Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extract from Fungal Material Preparation
2.3. Animals and Drug Administration
2.4. Behavioral Tasks for Cognitive Performance
2.4.1. Novel Tank Diving Test (NTT)
2.4.2. Y-Maze Test
2.4.3. Novel Object Recognition Test (NOR)
2.5. Biochemical Analysis
2.5.1. Acetylcholinesterase Activity
2.5.2. Superoxide Dismutase
2.5.3. Catalase
2.5.4. Glutathione Peroxidase
2.5.5. Glutathione
2.5.6. Malondialdehyde
2.5.7. Carbonylated Proteins
2.6. Statistical Analysis
3. Results
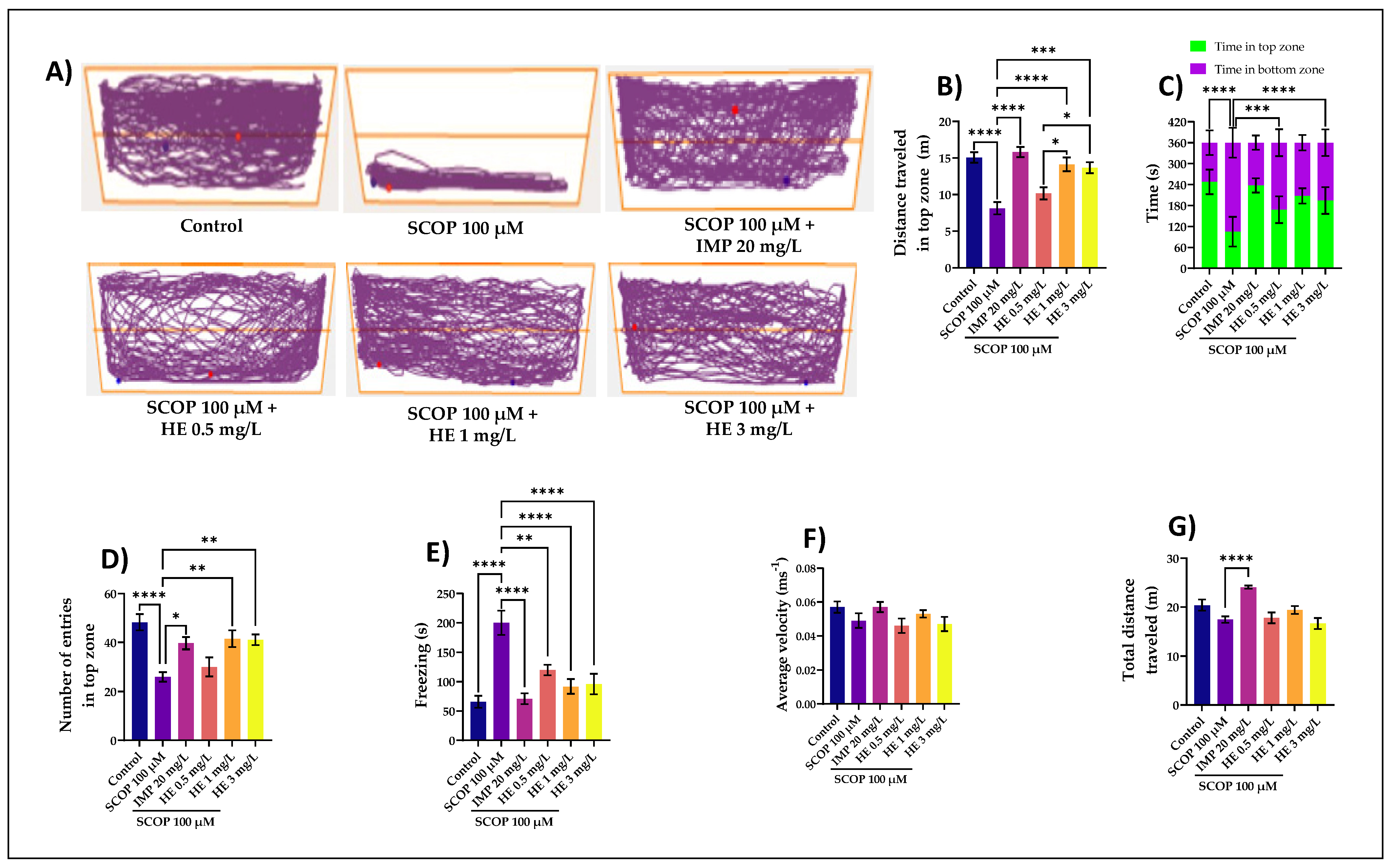
3.1. Effects of H. erinaceus Extracts on Anxiety in NTT Task
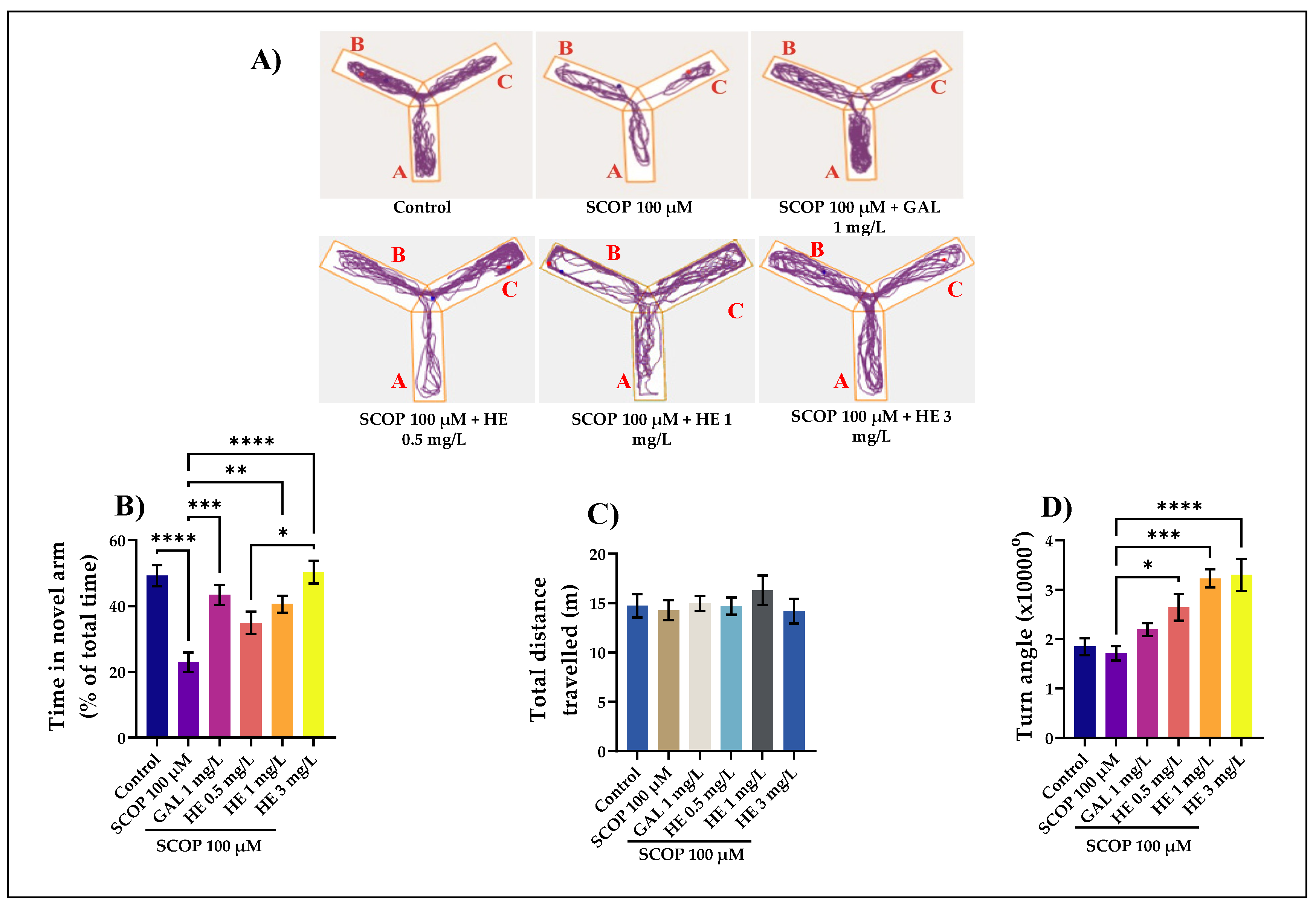
3.2. The Effects of H. erinaceus Extracts on Cognition in Y-Maze Task
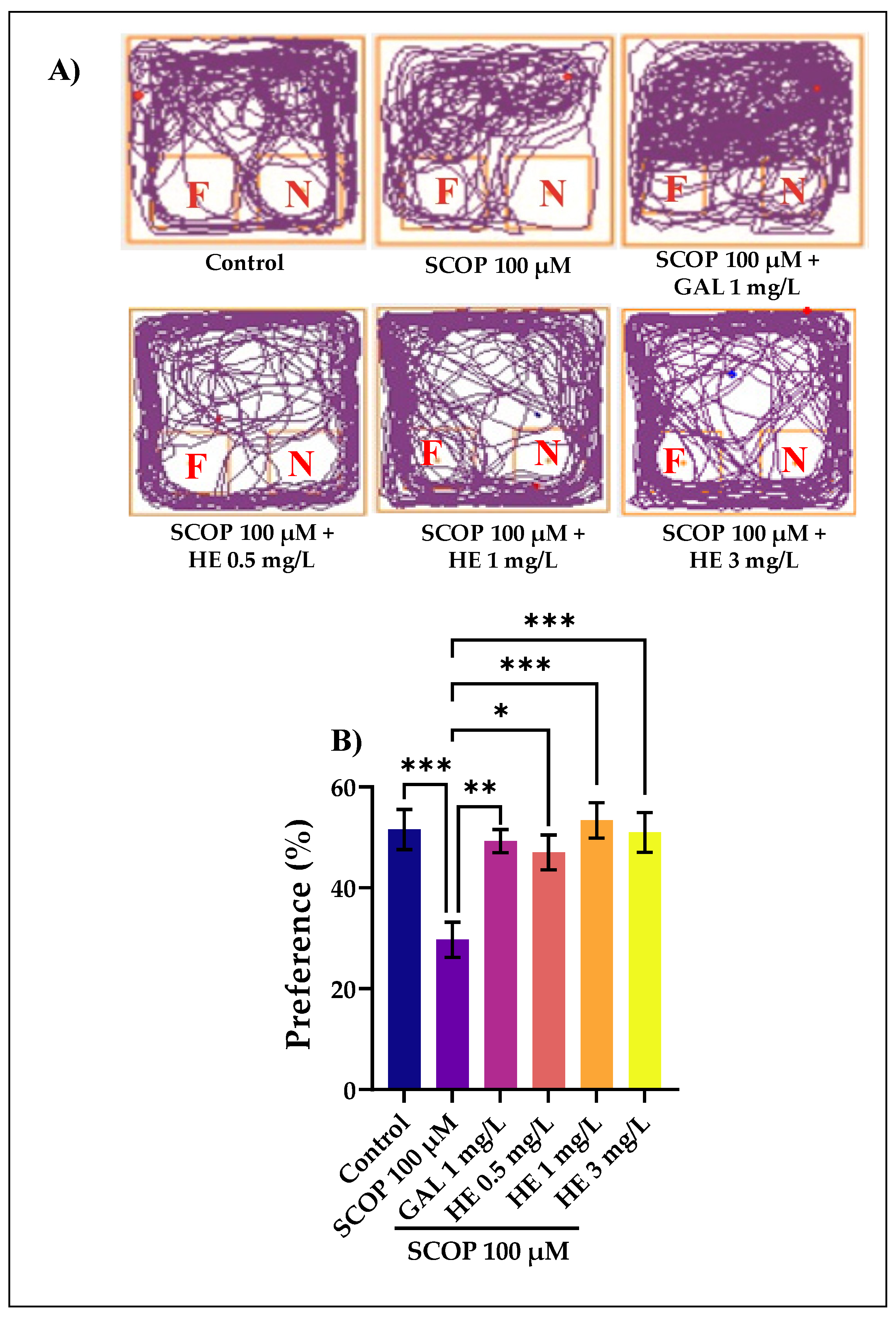
3.3. The Effects of H. erinaceus Extracts on Recognition Memory in NOR Test
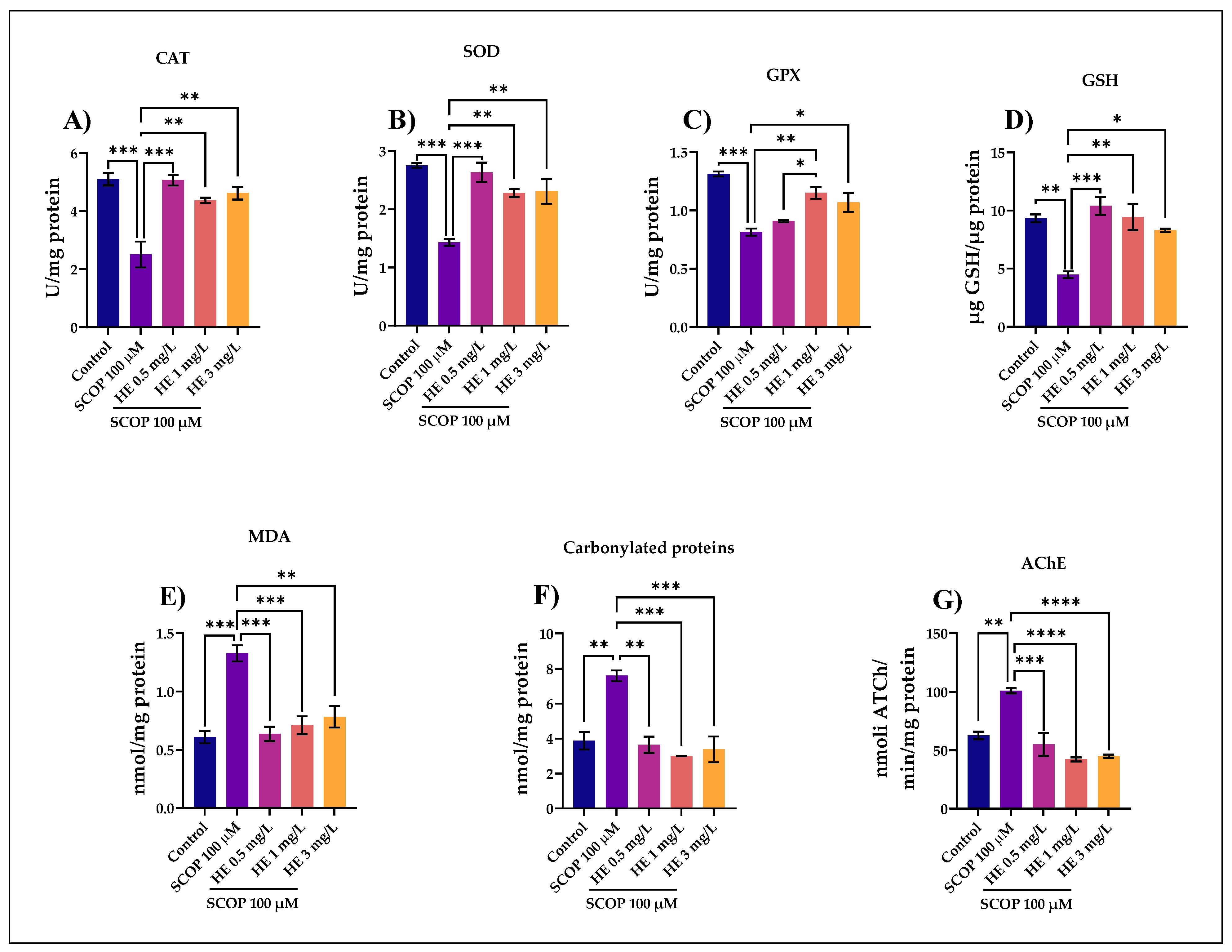
3.4. Biochemical Parameters Assay in the Brain Tissue
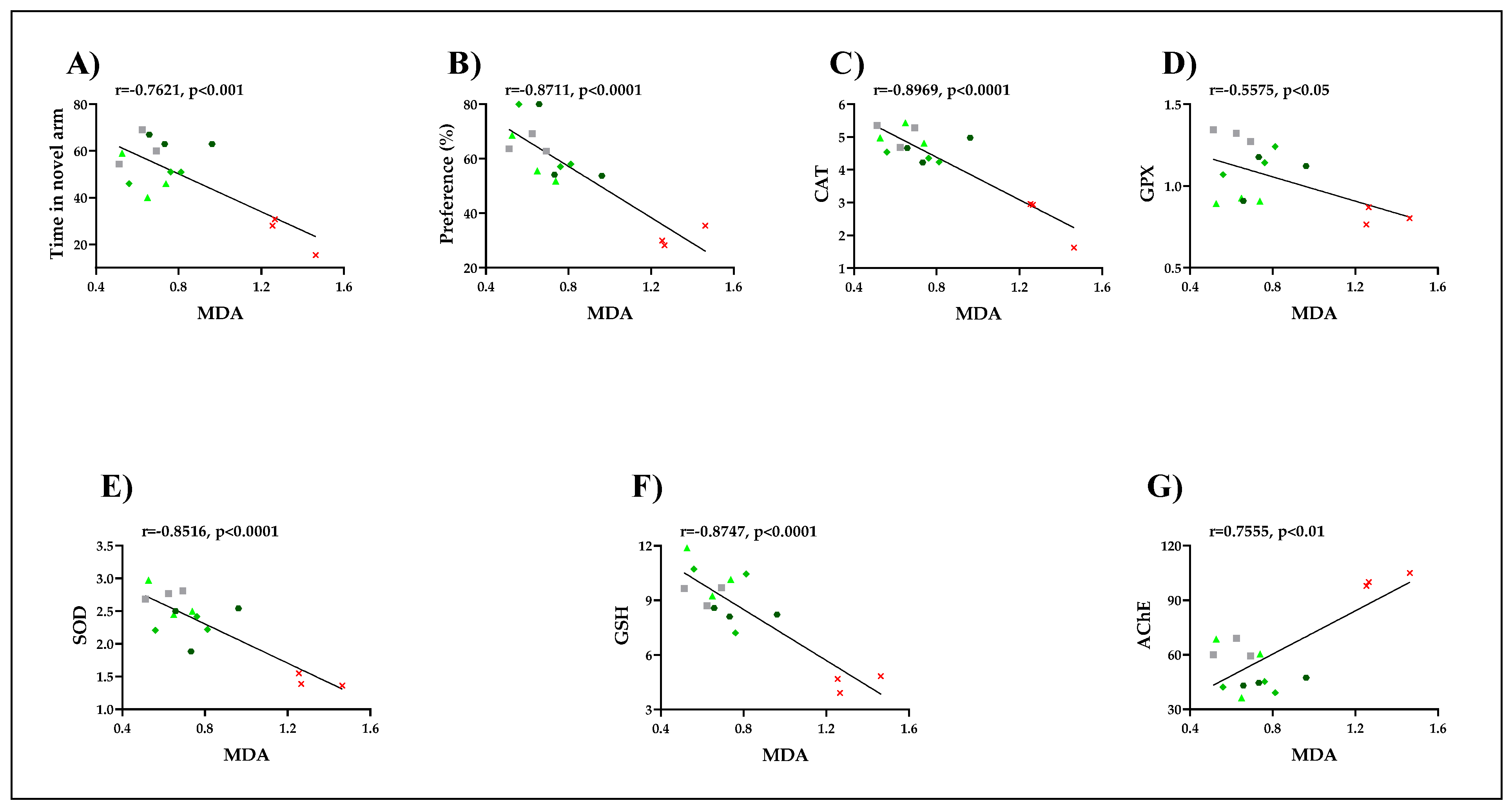
3.5. Pearson Correlations between Behavioral and Biochemical Parameters
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmquist, L.; Stuchbury, G.; Berbaum, K.; Muscat, S.; Young, S.; Hager, K.; Engel, J.; Münch, G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol. Ther. 2007, 113. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.K.; Kaul, C.L.; Ramarao, P. Dementia of Alzheimer’s disease and other neurodegenerative disorders—memantine, a new hope. Pharm. Res. 2005, 51. [Google Scholar] [CrossRef]
- Ionita, R.; Valu, V.M.; Postu, P.A.; Cioanca, O.; Hritcu, L.; Mihasan, M. 6-Hydroxy-l-nicotine effects on anxiety and depression in a rat model of chlorisondamine. Farmacia 2017, 65, 237–240. [Google Scholar]
- García-Borreguero, D.; Allen, R.P.; Kohnen, R.; Högl, B.; Trenkwalder, C.; Oertel, W.; Hening, W.A.; Paulus, W.; Rye, D.; Walters, A.; et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: Report from a world association of sleep medicine—International restless legs syndrome study group consensus conference at the max planck institute. Sleep Med. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Twamley, E.W. Cognitive rehabilitation therapies for Alzheimer’s disease: A review of methods to improve treatment engagement and self-efficacy. Neuropsychol. Rev. 2013, 23. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Jakaria, M.; Mathew, B.; Barreto, G.E.; Ashraf, G.M. Nootropic and anti-Alzheimer’s actions of medicinal plants: Molecular insight into therapeutic potential to alleviate Alzheimer’s neuropathology. Mol. Neurobiol. 2019, 56. [Google Scholar] [CrossRef]
- Sabaratnam, V.; Kah-Hui, W.; Naidu, M.; David, P.R. Neuronal health—Can culinary and medicinal mushrooms help? J. Tradit. Complementary Med. 2013, 3. [Google Scholar] [CrossRef]
- Tibuhwa, D.D. Wild mushroom—An underutilized healthy food resource and income generator: Experience from Tanzania rural areas. J. Ethnobiol. Ethnomedicine 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, FUNK-0052-2016. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015. [Google Scholar] [CrossRef]
- Schwartz, B.; Hadar, Y. Possible mechanisms of action of mushroom-derived glucans on inflammatory bowel disease and associated cancer. Ann. Transl. Med. 2014, 2. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Heather, L. Zwickey immune modulation from five major mushrooms: Application to integrative oncology. Integr. Med. Encinitas 2014, 13, 32–44. [Google Scholar]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18. [Google Scholar] [CrossRef]
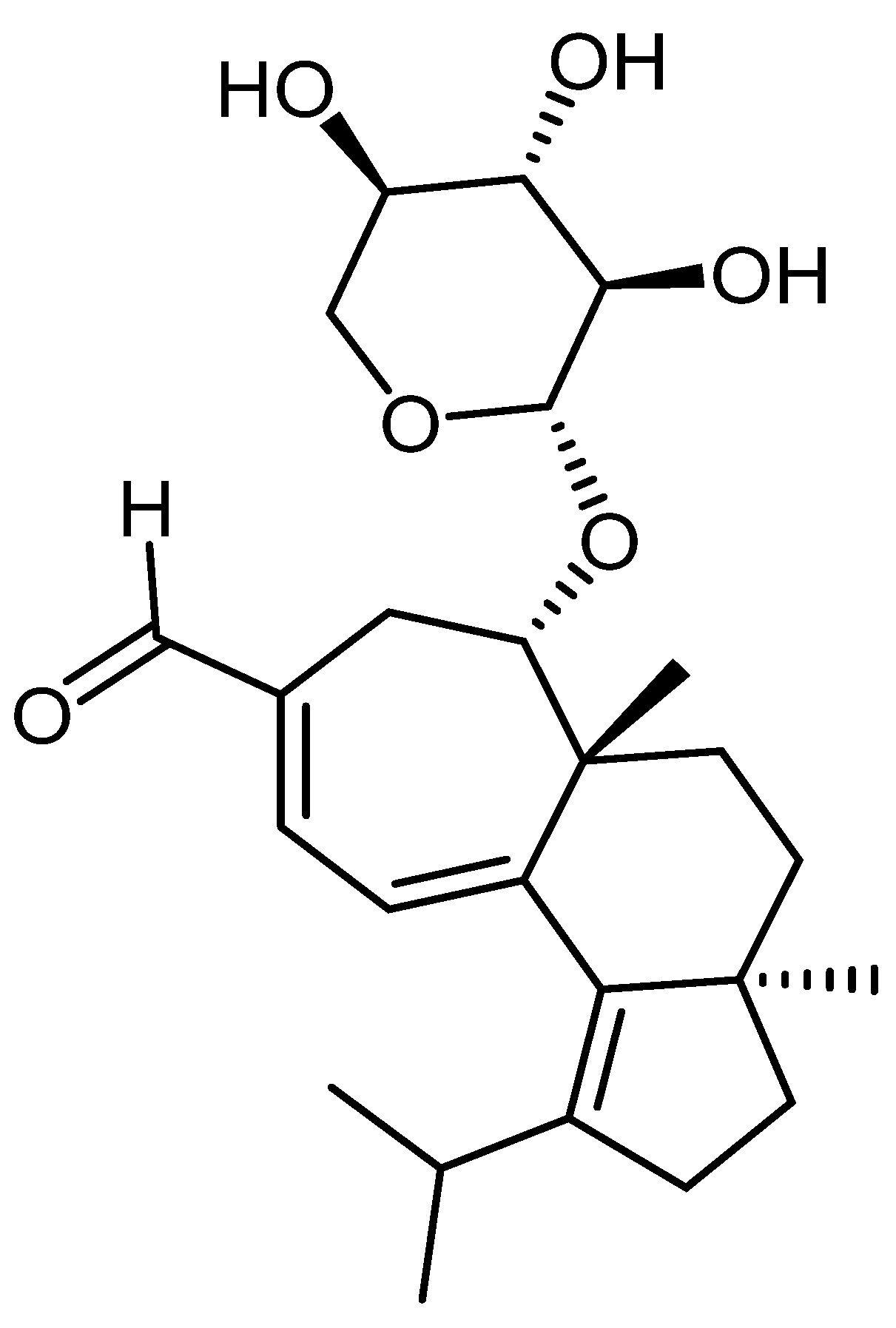
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom hericium erinaceus and the rare species, hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of INOS/P38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef]
- Li, I.-C.; Chang, H.-H.; Lin, C.-H.; Chen, W.-P.; Lu, T.-H.; Lee, L.-Y.; Chen, Y.-W.; Chen, Y.-P.; Chen, C.-C.; Lin, D.P.-C. Prevention of early Alzheimer’s disease by erinacine A-enriched hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. chemical constituents from hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorganic Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.-W.; Lee, G.-S.; Hong, S.-L.; Wong, Y.-T.; Brkljača, R.; Urban, S.; Abd Malek, S.N.; Sabaratnam, V. hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014, 5. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Tian, J.M.; Wei, J.; Gao, J.M. Bioactive metabolites from the mycelia of the basidiomycete hericium erinaceum. Nat. Prod. Res. 2014, 28, 1288–1292. [Google Scholar] [CrossRef]
- Ma, B.-J.; Shen, J.-W.; Yu, H.-Y.; Ruan, Y.; Wu, T.-T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium Erinaceus. Mycology 2010, 1. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of hericium erinaceus on amyloid β(25-35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011, 32. [Google Scholar] [CrossRef]
- Li, I.-C.; Lee, L.-Y.; Tzeng, T.-T.; Chen, W.-P.; Chen, Y.-P.; Shiao, Y.-J.; Chen, C.-C. Neurohealth properties of Hericium Erinaceus mycelia enriched with erinacines. Behav. Neurol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Santo, G.D.; Grotto, A.; Boligon, A.A.; da Costa, B.; Rambo, C.L.; Fantini, E.A.; Sauer, E.; Lazzarotto, L.M.V.; Bertoncello, K.T.; Júnior, O.T.; et al. Protective effect of Uncaria tomentosa extract against oxidative stress and genotoxicity induced by glyphosate-roundup® using zebrafish (Danio rerio) as a model. Environ. Sci. Pollut. Res. 2018, 25. [Google Scholar] [CrossRef]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of ultrasonic extraction to obtain erinacine a and polyphenols with antioxidant activity from the fungal biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Morlock, G.; Zorn, H. Production of cyathane type secondary metabolites by submerged cultures of Hericium erinaceus and evaluation of their antibacterial activity by direct bioautography. Fungal Biol. Biotechnol. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Hånell, A.; Marklund, N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Levin, E.D. Zebrafish assessment of cognitive improvement and anxiolysis: Filling the gap between in vitro and rodent models for drug development. Rev. Neurosci. 2011, 22, 75–84. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, J.; Lee, S. Exposure to copper (II) chloride induces behavioral and endocrine changes in zebrafish. J. Life Sci. 2020, 30, 321–330. [Google Scholar] [CrossRef]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Gee, N.R.; Reed, T.; Whiting, A.; Friedmann, E.; Snellgrove, D.; Sloman, K.A. Observing live fish improves perceptions of mood, relaxation and anxiety, but does not consistently alter heart rate or heart rate variability. Int. J. Environ. Res. Public Health 2019, 16, 3113. [Google Scholar] [CrossRef]
- Cognato, G.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze memory task in zebrafish (Danio rerio): The role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef]
- May, Z.; Morrill, A.; Holcombe, A.; Johnston, T.; Gallup, J.; Fouad, K.; Schalomon, M.; Hamilton, T.J. Object recognition memory in zebrafish. Behav. Brain Res. 2016, 296, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Gaspary, K.V.; Reolon, G.K.; Gusso, D.; Bonan, C.D. Novel object recognition and object location tasks in zebrafish: Influence of habituation and NMDA receptor antagonism. Neurobiol. Learn. Mem. 2018, 155, 249–260. [Google Scholar] [CrossRef]
- Lueptow, L.M. novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 2017, 55718. [Google Scholar] [CrossRef]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef]
- Faillace, M.; Pisera-Fuster, A.; Medrano, M.; Bejarano, A.; Bernabeu, R. Short- and long-term effects of nicotine and the histone deacetylase inhibitor phenylbutyrate on novel object recognition in zebrafish. Psychopharmacology 2017, 234, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The Novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Cheng, R.K.; Jesuthasan, S.J.; Penney, T.B. Zebrafish forebrain and temporal conditioning. Philos. Trans. Royal Soc. B Biol. Sci. 2014, 369, 20120462. [Google Scholar] [CrossRef]
- Soodi, M.; Naghdi, N.; Hajimehdipoor, H.; Choopani, S.; Sahraei, E. Memory-improving activity of melissa officinalis extract in naïve and scopolamine-treated rats. Res. Pharm. Sci. 2014, 9, 107–114. [Google Scholar]
- Lee, G.Y.; Lee, C.; Park, G.H.; Jang, J.H. Amelioration of scopolamine-induced learning and memory impairment by α -pinene in C57BL/6 Mice. Evid. Based Complementary Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Gorun, V.; Proinov, I.; Băltescu, V.; Balaban, G.; Bârzu, O. Modified ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal. Biochem. 1978, 86. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hawkins, R.; Brian, M.; Carrell, R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Massarsky, A.; Kozal, J.S.; di Giulio, R.T. Glutathione and zebrafish: Old assays to address a current issue. Chemosphere 2017, 168, 707–715. [Google Scholar] [CrossRef]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. Arab. Book 2011, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Wehr, N.B.; Levine, R.L. Quanti fication of protein carbonylation. Methods Mol. Biol. 2013, 965, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Starke-Reed, P.E.; Oliver, C.N. protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989, 275, 559–567. [Google Scholar] [CrossRef]
- Kysil, E.V.; Meshalkina, D.A.; Frick, E.E.; Echevarria, D.J.; Rosemberg, D.B.; Maximino, C.; Lima, M.G.; Abreu, M.S.; Giacomini, A.C.; Barcellos, L.J. Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 2017, 14, 197–208. [Google Scholar] [CrossRef]
- Fontana, B.D.; Gibbon, A.J.; Cleal, M.; Sudwarts, A.; Pritchett, D.; Miletto Petrazzini, M.E.; Brennan, C.H.; Parker, M.O. Moderate early life stress improves adult zebrafish (Danio rerio) working memory but does not affect social and anxiety-like responses. Dev. Psychobiol. 2021, 63, 54–64. [Google Scholar] [CrossRef]
- Imipramine—An Overview.ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/chemistry/imipramine (accessed on 2 May 2021).
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. Ameliorative effects of rhoifolin in scopolamine-induced amnesic zebrafish (Danio rerio) model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef]
- Dumitru, G.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A.N.B. Agathisflavone isolated from Schinus polygamus (Cav.) cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef]
- Ahmed, O.; Seguin, D.; Gerlai, R. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2011, 216, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.T.; Chen, C.C.; Chen, C.C.; Tsay, H.J.; Lee, L.Y.; Chen, W.P.; Shen, C.C.; Shiao, Y.J. The Cyanthin diterpenoid and sesterterpene constituents of hericium erinaceus mycelium ameliorate Alzheimer’s disease-related pathologies in APP/PS1 transgenic mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.V.; Fontana, B.D.; Ziani, P.R.; Müller, T.E.; Mezzomo, N.J.; Rosemberg, D.B. Exploring object discrimination in zebrafish: Behavioral performance and scopolamine-induced cognitive deficits at different retention intervals. Zebrafish 2019, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Magyary, I. Floating novel object recognition in adult zebrafish: A pilot study. Cogn. Process. 2019, 20, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Zhu, Y.; Carvey, P.M.; Ling, Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006, 1090, 35–44. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Chang, W.N.; Tsai, N.W.; Huang, C.C.; Kung, C.T.; Su, Y.J.; Lin, W.C.; Cheng, B.C.; Su, C.M.; Chiang, Y.F.; et al. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: A systematic review. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Acetylcholinesterase—An Overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/neuroscience/acetylcholinesterase (accessed on 2 May 2021).
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. In StatPearls [Internet]; StatPearls Publishing: St, Petersburd, FL, USA, 2019. [Google Scholar]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta 2007, 380, 50–58. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Bradley-Whitman, M.A.; Lovell, M.A. Biomarkers of lipid peroxidation in Alzheimer disease (AD): An update. Arch. Toxicol. 2015, 89, 1035–1044. [Google Scholar] [CrossRef]
- WeiYang, Y.; ShunKuo, S.; SuDer, C. Effects of extracts from Hericium erinaceus solid-state fermented products on protection and repair of brain cells in zebrafish embryos. Taiwan. J. Agric. Chem. Food Sci. 2016, 54, 98–106. [Google Scholar]
- Talita, H.F.-V.; Isabella, M.G.; Flavia, R.S.; Fabiola, M.R. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14. [Google Scholar] [CrossRef]
- Nonnis, S.; Angiulli, E.; Maffioli, E.; Frabetti, F.; Negri, A.; Cioni, C.; Alleva, E.; Romeo, V.; Tedeschi, G.; Toni, M. Acute environmental temperature variation affects brain protein expression, anxiety and explorative behaviour in adult zebrafish. Sci. Rep. 2021, 11, 2521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valu, M.-V.; Soare, L.C.; Ducu, C.; Moga, S.; Negrea, D.; Vamanu, E.; Balseanu, T.-A.; Carradori, S.; Hritcu, L.; Boiangiu, R.S. Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment. J. Fungi 2021, 7, 477. https://doi.org/10.3390/jof7060477
Valu M-V, Soare LC, Ducu C, Moga S, Negrea D, Vamanu E, Balseanu T-A, Carradori S, Hritcu L, Boiangiu RS. Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment. Journal of Fungi. 2021; 7(6):477. https://doi.org/10.3390/jof7060477
Chicago/Turabian StyleValu, Mihai-Vlad, Liliana Cristina Soare, Catalin Ducu, Sorin Moga, Denis Negrea, Emanuel Vamanu, Tudor-Adrian Balseanu, Simone Carradori, Lucian Hritcu, and Razvan Stefan Boiangiu. 2021. "Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment" Journal of Fungi 7, no. 6: 477. https://doi.org/10.3390/jof7060477
APA StyleValu, M.-V., Soare, L. C., Ducu, C., Moga, S., Negrea, D., Vamanu, E., Balseanu, T.-A., Carradori, S., Hritcu, L., & Boiangiu, R. S. (2021). Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment. Journal of Fungi, 7(6), 477. https://doi.org/10.3390/jof7060477










