Cytotoxicity and Nitric Oxide Production Inhibitory Activities of Compounds Isolated from the Plant Pathogenic Fungus Curvularia sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Material and Identification
2.3. Fermentation, Extraction, and Isolation
2.4. Bioassays
2.4.1. Nitric Oxide (NO) Production Inhibitory Assay
2.4.2. Cytotoxicity Assay in Mammalian Cells (RAW 264.7 Cells)
2.4.3. Cytotoxicity Assay against Lung Cancer A549, Colorectal Cancer SW480, and Leukemic K562 Cells
2.5. Computational Methods
3. Results and Discussion
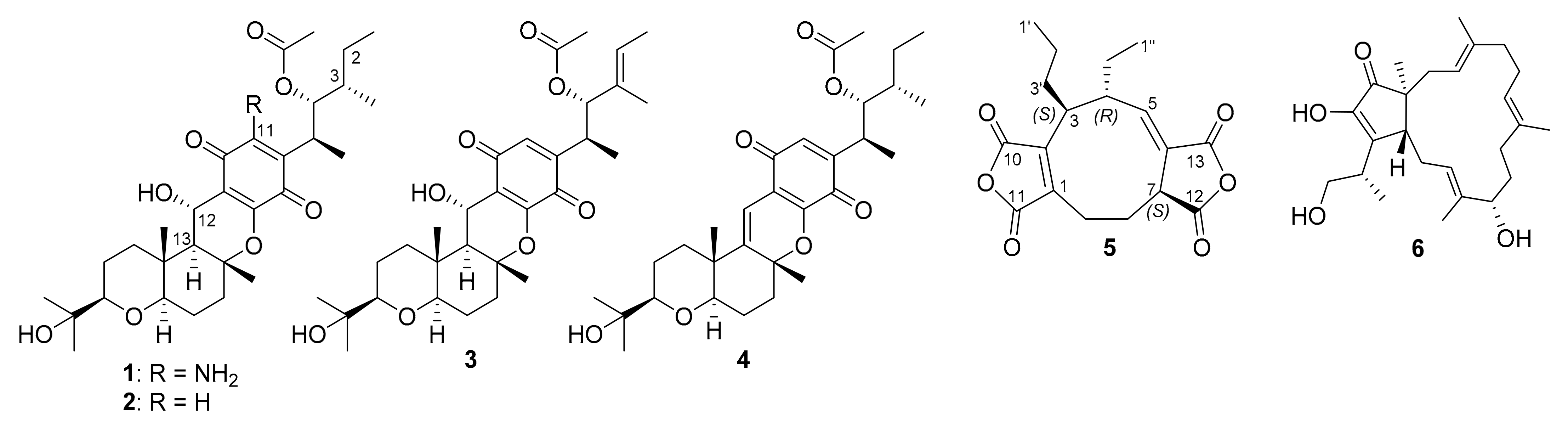
3.1. Isolated Compounds from Curvularia sp.
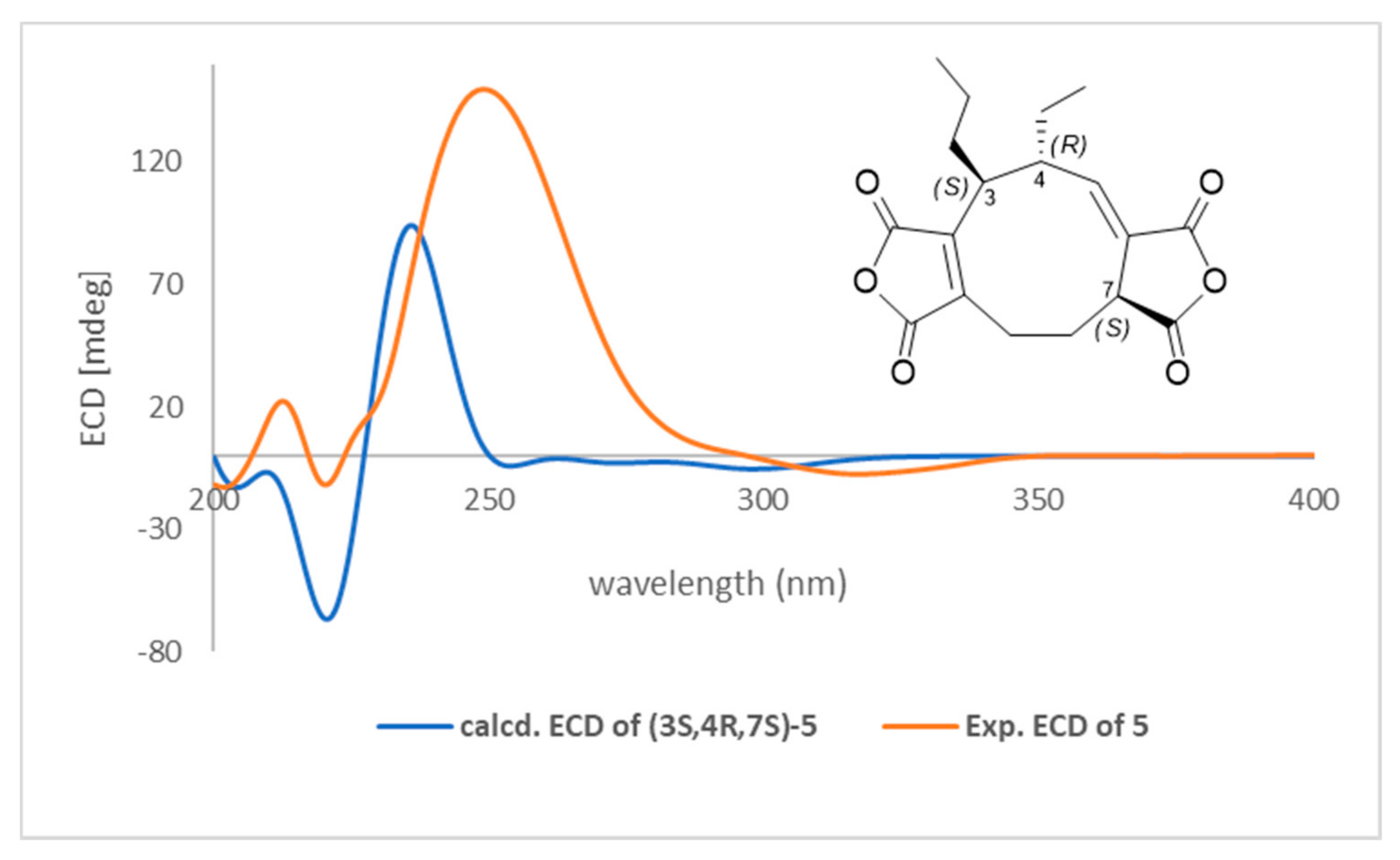
3.2. Structural Characterization of a New Compound
3.3. Cytotoxicity against the Three Cancer Cell Lines, including Lung Cancer A549, Colorectal Cancer SW480, and Leukemic K562 Cells and Mammalian Cells (RAW 264.7 Cells)
3.4. Nitric Oxide Production Inhibitory Activity of Isolated Compounds 1–6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.; Li, G.; Lou, H.-X. Structural diversity and biological activities of novel secondary metabolites from endophytes. Molecules 2018, 23, 646. [Google Scholar] [CrossRef] [PubMed]
- Uzma, F.; Mohan, C.D.; Siddaiah, C.N.; Chowdappa, S. Endophytic Fungi: Promising Source of Novel Bioactive Compounds. In Fungal Biology; Gupta, V.K., Tuohy, M.G., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 243–265. [Google Scholar] [CrossRef]
- Daley, S.-K.; Cordell, G.A. Biologically significant and recently isolated alkaloids from endophytic fungi. J. Nat. Prod. 2021, 84, 871–897. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers. 2011, 50, 3–19. [Google Scholar] [CrossRef]
- Rajamanikyam, M.; Vadlapudi, V.; Amanchy, R.; Upadhyayula, S.M. Endophytic fungi as novel resources of natural therapeutics. Braz. Arch. Biol. Technol. 2017, 60, 60. [Google Scholar] [CrossRef]
- Sánchez-Márquez, S.; Bills, G.F.; Zabalgogeazcoa, I. Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses. Fungal Divers. 2008, 3, 1–7. [Google Scholar]
- Sunpapao, A.; Kittimorakul, J.; Pornsuriya, C. Disease note: Identification of Curvularia oryzae as cause of leaf spot disease on oil palm seedlings in nurseries of Thailand. Phytoparasitica 2014, 42, 529–533. [Google Scholar] [CrossRef]
- Kusai, N.A.; Azmi, M.M.Z.; Zulkifly, S.; Yusof, M.T.; Zainudin, N.A.I.M. Morphological and molecular characteriza-tion of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rendiconti Lincei. Scienze Fisiche e Naturali 2016, 27, 205–214. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Senwanna, C.; Cheewangkoon, R.; Crous, P.W. New species and records of Bipolaris and Curvularia from Thailand. Mycosphere 2017, 8, 556–1574. [Google Scholar] [CrossRef]
- Kamalam, A.; Ajithadass, K.; Sentamilselvi, G.; Thambiah, A.S. Paronychia and black discoloration of a thumb nail caused by Curvularia lunata. Mycopathologia 1992, 118, 83–84. [Google Scholar] [CrossRef]
- Ebright, J.R.; Chandrasekar, P.H.; Marks, S.; Fairfax, M.R.; Aneziokoro, A.; McGinnis, M.R. Invasive sinusitis and cerebritis due to Curvularia clavata in an immunocompetent adult. Clin. Infect. Dis. 1999, 28, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Tupaki-Sreepurna, A.; Thanneru, V.; Sekar, U.; Shanthi, M.; Kindo, A.J. A rare case of Curvularia ha-waiiensis in the ear following trauma. J. Med. Sci. Clin. Res. 2017, 5, 28154–28158. [Google Scholar] [CrossRef]
- McAleer, R.; Kroenert, D.B.; Elder, J.L.; Froudist, J.H. Allergic bronchopulmonary disease caused by Curularia lunata and Drecbslera bawaiiensis. Thorax 1981, 36, 338–344. [Google Scholar] [CrossRef]
- Khiralla, A.; Spina, R.; Saliba, S.; Laurain-Mattar, D. Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biol. Rev. 2019, 33, 101–122. [Google Scholar] [CrossRef]
- Abraham, W.-R.; Meyer, H.; Abate, D. Curvupallides, a new class of alkaloids from the fungus Curvularia pallescens. Tetrahedron 1995, 51, 4947–4952. [Google Scholar] [CrossRef]
- Han, W.B.; Lu, Y.H.; Zhang, A.H.; Zhang, G.F.; Mei, Y.N.; Jiang, N.; Lei, X.; Song, Y.C.; Ng, S.W.; Tan, R.X. Curvulamine, a new antibacterial alkaloid incorporating two undescribed units from a Curvularia Species. Org. Lett. 2014, 16, 5366–5369. [Google Scholar] [CrossRef] [PubMed]
- Teles, H.L.; Silva, G.H.; Castro-Gamboa, I.; Bolzani, V.D.S.; Pereira, J.O.; Costa-Neto, C.M.; Haddad, R.; Eberlin, M.N.; Young, M.C.M.; Araújo, A.R. Benzopyrans from Curvularia sp., an endophytic fungus associated with Ocotea corymbosa (Lauraceae). Phytochemistry 2005, 66, 2363–2367. [Google Scholar] [CrossRef]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono, S.; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Xu, K.-X.; Xue, Y.; Cao, F.; Yang, L.-J.; Hou, X.-M.; Wang, C.-Y.; Shao, C.-L. Sordarin diterpene glycosides with an unusual 1,3-dioxolan-4-one ring from the zoanthid-derived fungus Curvularia hawaiiensis TA26-15. J. Nat. Prod. 2019, 82, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Kaaniche, F.; Hamed, A.; Abdel-Razek, A.S.; Wibberg, D.; Abdissa, N.; El Euch, I.Z.; Allouche, N.; Mellouli, L.; Shaaban, M.; Sewald, N. Bioactive secondary metabolites from new endophytic fungus Curvularia. sp isolated from Rauwolfia macrophylla. PLoS ONE 2019, 14, e0217627. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; König, G.M. Ten-membered lactones from the marine-derived fungus Curvularia sp. J. Nat. Prod. 2008, 71, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; Kelter, G.; Maier, A.; Koenig, G.M.; Fiebig, H.-H. Apralactone A and a new stereochemical class of curvularins from the marine fungus Curvularia sp. Eur. J. Org. Chem. 2008, 2008, 5085–5092. [Google Scholar] [CrossRef] [PubMed]
- Chomnunti, P.; Schoch, C.L.; Aguirre-Hudson, B.; Ko-Ko, T.W.; Hongsanan, S.; Jones, E.B.G.; Kodsueb, R.; Phookamsak, R.; Chukeatirote, E.; Bahkali, A.H.; et al. Capnodiaceae. Fungal Divers. 2011, 51, 103–134. [Google Scholar] [CrossRef] [PubMed]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.-Y.; Kim, S.; Jhoo, J.-W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Biol. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision A.02); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Crane, R.I.; Hedden, P.; MacMillan, J.; Turner, W.B. Fungal products. part IV. The structure of heveadride, a new nonadride from Helminthosporium heveae. J. Chem. Soc. Perkin Trans. 1973, 1, 194–200. [Google Scholar] [CrossRef]
- Szwalbe, A.J.; Williams, K.; O’Flynn, D.E.; Bailey, A.M.; Mulholland, N.P.; Vincent, J.L.; Willis, C.L.; Cox, R.J.; Simpson, T.J. Novel nonadride, heptadride and maleic acid metabolites from the byssochlamic acid producer Byssochlamys fulva IMI 40021–an insight into the biosynthesis of maleidrides. Chem. Commun. 2015, 51, 17088–17091. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Tang, T.; Wang, L.-Y.; He, B.; Gao, K. Absolute configuration and biological activities of meroterpenoids from an endophytic fungus of Lycium barbarum. J. Nat. Prod. 2019, 82, 2229–2237. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, H.B.; Lim, C.-H.; Kim, C.-J.; Kwon, H.J. Cochlioquinone A1, a new anti-angiogenic agent from Bipolaris zeicola. Bioorganic Med. Chem. 2003, 11, 4743–4747. [Google Scholar] [CrossRef]
- Huber, C.; Court, W.; Devlin, J.; Edwards, O.; Scott, P. Stemphone: A new type of natural quinone. Tetrahedron Lett. 1974, 15, 2545–2548. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Sawang, K.; Siripong, P.; Tip-Pyang, S. Anhydrocochlioquinone A, a new antitumor compound from Bipolaris oryzae. Tetrahedron Lett. 2007, 48, 5193–5195. [Google Scholar] [CrossRef]
- Oka, M.; Iimura, S.; Tenmyo, O.; Sawada, Y.; Sugawara, M.; Ohkusa, N.; Yamamoto, H.; Kawano, K.; Hu, S.-L.; Fukagawa, Y.; et al. Terpestacin, a new syncytium formation inhibitor from Arthrinium sp. J. Antibiot. 1993, 46, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Berestetskiy, A.O.; Dalinova, A.A.; Dubovik, V.R.; Grigoryeva, E.N.; Kochura, D.M.; Senderskiy, I.V.; Smirnov, S.N.; Stepanycheva, E.A.; Turaeva, S.M. Analysis and isolation of secondary metabolites of Bipolaris sorokiniana by different chromatography techniques and the spectrum of their biological activity. Appl. Biochem. Microbiol. 2020, 56, 569–582. [Google Scholar] [CrossRef]
- Miyagawa, H.; Nagai, S.; Tsurushima, T.; Sato, M.; Ueno, T.; Fukami, H. Phytotoxins produced by the plant pathogenic fungus Bipolaris bicolor El-1. Biosci. Biotech. Biochem. 1994, 58, 1143–1145. [Google Scholar] [CrossRef][Green Version]
- Campos, F.F.; Ramos, J.P.; De Oliveira, D.M.; Alves, T.M.A.; De Souza-Fagundes, E.M.; Zani, C.L.; Sampaio, F.C.; Converti, A.; Cota, B.B. In vitro leishmanicidal, antibacterial and antitumour potential of anhydrocochlioquinone A obtained from the fungus Cochliobolus sp. J. Biosci. 2017, 42, 657–664. [Google Scholar] [CrossRef]
- Carruthers, J.R.; Cerrini, S.; Fedeli, W.; Casinovi, C.G.; Galeffi, C.; Vaccaro, A.M.T.; Scala, A. Structures of cochlioquinones A and B, new metabolites of Cochliobolus miyabeanus: Chemical and X-ray crystallographic determination. J. Chem. Soc. D 1971, 3, 164–166. [Google Scholar] [CrossRef]
- Ogawara, H.; Higashi, K.; Machida, T.; Takashima, J.; Chiba, N.; Mikawa, T. Inhibitors of diacyl glycerol kinase from Drechslera sacchari. J. Antibiot. 1994, 47, 499–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, Q.-Y.; Huang, L.; He, L.-W.; Han, J.-J.; Chen, Q.; Cai, L.; Liu, H.-W. Cochlioquinone derivatives with apoptosis-inducing effects on HCT116 colon cancer cells from the phytopathogenic fungus Bipolaris luttrellii L439. Chem. Biodivers. 2014, 11, 1892–1899. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, X.; Wan, C.-P.; Yu, Z.-F.; Zhang, K.-Q.; Li, G.-H. Chemical constituents of the fungus Veronaea sp. Chem. Nat. Compd. 2015, 51, 270–272. [Google Scholar] [CrossRef]
- Ye, B.; Ding, W.; Wang, P.-M.; Xu, J. Two new sesterterpenes from marine-derived fungus Arthrinium sp. Chem. Nat. Compd. 2019, 55, 281–284. [Google Scholar] [CrossRef]
- Masi, M.; Aloi, F.; Nocera, P.; Cacciola, S.O.; Surico, G.; Evidente, A. Phytotoxic metabolites isolated from Neufusicoccum batangarum, the causal agent of the scabby canker of cactus pear (Opuntia ficus-indica L.). Toxins 2020, 12, 126. [Google Scholar] [CrossRef]
- Saetang, P.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Borwornpinyo, S.; Seemakhan, S.; Muanprasat, C. Depsidones and an α-pyrone derivative from Simpilcillium sp. PSU-H41, an endophytic fungus from Hevea brasiliensis leaf. Phytochemistry 2017, 143, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Lee, H.B.; Kim, C.J.; Rho, J.-R.; Shin, J.; Kwon, H.J. Anti-angiogenic activity of terpestacin, a bicyclo sesterterpene from Embellisia chlamydospora. J. Antibiot. 2003, 56, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Guo, Z.K.; Wang, W.; Cui, J.T.; Tan, R.X.; Ge, H.M. Neuraminidase inhibitory terpenes from endophytic Cochliobolus sp. J. Asian Nat. Prod. Res. 2011, 13, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, B.; Schmidtke, M.; Dörfelt, H.; Kleinwächter, P.; Gräfe, U. (–)-Terpestacin and L-tenuazonic acid, inducers of pigment and aerial mycelium formation by Fusarium culmorum JP 15. J. Basic Microbiol. 2001, 41, 179–183. [Google Scholar] [CrossRef]
- Nihashi, Y.; Lim, C.-H.; Tanaka, C.; Miyagawa, H.; Ueno, T. Phytotoxic sesterterpene, 11-epiterpestacin, from Bipolaris sorokiniana NSDR-011. Biosci. Biotechnol. Biochem. 2002, 66, 685–688. [Google Scholar] [CrossRef] [PubMed]

| Position | δC | δH [mult, J in Hz] | HMBC (1H→13C) |
|---|---|---|---|
| 1 | 142.7 | ||
| 2 | 146.9 | ||
| 3 | 40.4 | 2.82 (td, 10.5, 2.7) | 1, 2, 4, 10, 3′ |
| 4 | 46.3 | 2.68 (m) | 3 |
| 5 | 148.3 | 6.46 (d, 11.7) | 4, 6, 13, 2″ |
| 6 | 131.2 | ||
| 7 | 44.9 | 3.49 (d, 12.0) | 5, 6, 8, 9, 12, 13 |
| 8 | 24.3 | 2.61 (m); 1.76 (dd, 13.0, 12.6) | 5, 6, 7, 9, 12 |
| 9 | 24.4 | 2.93 (dd, 13.0, 7.3); 2.46 (t, 13.0) | 1, 2, 7, 8, 11 |
| 10 | 165.9 | ||
| 11 | 165.7 | ||
| 12 | 177.6 | ||
| 13 | 169.8 | ||
| 1’ | 14.0 | 0.92 (t, 7.6) | 2’, 3’ |
| 2’ | 21.0 | 1.25 (m) | 3, 1’, 3’ |
| 3’ | 34.9 | 1.89 (m); 1.53 (m) | 1’ |
| 1” | 12.3 | 0.93 (t, 7.2) | 4, 2” |
| 2” | 25.9 | 1.98 (ddd, 13.2, 7.2, 2.5); 1.38 (m) | 4, 5, 1” |
| Samples (100 µg/mL) | Cell Viability (%) | |||
|---|---|---|---|---|
| RAW 264.7 | A549 (Lung Cancer) | SW480 (Colorectal Cancer) | K562 (Leukemic Cells) | |
| 1 | 97.14 ± 0.81 | 88.51 ± 1.19 | 82.60 ± 1.68 | 81.07 ± 3.80 |
| 2 | 10.80 ± 0.09 | 13.37 ± 0.48 | 14.95 ± 0.57 | 12.54 ± 1.24 |
| 3 | 71.38 ± 2.67 | 96.32 ± 3.70 | 75.59 ± 2.00 | 79.23 ± 1.86 |
| 4 | 11.07 ± 0.43 | 8.40 ± 0.58 | 14.39 ± 0.40 | 10.56 ± 0.20 |
| 5 | 82.59 ± 1.72 | 84.08 ± 3.01 | 97.87 ± 1.70 | 75.65 ± 1.19 |
| 6 | 99.34 ± 2.51 | 92.03 ± 3.75 | 97.69 ± 1.04 | 99.15 ± 3.84 |
| 5% DMSO (control) | 100.00 ± 2.47 | 100.00 ± 0.41 | 100.00 ± 2.49 | 100.00 ± 1.89 |
| Sample | Concentration | Cell Viability (%) | |||
|---|---|---|---|---|---|
| RAW 264.7 | A549 (Lung Cancer) | SW480 (Colorectal Cancer) | K562 (Leukemic Cells) | ||
| 2 | 100 | 10.80 ± 0.09 | 13.37 ± 0.48 | 14.95 ± 0.57 | 12.54 ± 1.24 |
| 50 | 11.67 ± 0.08 | 14.77 ± 2.54 | 15.64 ± 0.77 | 13.35 ± 0.89 | |
| 25 | 15.00 ± 0.29 | 17.92 ± 1.93 | 17.56 ± 0.70 | 21.01 ± 2.61 | |
| 12.5 | 16.61 ± 0.52 | 23.07 ± 1.84 | 23.41 ± 0.25 | 38.42 ± 3.18 | |
| 6.25 | 28.48 ± 1.98 | 73.11 ± 1.09 | 30.75 ± 0.40 | 70.95 ± 1.69 | |
| IC50 (µM) | <11.73 | 14.34 | <11.73 | 17.59 | |
| 4 | 100 | 11.07 ± 0.43 | 8.40 ± 0.58 | 14.39 ± 0.40 | 10.56 ± 0.20 |
| 50 | 12.79 ± 0.70 | 9.35 ± 0.52 | 14.71 ± 0.24 | 11.27 ± 0.40 | |
| 25 | 12.14 ± 0.42 | 10.16 ± 0.17 | 15.37 ± 0.42 | 12.88 ± 0.34 | |
| 12.5 | 12.45 ± 0.47 | 11.58 ± 1.05 | 15.59 ± 0.39 | 14.03 ± 2.31 | |
| 6.25 | 14.32 ± 0.73 | 12.39 ± 0.17 | 17.29 ± 0.33 | 19.48 ± 2.62 | |
| IC50 (µM) | <12.14 | <12.14 | <12.14 | <12.14 | |
| Samples | NO Production Inhibition | |
|---|---|---|
| % of NO Inhibition at 100 µg/mL | IC50 (µM) | |
| 1 | 51.30 ± 1.65 | 53.7 |
| 2 | not tested * | not tested * |
| 3 | 73.41 ± 0.44 | 32.8 |
| 4 | not tested * | not tested * |
| 5 | 74.01 ± 1.14 | 12.8 |
| 6 | 41.03 ± 3.36 | inactive |
| Indomethacin | 77.45 ± 0.28 | 73.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suthiphasilp, V.; Raksat, A.; Maneerat, T.; Hadsadee, S.; Jungsuttiwong, S.; Pyne, S.G.; Chomnunti, P.; Jaidee, W.; Charoensup, R.; Laphookhieo, S. Cytotoxicity and Nitric Oxide Production Inhibitory Activities of Compounds Isolated from the Plant Pathogenic Fungus Curvularia sp. J. Fungi 2021, 7, 408. https://doi.org/10.3390/jof7060408
Suthiphasilp V, Raksat A, Maneerat T, Hadsadee S, Jungsuttiwong S, Pyne SG, Chomnunti P, Jaidee W, Charoensup R, Laphookhieo S. Cytotoxicity and Nitric Oxide Production Inhibitory Activities of Compounds Isolated from the Plant Pathogenic Fungus Curvularia sp. Journal of Fungi. 2021; 7(6):408. https://doi.org/10.3390/jof7060408
Chicago/Turabian StyleSuthiphasilp, Virayu, Achara Raksat, Tharakorn Maneerat, Sarinya Hadsadee, Siriporn Jungsuttiwong, Stephen G. Pyne, Putarak Chomnunti, Wuttichai Jaidee, Rawiwan Charoensup, and Surat Laphookhieo. 2021. "Cytotoxicity and Nitric Oxide Production Inhibitory Activities of Compounds Isolated from the Plant Pathogenic Fungus Curvularia sp." Journal of Fungi 7, no. 6: 408. https://doi.org/10.3390/jof7060408
APA StyleSuthiphasilp, V., Raksat, A., Maneerat, T., Hadsadee, S., Jungsuttiwong, S., Pyne, S. G., Chomnunti, P., Jaidee, W., Charoensup, R., & Laphookhieo, S. (2021). Cytotoxicity and Nitric Oxide Production Inhibitory Activities of Compounds Isolated from the Plant Pathogenic Fungus Curvularia sp. Journal of Fungi, 7(6), 408. https://doi.org/10.3390/jof7060408








