Identification of Volatile Sulfur Compounds Produced by Schizophyllum commune
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. Solid-Phase Microextraction—Gas Chromatography/Mass Spectrometry
2.3. H2S Detection Using Lead Acetate Paper
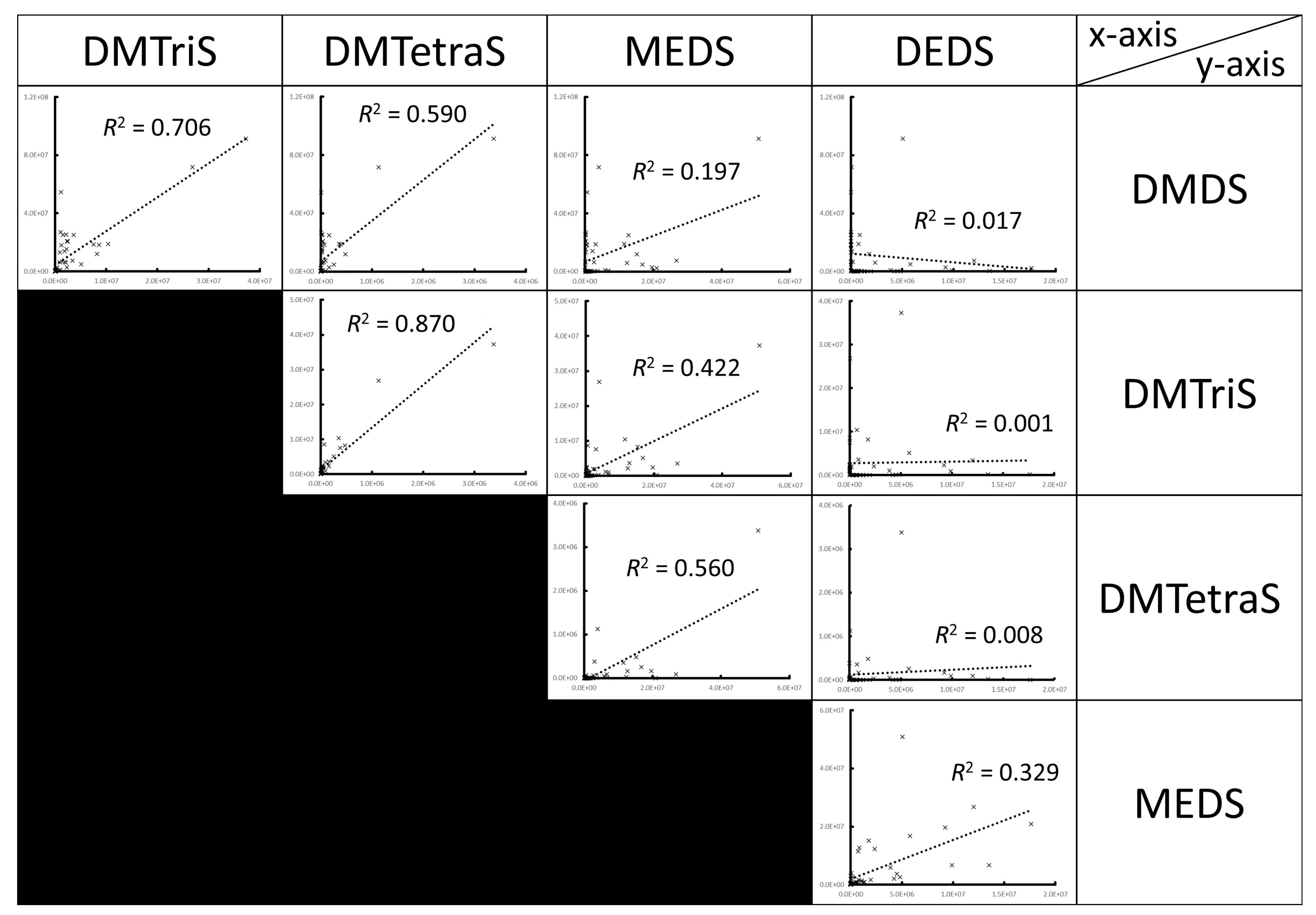
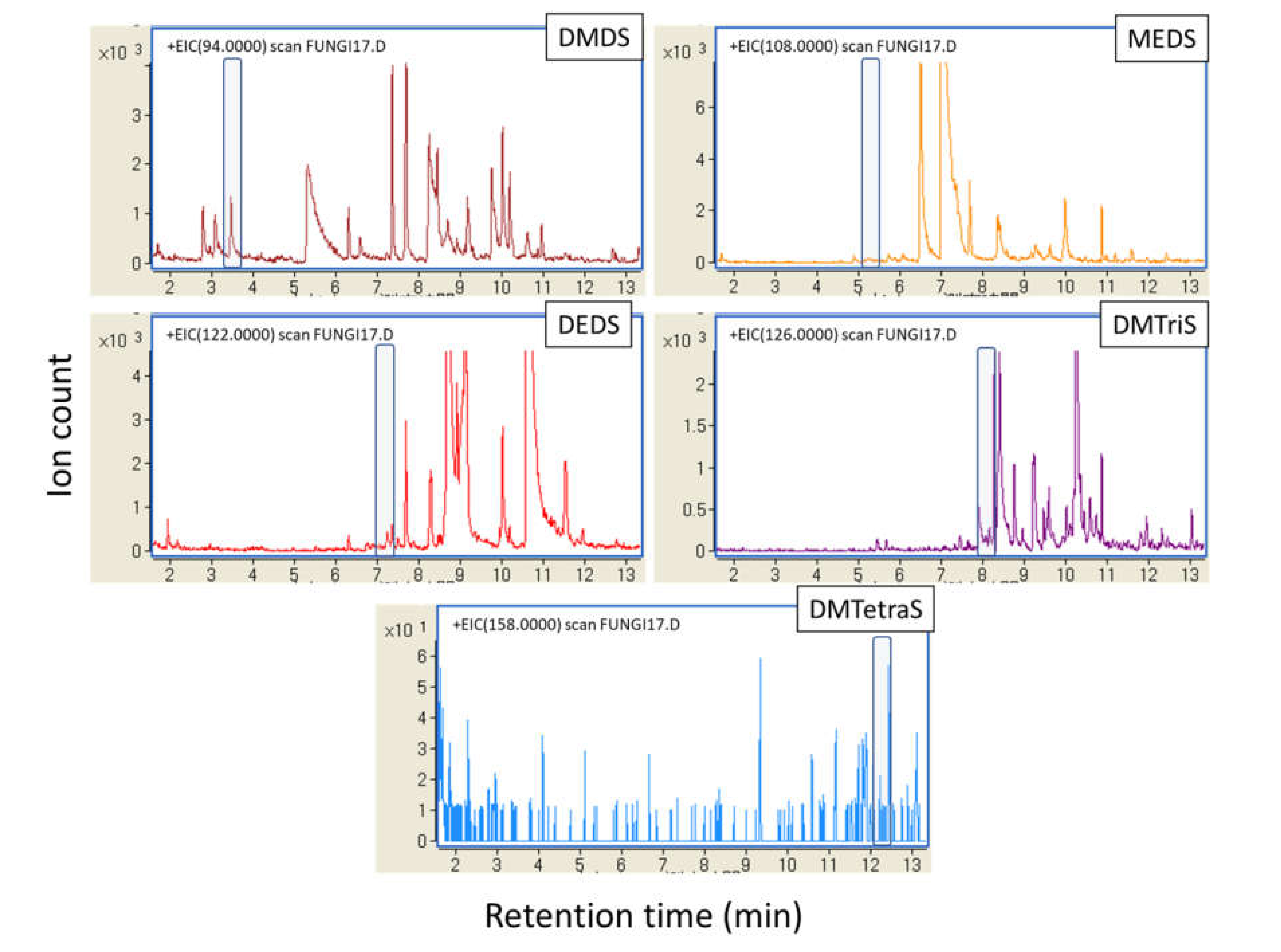
3. Results
3.1. Volatile Sulfur Compounds Are the Major VOCs of S. commune
3.2. H2S Detection by Lead Acetate Paper
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhary, A.; Randhawa, H.S.; Gaur, S.N.; Agarwal, K.; Kathuria, S.; Roy, P.; Klaassen, C.H.; Meis, J.F. Schizophyllum commune as an emerging fungal pathogen: A review and report of two cases. Mycoses 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Agarwal, K.; Meis, J.F. Recognizing filamentous basidiomycetes as agents of human disease: A review. Med. Mycol. 2014, 52, 782–797. [Google Scholar] [CrossRef]
- Kamei, K.; Unno, H.; Nagao, K.; Kuriyama, T.; Nishimura, K.; Miyaji, M. Allergic Bronchopulmonary Mycosis Caused by the Basidiomycetous Fungus Schizophyllum commune. Clin. Infect. Dis. 1994, 18, 305–309. [Google Scholar] [CrossRef]
- Oguma, T.; Taniguchi, M.; Shimoda, T.; Kamei, K.; Matsuse, H.; Hebisawa, A.; Takayanagi, N.; Konno, S.; Fukunaga, K.; Harada, K.; et al. Allergic bronchopulmonary aspergillosis in Japan: A nationwide survey. Allergol. Int. 2018, 67, 79–84. [Google Scholar] [CrossRef]
- Toyotome, T.; Satoh, M.; Yahiro, M.; Watanabe, A.; Nomura, F.; Kamei, K. Glucoamylase is a major allergen of Schizophyllum commune. Clin. Exp. Allergy 2014, 44, 450–457. [Google Scholar] [CrossRef]
- Ishiguro, T.; Kagiyama, N.; Kojima, A.; Yamada, M.; Nakamoto, Y.; Takaku, Y.; Shimizu, Y.; Kurashima, K.; Takayanagi, N. Allergic Bronchopulmonary Mycosis Due to Schizophyllum commune Treated Effectively with Voriconazole: A Case Report. Intern. Med. 2018, 57, 2553–2557. [Google Scholar] [CrossRef] [PubMed]
- Syhre, M.; Scotter, J.M.; Chambers, S.T. Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 2008, 46, 209–215. [Google Scholar] [CrossRef]
- Koo, S.; Thomas, H.R.; Daniels, S.D.; Lynch, R.C.; Fortier, S.M.; Shea, M.M.; Rearden, P.; Comolli, J.C.; Baden, L.R.; Marty, F.M. A breath fungal secondary metabolite signature to diagnose invasive aspergillosis. Clin. Infect. Dis. 2014, 59, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.A.; Stefanuto, P.; Beattie, S.R.; Bultman, K.M.; Cramer, R.A.; Hill, J.E. Sniffing out the hypoxia volatile metabolic signature of Aspergillus fumigatus. J. Breath Res. 2017, 11, 36003. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.T.; Bhandari, S.; Scott-Thomas, A.; Syhre, M. Novel diagnostics: Progress toward a breath test for invasive Aspergillus fumigatus. Med. Mycol. 2011, 49 (Suppl 1), S54–S61. [Google Scholar] [CrossRef]
- Chambers, S.T.; Syhre, M.; Murdoch, D.R.; McCartin, F.; Epton, M.J. Detection of 2-pentylfuran in the breath of patients with Aspergillus fumigatus. Med. Mycol. 2009, 47, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Freihorst, D.; Brunsch, M.; Wirth, S.; Krause, K.; Kniemeyer, O.; Linde, J.; Kunert, M.; Boland, W.; Kothe, E. Smelling the difference: Transcriptome, proteome and volatilome changes after mating. Fungal Genet. Biol. 2018, 112, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Schalchli, H.; Hormazabal, E.; Becerra, J.; Birkett, M.; Alvear, M.; Vidal, J.; Quiroz, A. Antifungal activity of volatile metabolites emitted by mycelial cultures of saprophytic fungi. Chem. Ecol. 2011, 27, 503–513. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Volatile sulfur compounds as the cause of bad breath: A review. In Proceedings of the Phosphorus, Sulfur and Silicon and the Related Elements; Taylor & Francis Group: Abingdon, UK, 2013; Volume 188, pp. 396–402. [Google Scholar]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.Ł.; Karpiński, T.M. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]
- Tokitomo, Y. Volatile Components of Cooked Onions. Nippon Shokuhin Kagaku Kogaku Kaishi 1995, 42, 279–287. [Google Scholar] [CrossRef]
- Maruyama, F.T. Identification of dimethyl trisulfide as a major aroma component of cooked brassicaceous vegetables. J. Food Sci. 1970, 35, 540. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Isogai, A.; Utsunomiya, H.; Kanda, R.; Iwata, H. Changes in the aroma compounds of sake during aging. J. Agric. Food Chem. 2005, 53, 4118–4123. [Google Scholar] [CrossRef]
- Cholet, O.; Hénaut, A.; Hébert, A.; Bonnarme, P. Transcriptional analysis of L-methionine catabolism in the cheese-ripening yeast Yarrowia lipolytica in relation to volatile sulfur compound biosynthesis. Appl. Environ. Microbiol. 2008, 74, 3356–3367. [Google Scholar] [CrossRef]
- Martin, N.; Berger, C.; Le Du, C.; Spinnler, H.E. Aroma compound production in cheese curd by coculturing with selected yeast and bacteria. J. Dairy Sci. 2001, 84, 2125–2135. [Google Scholar] [CrossRef]
- Del Castillo-Lozano, M.L.; Mansour, S.; Tâche, R.; Bonnarme, P.; Landaud, S. The effect of cysteine on production of volatile sulphur compounds by cheese-ripening bacteria. Int. J. Food Microbiol. 2008, 122, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Bezerra, A.R.; Almeida, A.; Rocha, S.M. Candida species (Volatile) metabotyping through advanced comprehensive two-dimensional gas chromatography. Microorganisms 2020, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle volatiles: From chemical ecology to aroma biosynthesis. New Phytol. 2011, 189, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Ohm, R.A.; De Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; De Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Kohler, A.; Murat, C.; Balestrini, R.; Coutinho, P.M.; Jaillon, O.; Montanini, B.; Morin, E.; Noel, B.; Percudani, R.; et al. Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 2010, 464, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, S-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef]
- Bobet, R.A.; Noble, A.C.; Boulton, R.B. Kinetics of the Ethanethiol and Diethyl Disulfide Interconversion in Wine-Like Solutions. J. Agric. Food Chem. 1990, 38, 449–452. [Google Scholar] [CrossRef]
- Monedeiro, F.; Milanowski, M.; Ratiu, I.-A.; Zmysłowski, H.; Ligor, T.; Buszewski, B. VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Shirasu, M.; Nagai, S.; Hayashi, R.; Ochiai, A.; Touhara, K. Dimethyl Trisulfide as a Characteristic odor associated with fungating cancer wounds. Biosci. Biotechnol. Biochem. 2009, 73, 2117–2120. [Google Scholar] [CrossRef]
- Scott, J.; Sueiro-Olivares, M.; Ahmed, W.; Heddergott, C.; Zhao, C.; Thomas, R.; Bromley, M.; Latgé, J.-P.; Krappmann, S.; Fowler, S.; et al. Pseudomonas aeruginosa-Derived Volatile Sulfur Compounds Promote Distal Aspergillus fumigatus Growth and a Synergistic Pathogen-Pathogen Interaction That Increases Pathogenicity in Co-infection. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Labows, J.N.; McGinley, K.J.; Webster, G.F.; Leyden, J.J. Headspace analysis of volatile metabolites of Pseudomonas aeruginosa and related species by gas chromatography-mass spectrometry. J. Clin. Microbiol. 1980, 12, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Ohno, H.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; Miyazaki, Y.; Tomono, K. Allergic bronchopulmonary mycosis due to co-infection with Aspergillus fumigatus and Schizophyllum commune. ID Cases 2014, 1, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Ishino, T.; Takeno, S.; Hirakawa, K. Bilateral allergic fungal rhinosinusitis caused by Schizophyllum commune and Aspergillus niger. A case report. Rhinology 2009, 47, 217–221. [Google Scholar] [PubMed]
- Ishiguro, T.; Takayanagi, N.; Saito, A.; Akiyama, K.; Wakayama, M.; Shibuya, K.; Shimizu, Y.; Sugita, Y.; Kamei, K. Allergic bronchopulmonary mycosis due to Schizophyllum commune and Aspergillus fumigatus. Nihon Kokyuki Gakkai Zasshi 2011, 49, 612–618. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyotome, T.; Takino, M.; Takaya, M.; Yahiro, M.; Kamei, K. Identification of Volatile Sulfur Compounds Produced by Schizophyllum commune. J. Fungi 2021, 7, 465. https://doi.org/10.3390/jof7060465
Toyotome T, Takino M, Takaya M, Yahiro M, Kamei K. Identification of Volatile Sulfur Compounds Produced by Schizophyllum commune. Journal of Fungi. 2021; 7(6):465. https://doi.org/10.3390/jof7060465
Chicago/Turabian StyleToyotome, Takahito, Masahiko Takino, Masahiro Takaya, Maki Yahiro, and Katsuhiko Kamei. 2021. "Identification of Volatile Sulfur Compounds Produced by Schizophyllum commune" Journal of Fungi 7, no. 6: 465. https://doi.org/10.3390/jof7060465
APA StyleToyotome, T., Takino, M., Takaya, M., Yahiro, M., & Kamei, K. (2021). Identification of Volatile Sulfur Compounds Produced by Schizophyllum commune. Journal of Fungi, 7(6), 465. https://doi.org/10.3390/jof7060465






