Characterizing the Assemblage of Wood-Decay Fungi in the Forests of Northwest Arkansas
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Specimens
2.2. Morphological Descriptions
2.3. DNA Extraction, PCR, and Sequencing.
2.4. Calculation of Coefficient of Community Indices
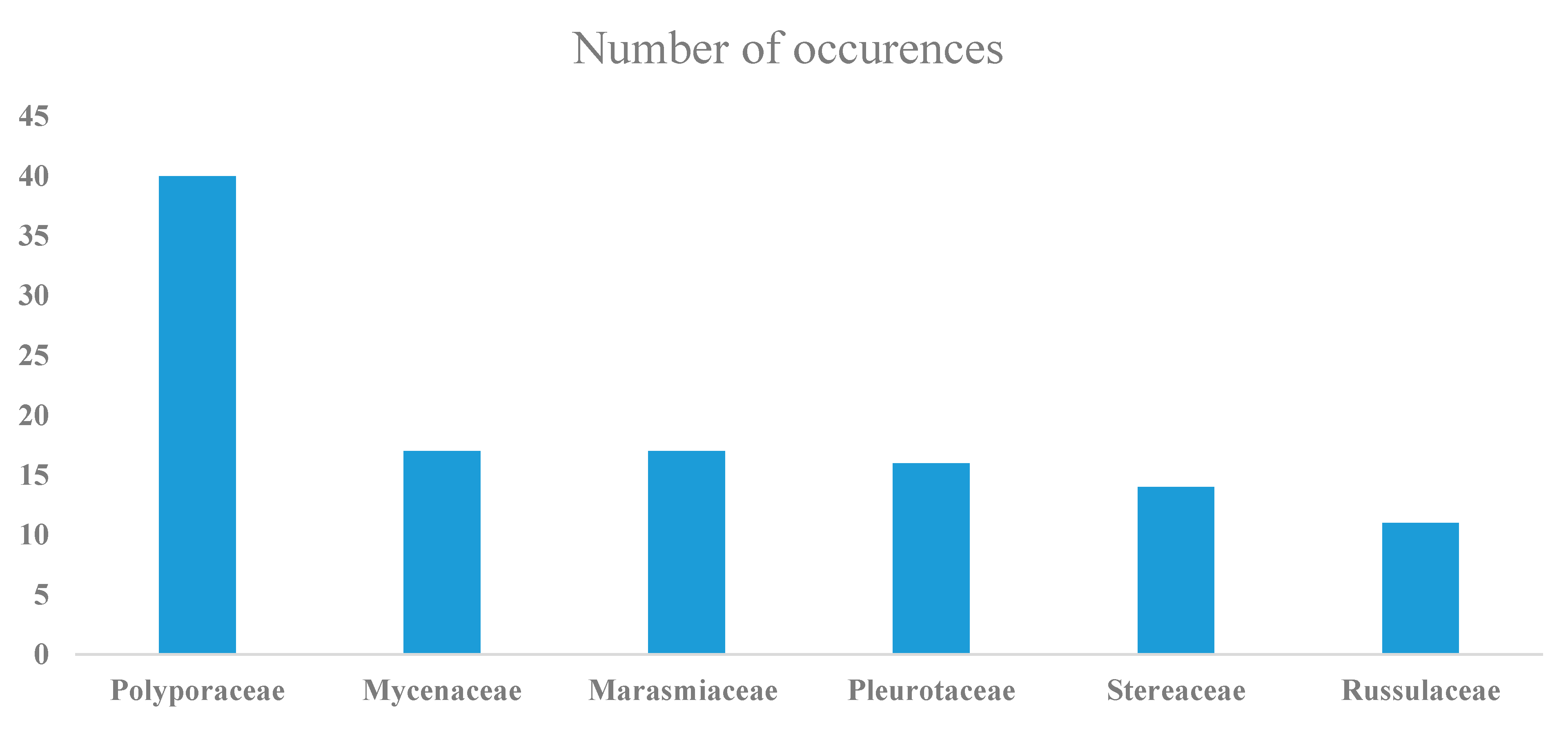
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gora, E.M.; Sayer, E.J.; Turner, B.L.; Tanner, E.V.J. Decomposition of coarse woody debris in a long-term litter manipulation experiment: A focus on nutrient availability. Funct. Ecol. 2018, 32, 1128–1138. [Google Scholar] [CrossRef]
- Kwaśna, H.; Mazur, A.; Kuźmiński, R.; Jaszczak, R.; Turski, M.; Behnke-Borowczyk, J.; Adamowicz, K.; Łakomy, P. Abundance and diversity of wood-decay fungi in managed and unmanaged stands in a Scots pine forest in Western Poland. For. Ecol. Manag. 2017, 400, 438–446. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Blanchette, R.A. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Eriksson, K.-E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer Science & Business Media; Springer: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Stephenson, S.L. The Kingdom Fungi: The Biology of Mushrooms, Molds, and Lichens; Timber Press: Portland, OR, USA, 2010. [Google Scholar]
- Stephenson, S.L.; Adams, H.S.; Huebner, C.D. Upland forest vegetation of the Ozark Mountains in northwestern Arkansas. Rhodora 2007, 109, 197–221. [Google Scholar] [CrossRef]
- Cannon, P.F.; Sutton, B.C. Microfungi on wood and plant debris. In Biodiversity of fungi; Mueller, G., Bills, G., Foster, M., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2004; pp. 217–239. [Google Scholar]
- Alshammari, N.I.; Veettil, V.N.; Sulieman, A.M.E.; Stephenson, S.L. Impact of Field and Laboratory Environmental Conditions on the Diversity of Wood-Decay Fungi in the Forests of Northwest Arkansas. J. Pure Appl. Microbiol. 2020, 14, 1801–1808. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Ryvarden, L. North American Polypores Vol. 2. Megasporoporia-Wrightoporia; Fungiflora: Oslo, Norway, 1987; pp. 437–885. [Google Scholar]
- Bessette, A.E.; Bessette, A.R.; Fischer, D.W. Mushrooms of Northeastern North America; Syracuse University Press: Syracuse, NY, USA, 1997. [Google Scholar]
- Barron, G.L. Mushrooms of Northeast North America: Midwest to New England; Lone Pine Publishing: Tukwila, WA, USA, 1999. [Google Scholar]
- Binion, D.E.; Stephenson, S.L.; Roody, W.C.; Burdsall, H.H.; Vasilyeva, L.N.; Miller, O.K. Macrofungi Associated with Oaks of Eastern North Americ; West Virginia University Press: Morgantown, WV, USA, 2008. [Google Scholar]
- Elliott, T.F.; Stephenson, S.L. Mushrooms of the Southeast; Timber Press: Portland, OR, USA, 2018. [Google Scholar]
- Ellenberg, D.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Leininger, T.D.; Wilson, A.D.; Lester, D.G. Hurricane Andrew damage in relation to wood decay fungi and insects in bottomland hardwoods of the Atchafalaya Basin, Louisiana. J. Coast. Res. 1997, 1, 1290–1293. [Google Scholar]
- Bhutia, L.P. A preliminary survey report on diversity of wood decaying fungi from East Sikkim. J. Mycopathol. Res. 2016, 54, 255–261. [Google Scholar]
- Alemu, F. Assessment of wild mushrooms and wood decaying fungi in Dilla University, main campus, Ethiopia. Int. J. Adv. Res. 2013, 1, 458–467. [Google Scholar]
- Lyngdoh, A.; Dkhar, M.S. Wood-rotting fungi in East Khasi Hills of Meghalaya, Northeast India, with special reference to Heterobasidion perplexa (a rare species-new to India). Curr. Res. Environ. Appl. Mycol. 2014, 4, 117–124. [Google Scholar] [CrossRef]
- Alshammari, N.; Stephenson, S. A preliminary study of wood-decay fungi in forests of northwest Arkansas. Curr. Res. Environ. Appl. Mycol. 2018, 8, 11. [Google Scholar] [CrossRef]
- Worrall, J.J.; Anagnost, S.E.; Zabel, R.A. Comparison of wood decay among diverse lignicolous fungi. Mycologia 1997, 89, 199–219. [Google Scholar] [CrossRef]
- Alshammari, N.; Albalawi, T.H.; Stephenson, S.L. Prescribed burning reduces species richness of wood-decay fungi in the forests of northwest Arkansas. Saudi J. Biol. Sci. 2021, 28, 936–941. [Google Scholar] [CrossRef] [PubMed]

| Number of Records | ||||||
|---|---|---|---|---|---|---|
| Size (Volume cm3) | Exidia Recisa | Sarcoscypha Occidentalis | Trametes Elegans | Trichaptum Biforme | Stereum Ostrea | Fuscoporia Gilva |
| Large | 0 | 0 | 4 | 3 | 9 | 2 |
| Intermediate | 0 | 0 | 6 | 6 | 14 | 13 |
| Small | 0 | 0 | 2 | 5 | 0 | 1 |
| Very small | 17 | 6 | 0 | 0 | 0 | 0 |
| Number of Records | ||||||
|---|---|---|---|---|---|---|
| Size (Diameter cm) | Exidia Recisa | Sarcoscypha Occidentalis | Trametes Elegans | Trichaptum Biforme | Stereum Ostrea | Fuscoporia Gilva |
| Thick | 1 | 0 | 2 | 1 | 3 | 0 |
| Intermediate | 0 | 0 | 2 | 4 | 5 | 5 |
| Thin | 0 | 0 | 5 | 4 | 14 | 7 |
| Very thin | 16 | 6 | 3 | 5 | 1 | 4 |
| Taxon | Percentage of Bark | ||||
|---|---|---|---|---|---|
| 75 to 100% | 50 to 75% | 50 to 25% | <25% | No Bark | |
| Stereum ostrea | 14 | 7 | 0 | 0 | 0 |
| Trichaptum biforme | 9 | 2 | 0 | 0 | 0 |
| Fuscoporia gilva | 5 | 6 | 3 | 0 | 0 |
| Trametes elegans | 6 | 2 | 1 | 0 | 0 |
| Sarcoscypha occidentalis | 6 | 0 | 0 | 0 | 0 |
| Exidia recisa | 9 | 7 | 0 | 0 | 0 |
| Phellinus stipticus | 7 | 0 | 0 | 0 | 0 |
| Auricularia americana | 5 | 0 | 0 | 0 | 0 |
| Morganella pyriformis | 5 | 0 | 0 | 0 | 0 |
| Deadaleopsis confragosa | 5 | 0 | 0 | 0 | 0 |
| All Three Study Areas | Only PRP | Only DDP | Only PFR |
|---|---|---|---|
| Trametopsis cervina | Phanerochaete pseudosanguinea | Daedaleopsis septentrionalis | Marasmiellus candidus |
| Panellus stypticus | Pluteus petasatus | Eurotium rubrum | Rhodotus palmatus |
| Stereum ostrea | Hymenochaete pinnatifida | Hericium erinaceum | Mycena zephirus |
| Trichaptum biforme | Gloeoporus dichrous | Merulius incarnatus | Phaeomarasmius erinaceellus |
| Crucibulum laeve | Gymnopus erythropus | Mycena haematopus | Lactarius rubrocinctus |
| Steccherinum murashkinskyi | Hydnochaete tabacina | Mycena haematopus | Pleurotus pulmonarius |
| Lenzites betulinus | Trametes conchifer | Mycena inclinata | Pluteus glaucotinctus |
| Exidia recisa | Tyromyces kmetii | Mycena leaiana | Diatrype stigma |
| Study Area | Coefficient of Community (CC) | ||
|---|---|---|---|
| PRP | DDP | BFR | |
| PRP | *** | 0.20 | 0.08 |
| DDP | 0.20 | *** | 0.07 |
| BFR | 0.08 | 0.07 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, N.; Ameen, F.; AlKahtani, M.D.F.; Stephenson, S. Characterizing the Assemblage of Wood-Decay Fungi in the Forests of Northwest Arkansas. J. Fungi 2021, 7, 309. https://doi.org/10.3390/jof7040309
Alshammari N, Ameen F, AlKahtani MDF, Stephenson S. Characterizing the Assemblage of Wood-Decay Fungi in the Forests of Northwest Arkansas. Journal of Fungi. 2021; 7(4):309. https://doi.org/10.3390/jof7040309
Chicago/Turabian StyleAlshammari, Nawaf, Fuad Ameen, Muneera D. F. AlKahtani, and Steven Stephenson. 2021. "Characterizing the Assemblage of Wood-Decay Fungi in the Forests of Northwest Arkansas" Journal of Fungi 7, no. 4: 309. https://doi.org/10.3390/jof7040309
APA StyleAlshammari, N., Ameen, F., AlKahtani, M. D. F., & Stephenson, S. (2021). Characterizing the Assemblage of Wood-Decay Fungi in the Forests of Northwest Arkansas. Journal of Fungi, 7(4), 309. https://doi.org/10.3390/jof7040309






