saccharomycetaceae | |||||
| Kluyveromyces lactis (Stell.-Dekk.) Van der Walt | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Louisiana | [56] |
| SORDARIOMYCETES | |||||
| Amphisphaeriales | |||||
| Amphisphaeriaceae | |||||
| Massariella sp. | – | – | Spartina townsendii | UK | [38] |
| Ommatomyces coronatus Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower parts of senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [97] |
| Pestalotia sp. | Living, senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Apiosporaceae | |||||
| Arthrinium arundinis (Corda) Dyko and B. Sutton | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Dead culms | Saprobic | Phragmites sp. | South Australia | [62] | |
| Arthrinium phaeospermum (Corda) M.B. Ellis | Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] |
| – | Saprobic | Spartina patens | USA: Rhode Island | [61] | |
| Inflorescence and upper leaves | Saprobic | Spartina alterniflora | USA: Rhode Island | [36] | |
| Arthrinium spp. | Living leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Nigrospora oryzae (Berk. and Broome) Petch | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Beltraniaceae | |||||
| Beltrania querna Harkn. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Hyponectriaceae | |||||
| Phragmitensis ellipsoidea M.K.M. Wong, Goh and K.D. Hyde | Intertidal to aerial culms | Saprobic | Phragmites australis | China: Hong Kong | [115] |
| Phragmitensis marina M.K.M. Wong, Poon and K.D. Hyde | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Physalospora citogerminans Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower and upper parts of senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [116] |
| Sporocadaceae | |||||
| Discostroma sp. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Pestalotiopsis juncestris Kohlm. and Volkm.-Kohlm. | Senescent involucral leaves and culms | Saprobic | Juncus roemerianus | USA: North Carolina | [117] |
| Pestalotiopsis planimi (Vize) Steyaert | – | – | Spartina alterniflora | USA: Rhode Island | [61] |
| Pestalotiopsis sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Coronophorales | |||||
| Ceratostomataceae | |||||
| Melanospora sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Microthecium fimicola (E.C. Hansen) Y. Marín, Stchigel, Guarro and Cano | – | – | Elymus pungens | UK | [38] |
| Microthecium levitum Udagawa and Cain | Dead leaves/culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [61] |
| Coronophorales genera incertae sedis | |||||
| Papulaspora halima Anastasiou | Living and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Papulosa amerospora Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [118] |
| Diaporthales | |||||
| Diaporthaceae | |||||
| Phomopsis spp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| – | – | Spartina sp. | – | [71] | |
| Gnomoniaceae | |||||
| Gnomonia salina E.B.G. Jones (probably a nomen dubiumand possibly a Halosarpheia species) | – | Saprobic | Spartina alterniflora | USA: Connecticut | [36] |
| Spartina spp. | [32,35] | ||||
| – | – | Spartina townsendii | UK | [35,65] | |
| Diaporthales incertae sedis | |||||
| Botryodiplodia sp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Glomerellales | |||||
| Glomerellaceae | |||||
| Colletotrichum sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Plectosphaerellaceae | |||||
| Stachylidium bicolor Link | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Hypocreales | |||||
| Bionectriaceae | |||||
| Acremonium spp. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert and W. Gams | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Fusariella obstipa (Pollack) S. Hughes | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Gliomastix spp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Hydropisphaera arenula (Berk. and Broome) Rossman and Samuels | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Hydropisphaera erubescens (Roberge ex Desm.) Rossman and Samuels | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] |
| – | – | Spartina spp. | – | [32] | |
| Clavicipitaceae | |||||
| Atkinsonella hypoxylon (Peck) Diehl | – | – | Spartina cynosuroides | – | [68] |
| Claviceps purpurea (Fr.) Tul. | – | Saprobic | Phragmites australis | UK: England (Southampton Hampshire, Sussex, Oxon) | [119,120] |
| Replaced seeds in the inflorescence, ovaries of the flowers | Saprobic, parasitic | Spartina alterniflora | USA: Rhode Island; Argentina | [36,61,68,73,121,122] | |
| – | Pathogenic | Spartina anglica | UK | [123] | |
| – | Saprobic, parasitic | Spartina cynosuroides | USA: New York, Florida, Mississippi | [44,68,121,124] | |
| Spartina patens | USA: Maryland, Mississippi | [44,68,124,125] | |||
| – | – | Spartina townsendii | UK: England | [120,126] | |
| – | – | Spartina sp. | Argentina | [122] | |
| Claviceps sp. | – | – | Spartina foliosa | USA: California | [127] |
| Metarhizium anisopliae (Metschn.) Sorokīn | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Hypocreaceae | |||||
| Cladobotryum sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Gliocladium sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Trichoderma citrinum (Pers.) Jaklitsch, W. Gams and Voglmayr | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Trichoderma sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Trichoderma viride Pers. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Nectriaceae | |||||
| Calonectria sp. | – | – | Elymus pungens | UK | [38] |
| Fusarium fujikuroi Nirenberg | – | Saprobic | Suaeda australis | South Australia | [62] |
| Fusarium graminearum Schwabe | Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Fusarium heterosporum Nees and T. Nees | – | – | Spartina maritima | – | [128] |
| Fusarium incarnatum (Desm.) Sacc. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Fusarium oxysporum Schltdl. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Leaves and roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| Fusarium poae (Peck) Wollenw. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Fusarium solani (Mart.) Sacc. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Fusarium spp. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | China: Hong Kong; Netherlands: Zeeland | [39,40,41] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve | [59] | |
| Gibberella sp. | – | Saprobic | Spartina alterniflora | Argentina: Buenos Aires | [36] |
| Nectria sp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Tubercularia pulverulenta Speg. | – | – | Sarcocornia perennis | – | [35] |
| – | – | Salicornia europaea | – | [35] | |
| – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [58] | |
| – | – | Sarcocornia fruticosa | – | [35] | |
| Tubercularia sp. | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] |
| Volutella ciliata (Alb. and Schwein.) Fr. | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Sarocladiaceae | |||||
| Sarocladium implicatum (J.C. Gilman and E.V. Abbott) A. Giraldo, Gené and Guarro | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Sarocladium sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Stachybotryaceae | |||||
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Paramyrothecium roridum (Tode) L. Lombard and Crous | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stachybotrys chartarum (Ehrenb.) S. Hughes | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stachybotrys cylindrosporus C.N. Jensen | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stachybotrys echinatus (Rivolta) G. Sm. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stachybotrys kampalensis Hansf. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stachybotrys nephrosporus Hansf. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stachybotrys spp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] | |
| Striaticonidium cinctum (Corda) L. Lombard and Crous | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Xepicula jollymannii (N.C. Preston) L. Lombard and Crous | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Hypocreales genera incertae sedis | |||||
| Cephalosporium spp. | Dead leaves/culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [61] |
| Lulworthiales | |||||
| Lulworthiaceae | |||||
| Cumulospora marina I. Schmidt | Dead culm | Saprobic | Phragmites australis | Iraq, Egypt, Germany, Thailand | [129] |
| – | – | Spartina spp. | – | [32] | |
| Halazoon fuscus (I. Schmidt) Abdel-Wahab, K.L. Pang, Nagah., Abdel-Aziz and E.B.G. Jones | Decaying rhizomes | Saprobic | Phragmites australis | France, Germany, Japan | [35,130] |
| Rhizomes and culms | Saprobic | Phragmites sp. | Sweden | [87] | |
| Halazoon melhae Abdel-Aziz, Abdel-Wahab and Nagahama | Decaying stem | Saprobic | Phragmites australis | Egypt: Port Said | [130] |
| Lulworthia floridana Meyers | – | Saprobic | Spartina alterniflora | USA: North Carolina, Rhode Island | [20,131] |
| Lulworthia medusa (Ellis and Everh.) Cribb and J.W. Cribb | – | – | Elymus pungens | UK | [38] |
| – | Saprobic | Spartina cynosuroides | USA: New Jersey | [89,132] | |
| – | Spartina spp. | USA: New Jersey | [32,89] | ||
| Stems | Saprobic | Spartina townsendii | UK: England (Wales); USA: Virginia, North Carolina, South Carolina, Florida, Texas | [38,49,71,72,89,132,133,134] | |
| Lulworthia spp. | – | – | Elymus pungens | – | [35] |
| – | – | Juncus roemerianus | – | [35,36] | |
| Dead culms | Saprobic | Spartina alterniflora | Argentina: Buenos Aires; USA: Rhode Island, North Carolina | [35,36,61,73,74] | |
| – | – | Spartina cynosuroides | – | [35] | |
| – | Saprobic | Spartina sp. | Argentina: Buenos Aires; Canada; USA: Maine, North Carolina | [36] | |
| – | – | Spartina townsendii | – | [35] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59,63] | |
| Moleospora maritima Abdel-Wahab, Abdel-Aziz and Nagah. | Decayed stems | Saprobic | Phragmites australis | Egypt: Port Said | [130] |
| Magnaporthales | |||||
| Ceratosphaeriaceae | |||||
| Ceratosphaeria sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Magnaporthaceae | |||||
| Buergenerula spartinae Kohlm. and R.V. Gessner | Lower stem and leaf sheath during the growth phase of the plant/living and dead; decaying leaf blades | Saprobic, parasitic | Spartina alterniflora | USA: Alabama, Rhode Island, Maine, New Hampshire, Connecticut, Mississippi, New Jersey, Virginia, North Carolina, Florida, Georgia | [20,35,36,55,56,58,61,73,74,82,92] |
| Leaves | Saprobic | Spartina spp. | Canada: Bay of Fundy; USA: South Carolina; UK | [32,35,36,48,65] this study | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59] | |
| Gaeumannomyces sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Kohlmeyeriopsis medullaris (Kohlm., Volkm.-Kohlm. and O.E. Erikss.) Klaubauf, M.-H. Lebrun and Crous | Lower parts of senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [97,135] |
| Utrechtiana roumeguerei (Cavara) Videira and Crous | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Pseudohalonectriaceae | |||||
| Pseudohalonectria falcata Shearer | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Pseudohalonectria halophila Kohlm. and Volkm.-Kohlm. | Fragments of leaves and culms in the wrack | Saprobic | Juncus roemerianus | USA: North Carolina | [105] |
| Meliolales | |||||
| Meliolaceae | |||||
| Meliola arundinis Pat. | – | – | Phragmites australis | Australia: Queensland | [62] |
| Microascales | |||||
| Halosphaeriaceae | |||||
| Aniptodera chesapeakensis Shearer and M.A. Mill. | Dead leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [35] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| – | – | Spartina alterniflora | – | [35] | |
| – | – | Spartina spp. | – | [32] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Alentejo, Algarve, Centro | [59,63] | |
| Aniptodera juncicola Volkm.-Kohlm. and Kohlm. | Dead standing culms of | Saprobic | Juncus roemerianus | India: Kerala, West Bengal, Tamil Nadu; USA: North Carolina | [52,136] |
| Aniptodera phragmiticola O. K. Poon et K. D. Hyde | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Ceriosporopsis halima Linde | – | – | Arundo donax | – | [35] |
| Submerged seeds | Saprobic | Spartina alterniflora | USA | [137] | |
| – | – | Spartina spp. | – | [32] | |
| – | – | Spartina townsendii | UK | [35,38] | |
| Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] | |
| Cirrenalia macrocephala (Kohlm.) Meyers and R.T. Moore | – | – | Ammophila arenaria | – | [35] |
| Decaying culms | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Decomposing culms, submerged seeds | Saprobic | Spartina alterniflora | USA: Rhode Island | [35,61,137] | |
| – | – | Spartina spp. | – | [32] | |
| Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] | |
| Cirrenalia pseudomacrocephala Kohlm. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Corollospora maritima Werderm. | Submerged seeds, decomposing culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [20,35,61,137] |
| – | – | Spartina spp. | – | [32] | |
| Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] | |
| – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [58] | |
| Corollospora ramulosa (Meyers and Kohlm.) E.B.G. Jones and Abdel-Wahab | – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [58] |
| – | Saprobic | Zostera marina | USA: North Carolina | [74] | |
| Haligena elaterophora Kohlm. | – | – | Spartina alterniflora | – | [35] |
| – | – | Spartina tonwsendii | UK | [38] | |
| – | – | Spartina spp. | – | [32] | |
| Halosarpheia culmiperda Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower parts of senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [97] |
| Halosarpheia sp. | Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] |
| Halosarpheia viscosa I. Schmidt ex Shearer and J.L. Crane | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] |
| – | Saprobic | Spartina maritima | Portugal: Lisbon | [54] | |
| Halosphaeria appendiculata Linder | – | – | Arundo donax | – | [35] |
| Halosphaeria sp. | Submerged seeds | Saprobic | Spartina alterniflora | USA | [137] |
| Lautisporopsis circumvestita (Kohlm.) E.B.G. Jones, Yusoff and S.T. Moss | – | – | Arundo donax | – | [35] |
| Lignincola laevis Höhnk | – | – | Elymus pungens | – | [35] |
| – | Saprobic | Spartina spp. | USA: North Carolina | [32,138] | |
| – | – | Spartina townsendii | – | [35] | |
| Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] | |
| Magnisphaera spartinae (E.B.G. Jones) J. Campb., J.L. Anderson and Shearer | – | – | Elymus farctus | – | [35] |
| – | – | Elymus pungens | – | [35] | |
| Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] | |
| – | Saprobic | Spartina alterniflora | USA: Rhode Island | [20,35,61] | |
| – | Spartina spp. | [32] | |||
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| Stem | Saprobic | Spartina townsendii | UK: Wales | [35,139] | |
| – | Typha sp. | [35] | |||
| Nais inornata Kohlm. | Decomposing culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [20,35,61] |
| Spartina spp. | [32] | ||||
| Natantispora unipolaris K.L. Pang, S.Y. Guo and E.B.G. Jones | Dead stem | Saprobic | Phragmites australis | Taiwan: Nankunshen | [140] |
| Natantispora retorquens (Shearer and J.L. Crane) J. Campb., J.L. Anderson and Shearer | Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Alentejo, Lisbon, Algarve, Centro | [31,54,59,63] |
| Oceanitis unicaudata (E.B.G. Jones and Camp.-Als.) J. Dupont and E.B.G. Jones | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] | |
| Panorbis viscosus (I. Schmidt) J. Campb., J.L. Anderson and Shearer | Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Alentejo, Algarve | [59,63] |
| Remispora hamata (Höhnk) Kohlm. | – | – | Elymus pungens | UK | [35,38] |
| Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] | |
| Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] | |
| – | Saprobic | Phragmites sp. | Sweden | [87] | |
| Dead leaves | Saprobic | Spartina alterniflora | USA: Rhode Island, Maine, Florida | [20,35,36,61,73] | |
| – | Saprobic | Spartina patens | USA: Rhode Island | [36] | |
| – | Saprobic | Spartina sp. | USA: North Carolina; Argentina: Buenos Aires | [36,138] | |
| – | – | Spartina townsendii | – | [35] | |
| – | – | Typha sp. | – | [35] | |
| Remispora trullifera Kohlm. | Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Centro | [59] |
| Tirispora unicaudata E.B.G. Jones and Vrijmoed | Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] |
| Microascaceae | |||||
| Scopulariopsis spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Myrmecridiales | |||||
| Myrmecridiaceae | |||||
| Myrmecridium schulzeri (Sacc.) Arzanlou, W. Gams and Crous | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Ophiostomatales | |||||
| Ophiostomataceae | |||||
| Sporothrix sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Phomatosporales | |||||
| Phomatosporaceae | |||||
| Phomatospora bellaminuta Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower parts of senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [116] |
| Phomatospora berkeleyi Sacc. | Living/decomposing leaf blades and sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40,50] |
| Phomatospora dinemasporium J. Webster | Decaying stems and leaf sheaths, stems | Saprobic | Phragmites australis | China: Hong Kong; Netherlands: Zeeland | [40,41] |
| Dead leaves | Saprobic | Phragmites sp. | South Australia | [62] | |
| – | – | Spartina townsendii | UK | [38] | |
| Phomatospora phragmiticola Poon and K.D. Hyde | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Phomatospora spp. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] | |
| Phyllachorales | |||||
| Phyllachoraceae | |||||
| Phyllachora graminis (Pers.) Fuckel | – | – | Elymus pungens | UK | [38] |
| – | Saprobic, pathogenic | Spartina alterniflora | USA: Massachusetts | [44] | |
| – | – | Spartina cynosuroides | – | [68] | |
| Phyllachora cynodontis Niessl. | – | Saprobic, pathogenic | Spartina alterniflora | USA | [68] |
| – | Saprobic, pathogenic | Spartina foliosa | USA: California | [44,112,141] | |
| Phyllachora paludicola Kohlm. and Volkm.-Kohlm. | Dead leaves (lower half of standing culms) | Saprobic | Spartina alterniflora | USA: Florida, Georgia, North Carolina, Maryland, Delaware | [142] |
| Phyllachora sylvatica Sacc. and Speg. | – | Saprobic | Spartina patens | USA: South Carolina | [141] |
| Savoryellales | |||||
| Savoryellaceae | |||||
| Savoryella paucispora (Cribb and J.W. Cribb) J. Koch | – | – | Elymus pungens | – | [35] |
| – | – | Juncus roemerianus | – | [35] | |
| – | – | Spartina alterniflora | – | [35] | |
| – | – | Spartina sp. | – | [35] | |
| – | – | Spartina townsendii | – | [35] | |
| Sordariales | |||||
| Chaetomiaceae | |||||
| Achaetomium sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Chaetomium elatum Kunze | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsendii | UK | [38] | |
| – | – | ||||
| Chaetomium globosum Kunze | – | – | Elymus pungens | UK | [38] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsendii | UK | [38] | |
| Chaetomium spirale Zopf | – | – | Elymus pungens | UK | [38] |
| Chaetomium thermophilum La Touche | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsendii | UK | [38] | |
| Chaetomium sp. | Stem | Saprobic | Typha sp. | UK | This study |
| Corynascus sepedonium (C.W. Emmons) Arx | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsendii | UK | [38] | |
| Dichotomopilus funicola (Cooke) X.Wei Wang and Samson | – | – | Elymus pungens | UK | [38] |
| – | – | Spartina alterniflora | USA: Rhode Island | [61] | |
| – | – | Spartina townsendii | UK | [38] | |
| Dichotomopilus indicus (Corda) X.Wei Wang and Samson | – | – | Elymus pungens | UK | [38] |
| Humicola sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] | |
| Thermothielavioides terrestris (Apinis) X. Wei Wang and Houbraken | – | – | Elymus pungens | UK | [38] |
| – | – | Puccinellia maritima | UK | [38] | |
| Trichocladium constrictum I. Schmidt | Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] |
| Trichocladium crispatum (Fuckel) X. Wei Wang and Houbraken | – | – | Elymus pungens | UK | [38] |
| – | – | Spartina townsendii | UK | [38] | |
| Lasiosphaeriaceae | – | – | |||
| Schizothecium hispidulum (Speg.) N. Lundq. | Living/decomposing leaf sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39] |
| Zopfiella latipes (N. Lundq.) Malloch and Cain | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Sordariaceae | |||||
| Neurospora calospora (Mouton) Dania García, Stchigel and Guarro | – | – | Elymus pungens | UK | [38] |
| Sordaria fimicola (Roberge ex Desm.) Ces. and De Not. | – | – | Elymus pungens | UK | [38] |
| Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] | |
| – | – | Puccinellia maritima | UK | [38] | |
| – | – | Spartina townsendii | UK | [38] | |
| Sordariomycetes families incertae sedis | |||||
| Koorchaloma galateae Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [117] |
| Koorchaloma spartinicola V.V. Sarma, S.Y. Newell and K.D. Hyde | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] |
| Koorchaloma sp. | Decaying leaf blades | Saprobic | Spartina alterniflora | USA: Georgia | [56] |
| Lautospora simillima Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower parts of senescent, soft culms | Saprobic | Juncus roemerianus | USA: North Carolina | [78] |
| Sordariomycetes genera incertae sedis | |||||
| Aquamarina speciosa Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms | Juncus roemerianus | USA: Georgia, North Carolina, Virginia | [77] | |
| Aropsiclus junci (Kohlm. and Volkm.-Kohlm.) Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [143] |
| Zalerion maritima (Linder) Anastasiou | Basal area of the sheath | Saprobic | Spartina densiflora | Argentina: Buenos Aires | [64] |
| – | – | Spartina spp. | – | [32] | |
| Ellisembia sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Torpedosporales | |||||
| Juncigenaceae | |||||
| Juncigena adarca Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [76] |
| Moheitospora adarca (Kohlm., Volkm.-Kohlm. and O.E. Erikss.) Abdel-Wahab, Abdel-Aziz and Nagah | Stems | Saprobic | Juncus roemerianus | USA | [130] |
| Moheitospora fruticosae Abdel-Wahab, Abdel-Aziz and Nagah. | Decayed stems | Saprobic | Suaeda vermiculata | Egypt: Alexandria | [130] |
| Torpedospora radiata Meyers | – | Saprobic | Unidentified saltmarsh plants | USA: Mississippi | [58] |
| Tracyllalales | |||||
| Tracyllaceae | |||||
| Tracylla spartinae (Peck) Tassi | – | Saprobic, pathogenic | Spartina patens | USA: Mississippi | [44,68] |
| Xylariales | |||||
| Diatrypaceae | |||||
| Cryptovalsa suaedicola Spooner | Dead twigs | Saprobic | Suaeda vermiculata | UK: Great Britain | [144] |
| Halocryptovalsa salicorniae Dayar. and K.D. Hyde | Dead stem | Saprobic | Salicornia sp. | Thailand: Prachuap Khiri Khan | [145] |
| Xylariaceae | |||||
| Anthostomella atroalba Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [60] |
| Anthostomella lugubris (Roberge ex Desm.) Sacc. | – | – | Elymus pungens | UK | [38] |
| Anthostomella phaeosticta (Berk.) Sacc. | – | – | Elymus pungens | UK | [38] |
| Anthostomella poecila Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Lower and upper parts of senescent culms, decaying leaves | Saprobic | Juncus roemerianus | USA: Alabama, Mississippi, North Carolina | [55,58,116] |
| Anthostomella punctulata (Roberge ex Desm.) Sacc. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Anthostomella semitecta Kohlm., Volkm.-Kohlm. and O.E. Erikss. | Senescent culms | – | Juncus roemerianus | USA: North Carolina | [116] |
| Anthostomella spissitecta Kohlm. and Volkm.-Kohlm. | Leaf sheaths of senescent culms | Saprobic | Spartina alterniflora, S. densiflora. | USA: Connecticut, Florida, North Carolina, Rhode Island; Argentina: Buenos Aires | [32] |
| – | – | Spartina sp. | – | [32] | |
| Leaf sheaths and blades, stem | Saprobic | Spartina maritima | Portugal: Algarve | [59] | |
| Anthostomella spp. | – | – | Elymus pungens | UK | [38] |
| – | Saprobic | Spartina alterniflora | USA: Connecticut, Florida, North Carolina, Rhode Island; Argentina | [36,61] | |
| – | – | Spartina townsendii | UK | [38] | |
| Anthostomella torosa Kohlm. and Volkm.-Kohlm. | Senescent culms (restricted to short culms) | Saprobic | Juncus roemerianus | USA: North Carolina | [32] |
| Geniculosporium sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Rosellinia sp. | Dead leaves/culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [61] |
| Virgaria nigra (Link) Nees | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Zygosporiaceae | |||||
| Zygosporium gibbum (Sacc., M. Rousseau and E. Bommer) S. Hughes | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Zygosporium masonii S. Hughes | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Zygosporium sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Xylariales genera incertae sedis | |||||
| Circinotrichum maculiforme Nees | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Xylariomycetidae family incertae sedis | |||||
| Cainiaceae | |||||
| Atrotorquata lineata Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [104] |
| Saprobic | Unidentified saltmarsh plant | USA: Mississippi | [58] | ||
| Ascomycota genera incertae sedis | |||||
| Asteromyces cruciatus C. Moreau and Moreau ex Hennebert | – | – | Agropyron sp. | – | [35] |
| – | – | Ammophila arenaria | – | [35] | |
| – | – | Spartina spp. | – | [32,35] | |
| – | Saprobic | Zostera sp. | USA: California | [74] | |
| Cremasteria cymatilis Meyers and R.T. Moore Nomen dubium | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Cytoplacosphaeria phragmiticola Poon and K.D. Hyde | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Cytoplacosphaeria rimosa (Oudem.) Petr. | Living/decomposing leaf sheaths, stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| Cytosporina sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Didymosamarospora euryhalina T.W. Johnson and H.S. Gold | Culms | Saprobic | Juncus roemerianus | USA: North Carolina | [146] |
| Haplobasidion lelebae Sawada ex M.B. Ellis | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Hymenopsis chlorothrix Kohlm. and Volkm.-Kohlm. | Senescent culms | Saprobic | Juncus roemerianus | USA: North Carolina | [147] |
| Hyphopolynema juncatile Kohlm. and Volkm.-Kohlm. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [148] |
| Kolletes undulatus Kohlm. and Volkm.-Kohlm. | Senescent leaves and culms | Saprobic | Juncus roemerianus | USA: North Carolina | [105] |
| Minimidochium parvum Cabello, Aramb. and Cazau | Leaves | Saprobic | Distichlis spicata | Argentina: Buenos Aires | [47] |
| Monodictys pelagica (T. Johnson) E.B.G. Jones | – | – | Juncus sp. | – | [35] |
| Decomposing culms | Saprobic | Spartina alterniflora | USA: Rhode Island | [20,35,61,73] | |
| – | – | Spartina spp. | – | [32] | |
| Neottiospora sp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Octopodotus stupendus Kohlm. and Volkm.-Kohlm. | Dead leaves (lower half of standing culms) | Saprobic | Spartina alterniflora | USA: North Carolina | [142] |
| Pycnodallia dupla Kohlm. and Volkm.-Kohlm. | Senescent inflorescences (involucral leaves and branchlets) | Saprobic | Juncus roemerianus | USA: North Carolina | [147] |
| Sphaeronaema sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Stauronema sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Tetranacriella papillata Kohlm. and Volkm.-Kohlm. | Senescent leaves | Saprobic | Juncus roemerianus | USA: North Carolina | [117] |
| Tetranacrium sp. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| Zythia spp. | Living, senescent, and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Psammina sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| BASIDIOMYCOTA | |||||
| AGARICOMYCETES | |||||
| Agaricales | |||||
| Niaceae | |||||
| Merismodes bresadolae (Grelet) Singer | Living/decomposing stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [40] |
| Nia globispora Barata and Basilio | Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] |
| Nia vibrissa R.T. Moore and Meyers | Old stem | Saprobic | Spartina alterniflora | USA: North Carolina | [35,149] |
| – | Saprobic | Spartina spp. | USA: North Carolina | [32,150] | |
| Stem | Saprobic | Spartina maritima | Portugal: Alentejo | [63] | |
| AGARICOSTILBOMYCETES | |||||
| Agaricostilbales | |||||
| Chionosphaeraceae | |||||
| Stilbum sp. | Decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| BARTHELETIOMYCETES | |||||
| Sebacinales | |||||
| Sebacinaceae | |||||
| Chaetospermum camelliae Agnihothr. | Decaying stems and leaf sheaths | Saprobic | Phragmites australis | China: Hong Kong | [41] |
| MICROBOTRYOMYCETES | |||||
| Sporidiobolales | |||||
| Sporidiobolaceae | |||||
| Sporobolomyces roseus Kluyver and C.B. Niel | Leaves | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] |
| Sporobolomyces spp. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| PUCCINIOMYCETES | |||||
| Pucciniales | |||||
| Pucciniaceae | |||||
| Puccinia distichlidis Ellis and Everh. | – | – | Distichlis spicata | USA | [151] |
| Puccinia magnusiana Körn. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Puccinia phragmitis (Schumach.) Tul. | Living/decomposing leaf blades and sheaths | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,50] |
| Puccinia sparganioidis Ellis and Barthol. | – | Saprobic, parasitic | Spartina alterniflora | USA: Maine, New Hampshire, Massachusetts, Rhode Island, Delaware, Virginia, North Carolina, Florida, Mississippi | [36,44,68,73,152] |
| – | – | Spartina cynosuroides | USA: New Jersey, Delaware, Maryland, South Carolina, Florida, Louisiana | [44,68,153] | |
| – | Saprobic, pathogenic | Spartina patens | USA: Connecticut, Maryland, New Jersey, New York | [44,68,153] | |
| Uromyces acuminatus Arthur | – | Saprobic, pathogenic | Spartina alterniflora | USA: Maine, New Hampshire, Massachusetts, Connecticut, New York, New Jersey, Delaware, Maryland, Florida | [44,68,152] |
| – | Saprobic, pathogenic | Spartina cynosuroides | USA: Florida | [44,68,153] | |
| – | Saprobic | Spartina patens | USA: Connecticut, Delaware, Florida, Maine, Maryland, Massachusetts, New Hampshire, New Jersey, | [44,68] | |
| Uromyces argutus F. Kern | – | Saprobic, pathogenic | Spartina alterniflora | France; USA: Florida | [44,68,152] |
| Uromyces salicorniae (DC.) de Bary | – | – | Salicornia sp. | South Australia | [95] |
| Pucciniales genera incertae sedis | |||||
| Aecidium suaedae Thüm. | Leaves | – | Suaeda verae | Egypt | [154] |
| TREMELLOMYCETES | |||||
| Tremellales | |||||
| Tremellaceae | |||||
| Tremella spicifera Van Ryck., Van de Put and P. Roberts | Living/decomposing leaf sheaths and stems | Saprobic | Phragmites australis | Netherlands: Zeeland | [39,40] |
| USTILAGINOMYCETES | |||||
| Ustilaginales | |||||
| Ustilaginaceae | |||||
| Tranzscheliella distichlidis (McAlpine) Vánky | – | Pathogenic | Distichlis spicata | Australia: Victoria | [155] |
| Ustilaginales genera incertae sedis | |||||
| Parvulago marina (Durieu) R. Bauer, M. Lutz, Piątek, Vánky and Oberw. | – | – | Eleocharis parvula | Finland, France, Germany, UK, Norway, Sweden | [156] |
| Urocystidales | |||||
| Urocystidaceae | |||||
| Flamingomyces ruppiae (Feldmann) R. Bauer, M. Lutz, Piątek, Vánky and Oberw. | – | Parasitic | Ruppia maritima | France | [156] |
| MUCOROMYCOTA | |||||
| MUCOROMYCETES | |||||
| Mucorales | |||||
| Choanephoraceae | |||||
| Blakeslea trispora Thaxt. | Senescent and decaying leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Mucoraceae | |||||
| Mucor sp. | Senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
| Roots | Saprobic | Spartina sp. | Canada: Bay of Fundy | [48] | |
| Rhizopodaceae | |||||
| Rhizopus stolonifer (Ehrenb.) Vuill. | Stems | Saprobic | Spartina townsendii | UK: England | [49] |
| Syncephalastraceae | |||||
| Syncephalastrum racemosum Cohn ex J. Schröt. | Living and senescent leaves | Saprobic | Juncus roemerianus | USA: Florida | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabon, M.S.; Jones, E.B.G.; Promputtha, I.; Hyde, K.D. Fungal Biodiversity in Salt Marsh Ecosystems. J. Fungi 2021, 7, 648. https://doi.org/10.3390/jof7080648
Calabon MS, Jones EBG, Promputtha I, Hyde KD. Fungal Biodiversity in Salt Marsh Ecosystems. Journal of Fungi. 2021; 7(8):648. https://doi.org/10.3390/jof7080648
Chicago/Turabian StyleCalabon, Mark S., E. B. Gareth Jones, Itthayakorn Promputtha, and Kevin D. Hyde. 2021. "Fungal Biodiversity in Salt Marsh Ecosystems" Journal of Fungi 7, no. 8: 648. https://doi.org/10.3390/jof7080648
APA StyleCalabon, M. S., Jones, E. B. G., Promputtha, I., & Hyde, K. D. (2021). Fungal Biodiversity in Salt Marsh Ecosystems. Journal of Fungi, 7(8), 648. https://doi.org/10.3390/jof7080648







