Immunological Cross-Reactivity of Proteins Extracted from the Oomycete Pythium insidiosum and the Fungus Basidiobolus ranarum Compromises the Detection Specificity of Immunodiagnostic Assays for Pythiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Uses of ICT and Culture Method for Diagnosis of Animal Pythiosis
2.2. Antigen Preparation
2.3. Serum Samples
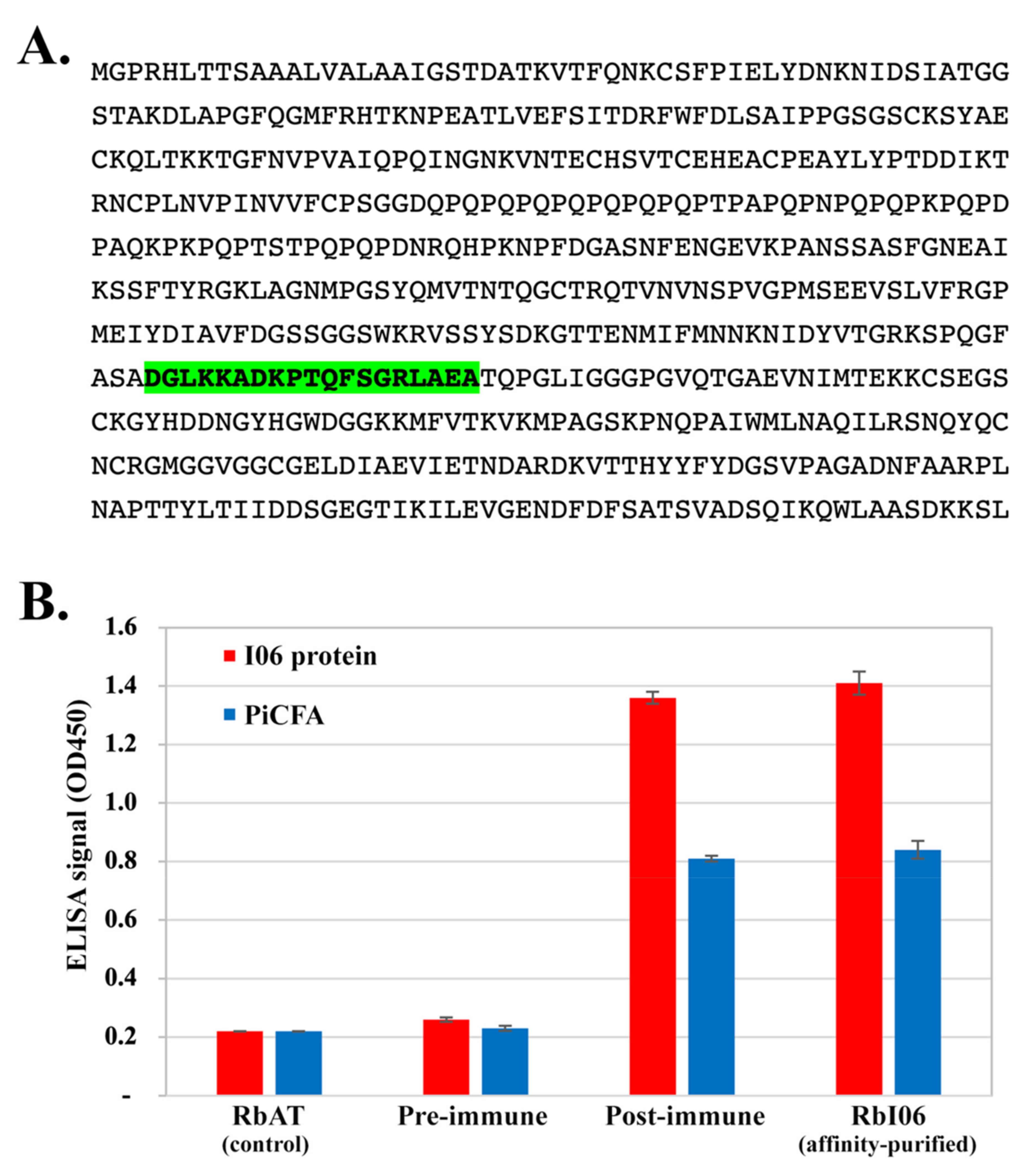
2.4. Cell-Free Synthesis of I06 Protein
2.5. Rabbit Anti-I06 Peptide Antibodies and ELISA Testing
2.6. Immunochromatographic Test
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical Application of ICT for Serodiagnosis of Animal Pythiosis
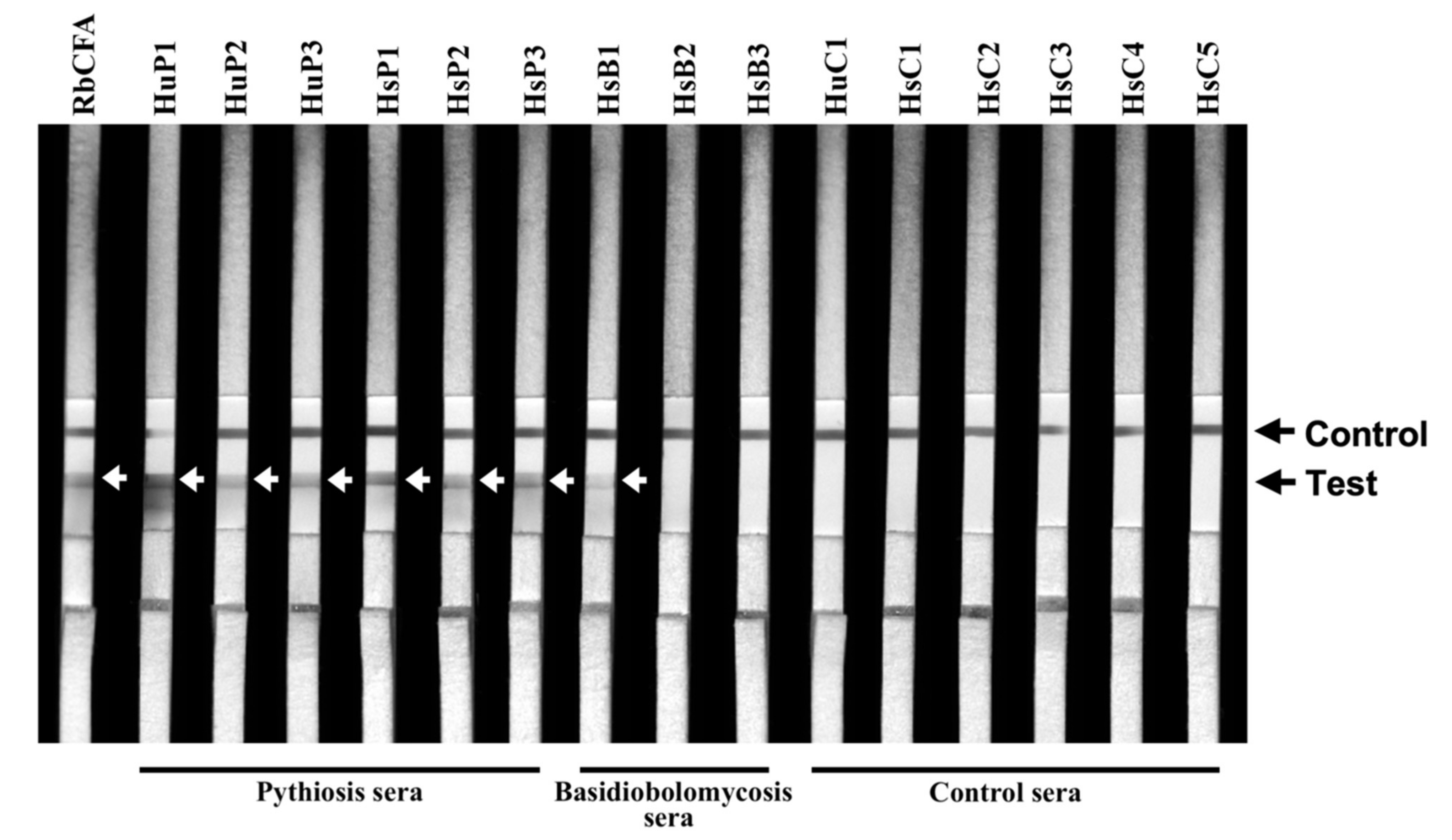
3.2. Retesting the ICT False-Positive Sera for Cross-Reactive Antibodies
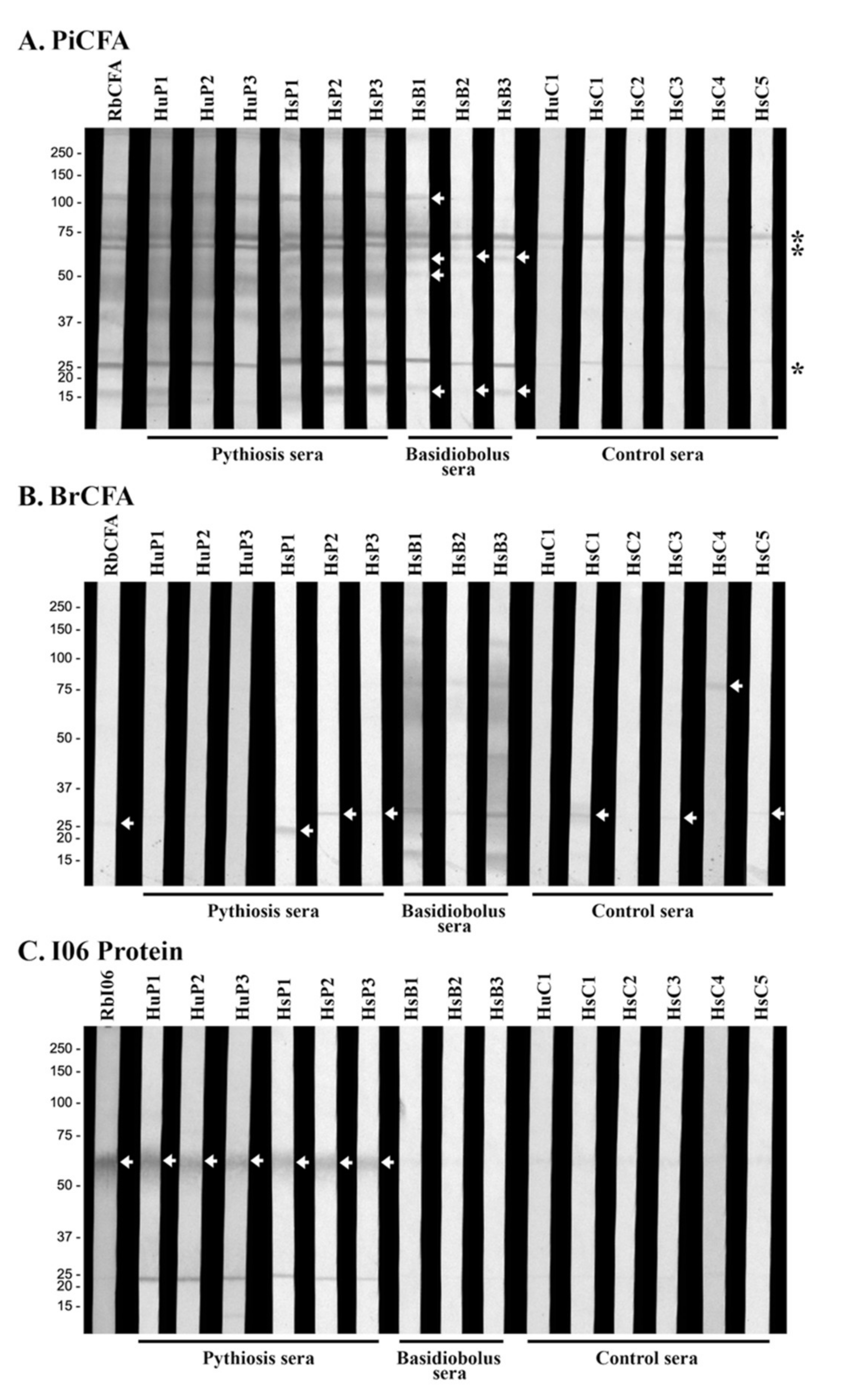
3.3. Western Blot Analyses Reveal Cross-Reactive Antigens of P. insidiosum
3.4. Investigation of the Cross-Reactive Antibodies Using the Synthesized I06 Protein
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Krajaejun, T.; Imkhieo, S.; Intaramat, A.; Ratanabanangkoon, K. Development of an Immunochromatographic Test for Rapid Serodiagnosis of Human Pythiosis. Clin. Vaccine Immunol. 2009, 16, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Intaramat, A.; Sornprachum, T.; Chantrathonkul, B.; Chaisuriya, P.; Lohnoo, T.; Yingyong, W.; Jongruja, N.; Kumsang, Y.; Sandee, A.; Chaiprasert, A.; et al. Protein A/G-Based Immunochromatographic Test for Serodiagnosis of Pythiosis in Human and Animal Subjects from Asia and Americas. Med. Mycol. 2016, 54, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Jaturapaktrarak, C.; Payattikul, P.; Lohnoo, T.; Kumsang, Y.; Laikul, A.; Pathomsakulwong, W.; Yurayart, C.; Tonpitak, W.; Krajaejun, T. Protein A/G-Based Enzyme-Linked Immunosorbent Assay for Detection of Anti-Pythium Insidiosum Antibodies in Human and Animal Subjects. BMC Res. Notes 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Chareonsirisuthigul, T.; Khositnithikul, R.; Intaramat, A.; Inkomlue, R.; Sriwanichrak, K.; Piromsontikorn, S.; Kitiwanwanich, S.; Lowhnoo, T.; Yingyong, W.; Chaiprasert, A.; et al. Performance Comparison of Immunodiffusion, Enzyme-Linked Immunosorbent Assay, Immunochromatography and Hemagglutination for Serodiagnosis of Human Pythiosis. Diagn. Microbiol. Infect. Dis. 2013, 76, 42–45. [Google Scholar] [CrossRef]
- Jindayok, T.; Piromsontikorn, S.; Srimuang, S.; Khupulsup, K.; Krajaejun, T. Hemagglutination Test for Rapid Serodiagnosis of Human Pythiosis. Clin. Vaccine Immunol. 2009, 16, 1047–1051. [Google Scholar] [CrossRef]
- Krajaejun, T.; Kunakorn, M.; Niemhom, S.; Chongtrakool, P.; Pracharktam, R. Development and Evaluation of an In-House Enzyme-Linked Immunosorbent Assay for Early Diagnosis and Monitoring of Human Pythiosis. Clin. Diagn. Lab. Immunol. 2002, 9, 378–382. [Google Scholar] [CrossRef]
- Pracharktam, R.; Changtrakool, P.; Sathapatayavongs, B.; Jayanetra, P.; Ajello, L. Immunodiffusion Test for Diagnosis and Monitoring of Human Pythiosis Insidiosi. J. Clin. Microbiol. 1991, 29, 2661–2662. [Google Scholar] [CrossRef]
- Supabandhu, J.; Vanittanakom, P.; Laohapensang, K.; Vanittanakom, N. Application of Immunoblot Assay for Rapid Diagnosis of Human Pythiosis. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2009, 92, 1063–1071. [Google Scholar]
- Chitasombat, M.N.; Jongkhajornpong, P.; Lekhanont, K.; Krajaejun, T. Recent Update in Diagnosis and Treatment of Human Pythiosis. Peer J. 2020, 8, e8555. [Google Scholar] [CrossRef]
- Gaastra, W.; Lipman, L.J.; de Cock, A.W.; Exel, T.K.; Pegge, R.B.; Scheurwater, J.; Vilela, R.; Mendoza, L. Pythium Insidiosum: An Overview. Vet. Microbiol. 2010, 146, 1–16. [Google Scholar] [CrossRef]
- Krajaejun, T.; Sathapatayavongs, B.; Pracharktam, R.; Nitiyanant, P.; Leelachaikul, P.; Wanachiwanawin, W.; Chaiprasert, A.; Assanasen, P.; Saipetch, M.; Mootsikapun, P.; et al. Clinical and Epidemiological Analyses of Human Pythiosis in Thailand. Clin. Infect. Dis. 2006, 43, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Ajello, L.; McGinnis, M.R. Infection Caused by the Oomycetous Pathogen Pythium Insidiosum. J. Mycol. Med. 1996, 6, 151–164. [Google Scholar]
- Krajaejun, T.; Kunakorn, M.; Pracharktam, R.; Chongtrakool, P.; Sathapatayavongs, B.; Chaiprasert, A.; Vanittanakom, N.; Chindamporn, A.; Mootsikapun, P. Identification of a Novel 74-KiloDalton Immunodominant Antigen of Pythium Insidiosum Recognized by Sera from Human Patients with Pythiosis. J. Clin. Microbiol. 2006, 44, 1674–1680. [Google Scholar] [CrossRef]
- Yolanda, H.; Krajaejun, T. Review of Methods and Antimicrobial Agents for Susceptibility Testing against Pythium Insidiosum. Heliyon 2020, 6, e03737. [Google Scholar] [CrossRef]
- Lerksuthirat, T.; Sangcakul, A.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Krajaejun, T. Evolution of the Sterol Biosynthetic Pathway of Pythium Insidiosum and Related Oomycetes Contributes to Antifungal Drug Resistance. Antimicrob. Agents Chemother. 2017, 61, e02352-16. [Google Scholar] [CrossRef]
- Tonpitak, W.; Pathomsakulwong, W.; Sornklien, C.; Krajaejun, T.; Wutthiwithayaphong, S. First Confirmed Case of Nasal Pythiosis in a Horse in Thailand. JMM Case Rep. 2018, 5, e005136. [Google Scholar] [CrossRef] [PubMed]
- Rujirawat, T.; Sridapan, T.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Sae-Chew, P.; Tonpitak, W.; Krajaejun, T. Single Nucleotide Polymorphism-Based Multiplex PCR for Identification and Genotyping of the Oomycete Pythium Insidiosum from Humans, Animals and the Environment. Infect. Genet. Evol. 2017, 54, 429–436. [Google Scholar] [CrossRef]
- Keeratijarut, A.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Srichunrusami, C.; Onpeaw, P.; Chongtrakool, P.; Brandhorst, T.T.; Krajaejun, T. Detection of the Oomycete Pythium Insidiosum by Real-Time PCR Targeting the Gene Coding for Exo-1,3-β-Glucanase. J. Med. Microbiol. 2015, 64, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Keeratijarut, A.; Lohnoo, T.; Yingyong, W.; Nampoon, U.; Lerksuthirat, T.; Onpaew, P.; Chongtrakool, P.; Krajaejun, T. PCR Amplification of a Putative Gene for Exo-1, 3-Beta-Glucanase to Identify the Pathogenic Oomycete Pythium Insidiosum. Asian Biomed. 2014, 8, 637–644. [Google Scholar] [CrossRef]
- Htun, Z.M.; Rotchanapreeda, T.; Rujirawat, T.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Sae-Chew, P.; Payattikul, P.; Yurayart, C.; Limsivilai, O.; et al. Loop-Mediated Isothermal Amplification (LAMP) for Identification of Pythium Insidiosum. Int. J. Infect. Dis. 2020, 101, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sae-Chew, P.; Rujirawat, T.; Kumsang, Y.; Payattikul, P.; Lohnoo, T.; Yingyong, W.; Jaturapaktrarak, C.; Rotchanapreeda, T.; Reamtong, O.; Srisuk, T.; et al. Automated Cell-Free Multiprotein Synthesis Facilitates the Identification of a Secretory, Oligopeptide Elicitor-Like, Immunoreactive Protein of the Oomycete Pythium Insidiosum. mSystems 2020, 5, e00196-20. [Google Scholar] [CrossRef]
- Irinyi, L.; Lackner, M.; de Hoog, G.S.; Meyer, W. DNA Barcoding of Fungi Causing Infections in Humans and Animals. Fungal Biol. 2016, 120, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mar Htun, Z.; Laikul, A.; Pathomsakulwong, W.; Yurayart, C.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Payattikul, P.; Sae-Chew, P.; Rujirawat, T.; et al. Identification and Biotyping of Pythium Insidiosum Isolated from Urban and Rural Areas of Thailand by Multiplex PCR, DNA Barcode, and Proteomic Analyses. J. Fungi 2021, 7, 242. [Google Scholar] [CrossRef]
- Keeratijarut, A.; Karnsombut, P.; Aroonroch, R.; Srimuang, S.; Sangruchi, T.; Sansopha, L.; Mootsikapun, P.; Larbcharoensub, N.; Krajaejun, T. Evaluation of an In-House Immunoperoxidase Staining Assay for Histodiagnosis of Human Pythiosis. Southeast Asian J. Trop. Med. Public Health 2009, 40, 1298–1305. [Google Scholar] [PubMed]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Orlando, FL, USA, 1990; pp. 315–322. [Google Scholar]
- Lohnoo, T.; Jongruja, N.; Rujirawat, T.; Yingyon, W.; Lerksuthirat, T.; Nampoon, U.; Kumsang, Y.; Onpaew, P.; Chongtrakool, P.; Keeratijarut, A.; et al. Efficiency Comparison of Three Methods for Extracting Genomic DNA of the Pathogenic Oomycete Pythium Insidiosum. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2014, 97, 342–348. [Google Scholar]
- Kamoun, S. Molecular Genetics of Pathogenic Oomycetes. Eukaryot. Cell 2003, 2, 191–199. [Google Scholar] [CrossRef]
- Prabhu, R.M.; Patel, R. Mucormycosis and Entomophthoramycosis: A Review of the Clinical Manifestations, Diagnosis and Treatment. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004, 10, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Hussain, K.A.; Petraitiene, R.; Schuetz, A.N.; Walsh, T.J. Entomophthoramycosis: A Neglected Tropical Mycosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 688–694. [Google Scholar] [CrossRef]
- Gugnani, H.C. Entomophthoromycosis Due to Conidiobolus. Eur. J. Epidemiol. 1992, 8, 391–396. [Google Scholar] [CrossRef]
- Vilela, R.; Mendoza, L. Human Pathogenic Entomophthorales. Clin. Microbiol. Rev. 2018, 31, e00014-18. [Google Scholar] [CrossRef]
- Rujirawat, T.; Patumcharoenpol, P.; Lohnoo, T.; Yingyong, W.; Lerksuthirat, T.; Tangphatsornruang, S.; Suriyaphol, P.; Grenville-Briggs, L.J.; Garg, G.; Kittichotirat, W.; et al. Draft Genome Sequence of the Pathogenic Oomycete Pythium Insidiosum Strain Pi-S, Isolated from a Patient with Pythiosis. Genome Announc. 2015, 3, e00574-15. [Google Scholar] [CrossRef] [PubMed]
- Rujirawat, T.; Patumcharoenpol, P.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Payattikul, P.; Tangphatsornruang, S.; Suriyaphol, P.; Reamtong, O.; Garg, G.; et al. Probing the Phylogenomics and Putative Pathogenicity Genes of Pythium Insidiosum by Oomycete Genome Analyses. Sci. Rep. 2018, 8, 4135. [Google Scholar] [CrossRef] [PubMed]
- Rujirawat, T.; Patumcharoenpol, P.; Kittichotirat, W.; Krajaejun, T. Oomycete Gene Table: An Online Database for Comparative Genomic Analyses of the Oomycete Microorganisms. Database 2019, 2019, baz082. [Google Scholar] [CrossRef] [PubMed]
| Year | Case ID | Affected Animal | Clinical Diagnosis | Diagnostic Method | |
|---|---|---|---|---|---|
| ICT for Pythiosis | Culture/Molecular Assay | ||||
| 2017 | 01 | Dog | Pythiosis | Positive | Pythium insidiosum |
| 2017 | 02 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2017 | 03 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2017 | 04 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2017 | 05 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2017 | 06 | Horse | Fungal granuloma | Weakly positive * | Basidiobolus ranarum |
| 2018 | 07 | Horse | Guttural pouch empyema | (Negative) | Aspergillus flavus |
| 2018 | 08 | Horse | Chronic wound | (Negative) | Dark fungus |
| 2018 | 09 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2018 | 10 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2018 | 11 | Horse | Habronemiasis | Weakly positive * | Nocardia species |
| 2018 | 12 | Horse | Chronic wound | Weakly positive * | Basidiobolus ranarum |
| 2018 | 13 | Horse | Chronic wound | Weakly positive * | Basidiobolus ranarum |
| 2019 | 14 | Cat | Spinal cord granuloma | (Negative) | Microsporum canis |
| 2019 | 15 | Dog | Cutaneous granuloma | (Negative) | Hyaline septate fungus |
| 2019 | 16 | Dog | Chronic wound | (Negative) | No growth |
| 2019 | 17 | Dog | Healthy | (Negative) | None |
| 2019 | 18 | Dog | Healthy | (Negative) | None |
| 2019 | 19 | Dog | Healthy | (Negative) | None |
| 2019 | 20 | Dog | Healthy | (Negative) | None |
| 2019 | 21 | Dog | Healthy | (Negative) | None |
| 2019 | 22 | Dog | Healthy | (Negative) | None |
| 2019 | 23 | Dog | Healthy | (Negative) | None |
| 2019 | 24 | Dog | GI granuloma | Positive | Pythium insidiosum |
| 2019 | 25 | Dog | GI granuloma | Positive | Pythium insidiosum |
| 2019 | 26 | Dog | Chronic wound | Weakly positive | Pythium insidiosum |
| 2019 | 27 | Horse | Chronic wound | (Negative) | Nocardia species |
| 2019 | 28 | Horse | Chronic wound | (Negative) | None |
| 2020 | 29 | Cat | Chronic wound | (Negative) | Hyaline septate fungus |
| 2020 | 30 | Dog | Chronic wound | (Negative) | No growth |
| 2020 | 31 | Dog | Chronic wound | (Negative) | No growth |
| 2020 | 32 | Dog | GI granuloma | Positive | None |
| 2020 | 33 | Dog | Cutaneous granuloma | Positive | Pythium insidiosum |
| 2020 | 34 | Dog | Pythiosis | Positive | Pythium insidiosum |
| 2020 | 35 | Dog | GI infection | Positive * | Basidiobolus ranarum |
| 2020 | 36 | Elephant | Unknown | (Negative) | None |
| 2020 | 37 | Horse | Pythiosis | Positive | Pythium insidiosum |
| 2021 | 38 | Dog | Gastric mass | Positive | None |
| Serum ID | Host | Cause of Infection | ICT Result |
|---|---|---|---|
| HuP1 | Human | Pythium insidiosum | Positive |
| HuP2 | Human | Pythium insidiosum | Positive |
| HuP3 | Human | Pythium insidiosum | Positive |
| HsP1 | Horse | Pythium insidiosum | Positive |
| HsP2 | Horse | Pythium insidiosum | Positive |
| HsP3 | Horse | Pythium insidiosum | Positive |
| HsB1 | Horse | Basidiobolus ranarum | Weakly positive |
| HsB2 | Horse | Basidiobolus ranarum | (Negative) |
| HsB3 | Horse | Basidiobolus ranarum | (Negative) |
| HuC1 | Human | None (Healthy) | (Negative) |
| HsC1 | Horse | None (Healthy) | (Negative) |
| HsC2 | Horse | None (Healthy) | (Negative) |
| HsC3 | Horse | None (Healthy) | (Negative) |
| HsC4 | Horse | Aspergillus flavus | (Negative) |
| HsC5 | Horse | Actinomyces species | (Negative) |
| RbCFA | Rabbit | None (anti-PiCFA serum) | Positive |
| RbI06 | Rabbit | None (anti-I06 peptide serum) | None |
| RbAT | Rabbit | None (anti-alpha tubulin serum) | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotchanapreeda, T.; Sae-Chew, P.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Kumsang, Y.; Payattikul, P.; Jaturapaktrarak, C.; Intaramat, A.; Pathomsakulwong, W.; et al. Immunological Cross-Reactivity of Proteins Extracted from the Oomycete Pythium insidiosum and the Fungus Basidiobolus ranarum Compromises the Detection Specificity of Immunodiagnostic Assays for Pythiosis. J. Fungi 2021, 7, 474. https://doi.org/10.3390/jof7060474
Rotchanapreeda T, Sae-Chew P, Lohnoo T, Yingyong W, Rujirawat T, Kumsang Y, Payattikul P, Jaturapaktrarak C, Intaramat A, Pathomsakulwong W, et al. Immunological Cross-Reactivity of Proteins Extracted from the Oomycete Pythium insidiosum and the Fungus Basidiobolus ranarum Compromises the Detection Specificity of Immunodiagnostic Assays for Pythiosis. Journal of Fungi. 2021; 7(6):474. https://doi.org/10.3390/jof7060474
Chicago/Turabian StyleRotchanapreeda, Tiwa, Pattarana Sae-Chew, Tassanee Lohnoo, Wanta Yingyong, Thidarat Rujirawat, Yothin Kumsang, Penpan Payattikul, Chalisa Jaturapaktrarak, Akarin Intaramat, Watcharapol Pathomsakulwong, and et al. 2021. "Immunological Cross-Reactivity of Proteins Extracted from the Oomycete Pythium insidiosum and the Fungus Basidiobolus ranarum Compromises the Detection Specificity of Immunodiagnostic Assays for Pythiosis" Journal of Fungi 7, no. 6: 474. https://doi.org/10.3390/jof7060474
APA StyleRotchanapreeda, T., Sae-Chew, P., Lohnoo, T., Yingyong, W., Rujirawat, T., Kumsang, Y., Payattikul, P., Jaturapaktrarak, C., Intaramat, A., Pathomsakulwong, W., Yurayart, C., & Krajaejun, T. (2021). Immunological Cross-Reactivity of Proteins Extracted from the Oomycete Pythium insidiosum and the Fungus Basidiobolus ranarum Compromises the Detection Specificity of Immunodiagnostic Assays for Pythiosis. Journal of Fungi, 7(6), 474. https://doi.org/10.3390/jof7060474







