Disseminated Mucormycosis in Immunocompromised Children: Are New Antifungal Agents Making a Difference? A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnostic Methods
3. Results
3.1. Patient Characteristics
3.2. Clinical Presentation and Pathogens
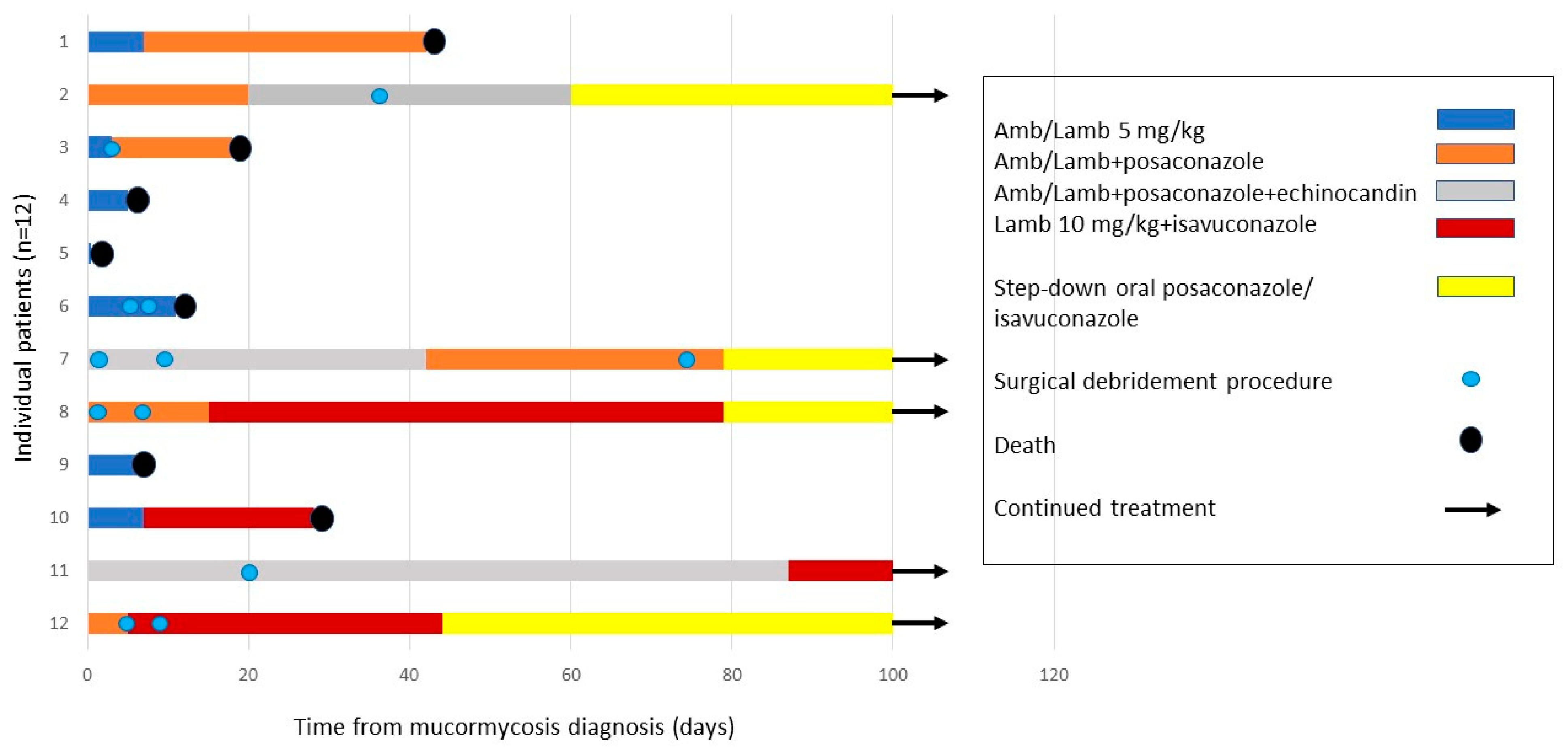
3.3. Treatment
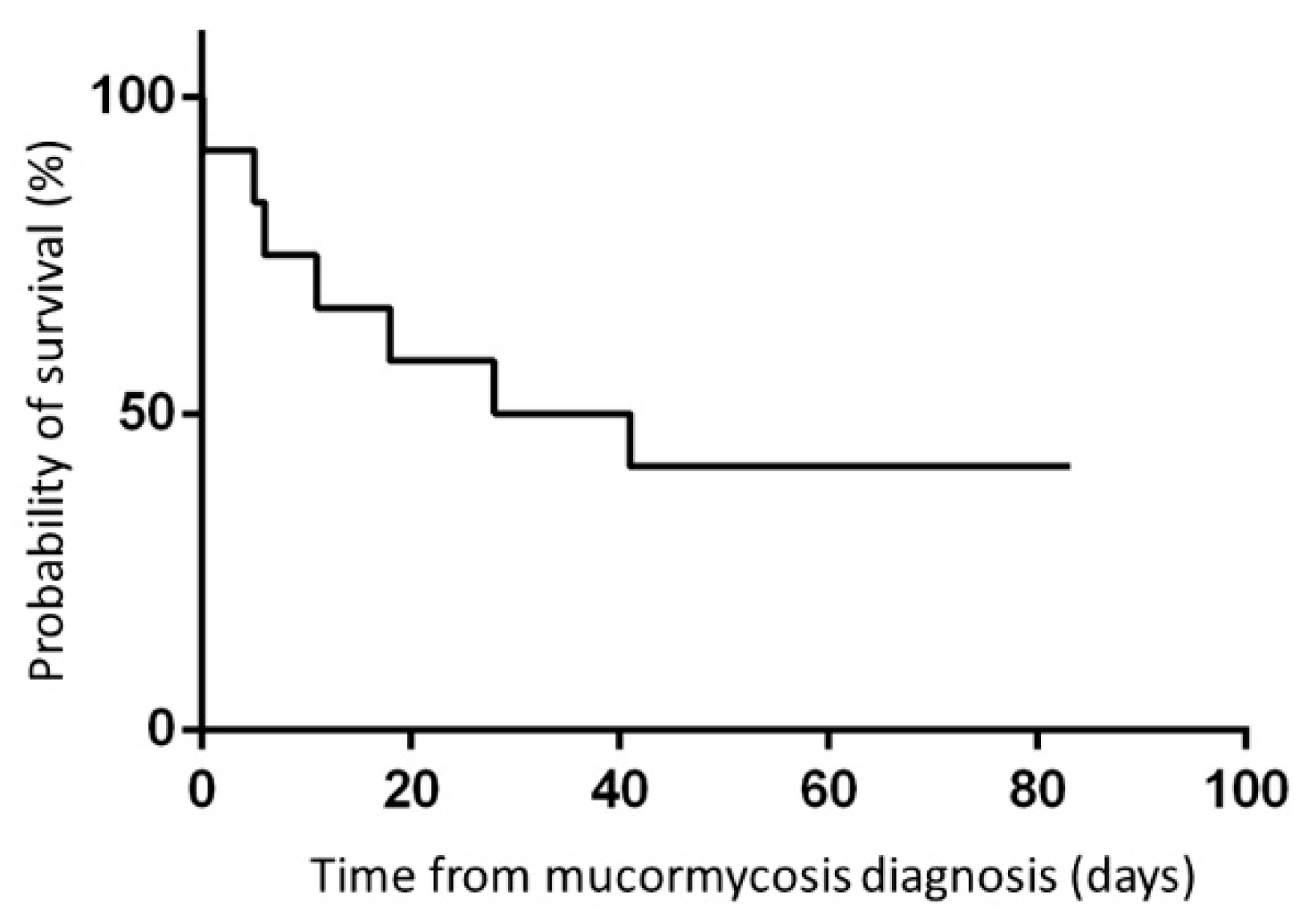
3.4. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study Group. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54, 35–43. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef]
- Elitzur, S.; Arad-Cohen, N.; Barg, A.; Litichever, N.; Bielorai, B.; Elhasid, R.; Fischer, S.; Fruchtman, Y.; Gilad, G.; Kapelushnik, J.; et al. Mucormycosis in children with haematological malignancies is a salvageable disease: A report from the Israeli Study Group of Childhood Leukemia. Br. J. Haematol. 2020, 189, 339–350. [Google Scholar] [CrossRef]
- Styczynski, J.; Czyzewski, K.; Fraczkiewicz, J.; Salamonowicz, M.; Piekarska, A.; Adamska, M.; Galazka, P.; Mensah-Glanowska, P.; Drozd-Sokolowska, J.; Waszczuk-Gajda, A.; et al. Clinical spectrum and outcome of invasive mucormycosis in children and adults: Polish experience of the decade 2010–2019. Acta Haematol. Pol. 2020, 51, 157–163. [Google Scholar] [CrossRef]
- Zaoutis, T.E.; Roilides, E.; Chiou, C.C.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Scaufer, R.L.; Sein, M.; Sein, T.; Prasad, P.A.; et al. Zygomycosis in children: A systematic review and analysis of reported cases. Pediatr Infect. Dis. J. 2007, 26, 723–727. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Groll, A.H.; Castagnola, E.; Cesaro, S.; Dalle, J.H.; Engelhard, D.; Hope, W.; Roilides, E.; Styczynski, J.; Warris, A.; Lehrnbecher, T. Fourth European Conference on Infections in Leukemia (ECIL-4): Guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haematopoietic stem-cell transplantation. Lancet Oncol. 2014, 15, e327–e340. [Google Scholar] [CrossRef]
- Eighth European Conference on Infections in Leukemia (ECIL-8) Guidelines. Available online: http://www.ecil-leukaemia.com/resources.htm (accessed on 16 February 2021).
- Davoudi, S.; Anderlini, P. A Long-Term Survivor of Disseminated Aspergillus and Mucorales Infection: An Instructive Case. Mycopathologica 2014, 178, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.; Gagne, L.S.; Hammond, S.P.; Gilmore, E.T.; Joyce, A.C.; Soiffer, R.J.; Marty, F.M. Isavuconazole Treatment of a Patient with Disseminated Mucormycosis. J. Clin. Microbiol. 2014, 52, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Pomorska, A.; Malecka, A.; Jaworski, R.; Radon-Proskura, J.; Hare, K.R.; Nielsen, H.V.; Andersen, L.O.; Jensen, H.E.; Arendrup, M.C.; Irga-Jaworska, N. Isavuconazole in a Successful Combination Treatment of Disseminated Mucormycosis in a Child with Acute Lymphoblastic Leukaemia and Generalized Haemochromatosis: A Case Report and Review of the Literature. Mycopathologia 2019, 184, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cornu, M.; Bruno, B.; Loridant, S.; Navarin, P.; François, N.; Lanternier, F.; Amzallag-Bellenger, E.; Dubos, F.; Mazingue, F.; Sendid, B. Successful outcome of disseminated mucormycosis in a 3-year-old child suffering from acute leukaemia: The role of isavuconazole? A case report. BMC Pharmacol. Toxicol. 2018, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Guymer, C.; Khurana, S.; Suppiah, R.; Hennessey, I.; Cooper, C. Successful treatment of disseminated mucormycosis in a neutropenic patient with T-cell acute lymphoblastic leukaemia. BMJ Case Rep. 2013, 1–5. [Google Scholar] [CrossRef]
- Rickerts, V.; Atta, J.; Herrmann, S.; Jacobi, V.; Lambrecht, E.; Bialek, R. Successful treatment of disseminated mucormycosis with a combination of liposomal amphotericin B and posaconazole in a patient with acute myeloid leukaemia. Mycoses 2006, 49, 27–30. [Google Scholar] [CrossRef]
- Aftandilian, C.; Mathew, R.; Messner, A. Mucormycosis diagnosed during induction chemotherapy in five pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer 2019, 66, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.J.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, M.; Levi, I.; Steinberg, R.; Shonfeld, T.; Shapiro, R.; Israeli, M.; Sprecher, H.; Shalit, I.; Mor, E. Mucormycosis in a liver allograft: Salvage re-transplantation and targeted immunosuppressive management. Transpl. Infect. Dis. 2012, 14, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Albert, N.D.; Liao, G.; Hou, J.; Prince, R.A.; Kontoyiannis, D.P. Comparative Pharmacodynamics of Amphotericin B Lipid Complex and Liposomal Amphotericin B in a Murine Model of Pulmonary Mucormycosis. Antimicrob. Agents Chemother. 2010, 54, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Poiree, S.; Elie, C.; Bakouboula, P.; Sitbon, K.; Herbrecht, R.; Wolff, M.; Ribaud, P.; Lortholary, O.; French Mycosis Study Group. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J. Antimicrob. Chemother. 2015, 70, 3116–3123. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Kyvernitakis, A.; Torres, H.A.; Jiang, Y.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: A propensity score analysis. Clin. Microbiol. Infect. 2016, 22, 811.e1–811.e8. [Google Scholar] [CrossRef]
- Lamoth, F.; Kontoyiannis, D.P. Therapeutic Challenges of Non- Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob. Agents Chemother. 2019, 63, e01244-19. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying Amphotericin B–Based Frontline Therapy Significantly Increases Mortality among Patients with Hematologic Malignancy Who Have Zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Cassaing, S.; Chouaki, T.; Kauffman-Lacroix, C.; Poirier, P.; Toubas, D.; et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: Retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, 810.e1–810.e8. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology ( ECMM ) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Vironneau, P.; Kania, R.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Herman, P.; Lortholary, O.; Lanternier, F.; French Mycosis Study Group. Local control of rhino-orbito-cerebral mucormycosis dramatically impacts survival. Clin. Microbiol. Infect. 2014, 20, 336–339. [Google Scholar] [CrossRef]
- Lelievre, L.; Garcia-Hermoso, D.; Abdoul, H.; Hivelin, M.; Chouaki, T.; Toubas, D.; Mamez, A.C.; Lantieri, L.; Lortholary, O.; Lanternier, F. Posttraumatic mucormycosis: A nationwide study in France and review of the literature. Medicine 2014, 93, 395–404. [Google Scholar] [CrossRef]
- Chretien, M.L.; Legouge, C.; Pagès, P.B.; Lafon, I.; Ferrant, E.; Plocque, A.; Favennec, C.; Estivalet, L.; Bottolier-Lemallaz, E.; Dalle, F.; et al. Emergency and elective pulmonary surgical resection in haematological patients with invasive fungal infections: A report of 50 cases in a single centre. Clin. Microbiol. Infect. 2016, 22, 782–787. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [PubMed]
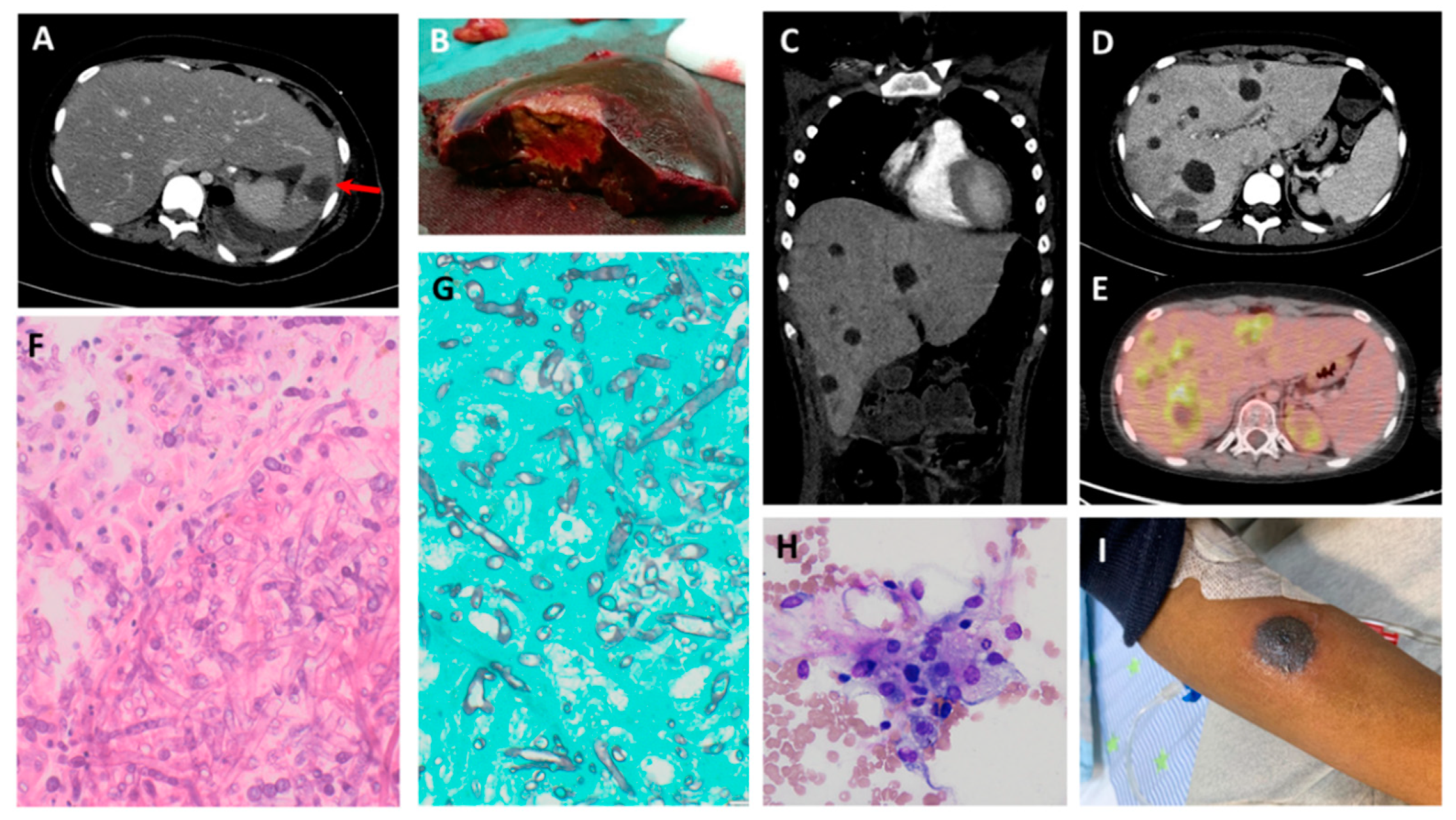
| Sex | Age (Years) | Underlying Condition | Phase of Therapy | Duration of Neutropenia (Days) | Clinical Presentation | Sites of Involvement | Pathogen | Diagnostic Method | Preceding Bacterial Infection (within 14 Days) | Fungal Co-Infection | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 19.2 | Relapsed ALL | Post-allogeneic HSCT (19 days) | 21 | Pleuritic chest pain | Lung, skin, and soft tissue | Rhizopus oryzae | Culture | ||
| 2 | F | 10 | SOT—liver | Post liver-transplant (8 weeks) | 0 | Prolonged fever | Liver allograft, pericardial effusion, diaphragm, and gastric wall | Rhizopus oryzae | Pathology and PCR | E. coli peritonitis | Candida krusei peritonitis (proven) |
| 3 | M | 8.9 | ALL | Delayed intensification | 8 | Prolonged fever and obtundation | Lung, sinus, and brain | Rhizopus oryzae | Pathology and PCR | ||
| 4 | F | 16.5 | AML | Consolidation | 14 | Prolonged fever and convulsions | Lung, brain, liver, and kidneys | Rhizopus pusillus | Pathology and PCR | K. pneumoniae sepsis | |
| 5 | F | 16 | ALL | Induction | 35 | Hemiparesis and convulsions | Lung, heart, bowel, brain, kidneys, and liver | Mucor spp | Pathology and culture | S. aureus sepsis | |
| 6 | M | 11.5 | ALL | Induction | 22 | Fever and abdominal pain | Bowel, liver, spleen, and gastric wall | Unknown | Pathology | ||
| 7 | M | 11.2 | Metastatic medulloblastoma post-autologous HSCT | Post- fourth autologous HSCT (10 days) | 8 | Fever and abdominal pain | Bowel, liver, abdominal abscesses, and lung | Lichtheimia ramosa | Culture, pathology, and PCR | ||
| 8 | F | 13.4 | ALL | Induction | 25 | Prolonged fever and signs of peritoneal irritation | Liver, spleen, bowel, and kidney | Lichtheimia corymbifera | Culture, pathology, and PCR | P. aeruginosa sepsis | Pulmonary aspergillosis (probable) |
| 9 | F | 15 | AML | Induction | 28 | Acute renal failure requiring hemodialysis | Brain, lung, bowel, kidneys, liver, and spleen | Unknown | Pathology | A. baumani sepsis | |
| 10 | F | 10 | Mature B-cell (Burkitt) leukemia | Induction | 11 | Fever and abdominal pain | Sinus, liver, and bowel | Lichtheimia ramosa | Culture and pathology | A lwoffii sepsis | Pulmonary aspergillosis (probable) |
| 11 | F | 14.4 | AML | Consolidation | 11 | Fever and abdominal pain | Liver, bowel, and lung | Mucor spp. | Culture, pathology, and PCR | K pneumoniae sepsis | |
| 12 | M | 13.5 | AML | Consolidation | 7 | Necrotic cutaneous lesions | Disseminated skin (right arm, right foot, right ankle, and scalp) | Lichtheimia ramosa | Culture, pathology, and PCR | Pulmonary aspergillosis (Aspergillus niger) (proven) |
| Amphotericin B | Posaconazole | Voriconazole | Itraconazole | Caspofungin | Anidulafungin | ||
|---|---|---|---|---|---|---|---|
| Patient no. 8 | Lichtheimia corymbifera | 0.125 | 0.5 | 0.5 | 0.25 | >8 | >4 |
| Patient no. 10 | Lichtheimia ramosa | 0.25 | 2 | >16 | >16 | >8 | 2 |
| Patient no. 11 | Mucor spp. | 0.06 | 1 | >16 | >16 | >8 | >16 |
| Patient no. 12 | Lichtheimia ramosa | 0.125 | 1 | >16 | 2 | >5 | >16 |
| Year of Mucormycosis Diagnosis | Antifungal Prophylaxis | First-Line Antifungal Treatment | Second-Line Antifungal Treatment | Step-Down Treatment | Surgical Debridement Procedures | Outcome | Follow-Up (Days) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 2009 | Voricinazole | AmB | L-AmB 5 mg/kg/d posaconazole susp | None | Death | 42 | |
| 2 | 2009 | Fluconazole | L-AmB 5 mg/kg/d posaconazole susp | L-AmB 5 mg/kg/d caspofunginposaconazole susp | Posaconazole susp (12 months) | Liver re-transplantation, splenectomy, partial gastrectomy, thoracotomy, and pericardial window | Survival | 4188 (137.5 months) |
| 3 | 2010 | None | AmB caspofungin | L-AmB 5 mg/kg/d posaconazol susp | FESS | Death | 18 | |
| 4 | 2012 | Itraconazole | AmB | L-AmB 5 mg/kg/d | None | Death | 5 | |
| 5 | 2012 | Fluconazole | L-AmB 5 mg/kg/d | - | None | Death | 0 | |
| 6 | 2015 | Itraconazole | AmB | - | 1. Resection of transverse colon 2. Resection of small intestine 3. Exploratory laparotomy | Death | 11 | |
| 7 | 2016 | Fluconazole | L-AmB 7.5 mg/kg anidulafungin/ caspofungin | L-AmB IV posaconazole | PO posaconazole TAB | 1. Appendectomy and drainage of hepatic abscess 2. Right hemicolectomy, partial hepatectomy, debridement of diaphragm and abdominal wall, and thoracoscopic decortication of right lung =3. Repeat partial hepatectomy | Survival | 1797 (59 months) |
| 8 | 2017 | Itraconazole | L-AmB 5 mg/kg/d IV posaconazole | L-AmB 10 mg/kg/d IV isavuconazole | PO isavuconazole, followed byposaconazole TAB | 1. Bowel resections 2. Splenectomy, partial hepatectomy, left nephrectomy, and debridement of abdominal wall | Survival | 1260 (41.4 months) |
| 9 | 2019 | Fluconazole | L-AmB 5mg/kg/d | - | None | Death | 6 | |
| 10 | 2019 | Anidulafungin | L-AmB 5mg/kg/d | L-AmB 10 mg/kg/d IV isavuconazole | None | Death | 28 | |
| 11 | 2020 | Itraconazole | L-AmB 10 mg/kg/d IV posaconazole caspofungin | L-AmB 10 mg/kg/d IV isavuconazole | Posaconazole TAB | Right hemicolectomy, left hemicolectomy, partial resection of small intestine, partial hepatectomy, and omentectomy | Survival | 363 (11.9 months) |
| 12 | 2020 | None | L-AmB 5 mg/kg/d IV posaconazole | L-AmB 7.5 mg/kg IV isavuconazole | PO isavuconazole | 1. Excisions of skin lesions 2. FESS 3. Pulmonary left lower lobe lobectomy | Survival | 261 days (8.6 months) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elitzur, S.; Fischer, S.; Arad-Cohen, N.; Barg, A.; Ben-Harosh, M.; Danino, D.; Elhasid, R.; Gefen, A.; Gilad, G.; Levy, I.; et al. Disseminated Mucormycosis in Immunocompromised Children: Are New Antifungal Agents Making a Difference? A Multicenter Retrospective Study. J. Fungi 2021, 7, 165. https://doi.org/10.3390/jof7030165
Elitzur S, Fischer S, Arad-Cohen N, Barg A, Ben-Harosh M, Danino D, Elhasid R, Gefen A, Gilad G, Levy I, et al. Disseminated Mucormycosis in Immunocompromised Children: Are New Antifungal Agents Making a Difference? A Multicenter Retrospective Study. Journal of Fungi. 2021; 7(3):165. https://doi.org/10.3390/jof7030165
Chicago/Turabian StyleElitzur, Sarah, Salvador Fischer, Nira Arad-Cohen, Assaf Barg, Miriam Ben-Harosh, Dana Danino, Ronit Elhasid, Aharon Gefen, Gil Gilad, Itzhak Levy, and et al. 2021. "Disseminated Mucormycosis in Immunocompromised Children: Are New Antifungal Agents Making a Difference? A Multicenter Retrospective Study" Journal of Fungi 7, no. 3: 165. https://doi.org/10.3390/jof7030165
APA StyleElitzur, S., Fischer, S., Arad-Cohen, N., Barg, A., Ben-Harosh, M., Danino, D., Elhasid, R., Gefen, A., Gilad, G., Levy, I., Shachor-Meyouhas, Y., Weinreb, S., Izraeli, S., & Barzilai-Birenboim, S. (2021). Disseminated Mucormycosis in Immunocompromised Children: Are New Antifungal Agents Making a Difference? A Multicenter Retrospective Study. Journal of Fungi, 7(3), 165. https://doi.org/10.3390/jof7030165







