A Pilot Study on Baseline Fungi and Moisture Indicator Fungi in Danish Homes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Locations
2.2. Sampling and Treatment Protocols
2.3. Additional Samples
2.4. Culture-Independent Method: DNA Sequencing
2.5. Data Treatment
| Genus | Total DNA Reads | Outdoors | Indoors | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Out | In | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | H-9 | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | H-9 | |
| Alternaria | 2785 | 7596 | 0 | 908 | 189 | 225 | 50 | 452 | 248 | 7 | 706 | 244 | 461 | 865 | 1931 | 55 | 0 | 2418 | 1619 | 3 |
| Aspergillus | 364 | 6784 | 1 | 6 | 36 | 20 | 1 | 269 | 17 | 0 | 14 | 0 | 2767 | 405 | 386 | 2772 | 101 | 295 | 57 | 1 |
| Aureobasidium * | 3724 | 1893 | 149 | 1259 | 4 | 990 | 22 | 5 | 1079 | 0 | 216 | 24 | 239 | 245 | 509 | 110 | 0 | 555 | 211 | 0 |
| Blumeria * | 4275 | 1837 | 147 | 559 | 590 | 146 | 32 | 1569 | 324 | 5 | 903 | 535 | 5 | 244 | 0 | 0 | 0 | 0 | 1053 | 0 |
| Botrytis * | 1488 | 5652 | 0 | 711 | 0 | 1 | 4 | 701 | 71 | 0 | 0 | 594 | 3 | 702 | 831 | 0 | 1832 | 942 | 748 | 0 |
| Cladosporium | 5193 | 10,645 | 67 | 1202 | 327 | 1558 | 52 | 579 | 614 | 56 | 738 | 1110 | 1942 | 1297 | 2023 | 173 | 57 | 1313 | 2666 | 64 |
| Devriesia * | 3740 | 410 | 130 | 965 | 124 | 301 | 1896 | 79 | 220 | 0 | 25 | 0 | 89 | 65 | 41 | 78 | 0 | 89 | 48 | 0 |
| Exophiala * | 7198 | 129 | 0 | 124 | 510 | 773 | 224 | 4395 | 438 | 17 | 717 | 0 | 0 | 39 | 47 | 0 | 0 | 0 | 43 | 0 |
| Itersonilia * | 3242 | 1693 | 7 | 11 | 1440 | 534 | 48 | 25 | 1168 | 8 | 1 | 439 | 7 | 822 | 4 | 0 | 0 | 214 | 207 | 0 |
| Knufia * | 2618 | 234 | 0 | 0 | 247 | 320 | 84 | 38 | 867 | 0 | 1062 | 0 | 86 | 50 | 0 | 0 | 0 | 0 | 98 | 0 |
| Penicillium | 466 | 5103 | 0 | 52 | 42 | 1 | 0 | 139 | 162 | 9 | 61 | 1065 | 258 | 480 | 179 | 2081 | 0 | 908 | 43 | 89 |
| Pseudopithomyces * | 264 | 7069 | 69 | 82 | 0 | 7 | 9 | 0 | 97 | 0 | 0 | 97 | 545 | 1988 | 1626 | 0 | 0 | 1728 | 1085 | 0 |
| Scoliciosporum * | 5317 | 25 | 108 | 139 | 911 | 160 | 4 | 0 | 85 | 1 | 3909 | 22 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 |
| Taphrina * | 3420 | 353 | 0 | 0 | 50 | 282 | 1235 | 53 | 71 | 0 | 1729 | 29 | 0 | 165 | 0 | 0 | 0 | 95 | 64 | 0 |
| Wallemia * | 1 | 746 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 107 | 0 | 0 | 548 | 0 | 91 | 0 | 0 |
| Total DNA reads † | 104,858 | 85,701 | 5853 | 15,950 | 10,571 | 10,491 | 17,566 | 14,328 | 13,337 | 761 | 16,001 | 8928 | 11,366 | 17,046 | 10,082 | 8604 | 5977 | 12,245 | 11,273 | 180 |
| Species | Total DNA Reads | Indoors | Fungal Growth | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Out | In | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | H-9 | H-3-G | H-4-Ga | H-4-Gb | |
| Acremonium charticola | 5 | 59 | 0 | 0 | 0 | 0 | 0 | 59 | 0 | 0 | 0 | 0 | 3483 | 50 |
| Akanthomyces lecanii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1289 |
| Alternaria sect. Alternaria * | 1542 | 3275 | 119 | 161 | 494 | 258 | 1 | 0 | 1547 | 694 | 1 | 0 | 2 | 0 |
| Alternaria sect. Infectoriae * | 1201 | 4051 | 125 | 297 | 360 | 1656 | 0 | 0 | 826 | 786 | 1 | 0 | 0 | 0 |
| Alternaria sect. Ulocladium | 14 | 59 | 0 | 3 | 0 | 0 | 55 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Aspergillus canadensis | 64 | 361 | 0 | 271 | 0 | 0 | 0 | 0 | 89 | 0 | 1 | 1 | 1856 | 0 |
| Aspergillus domesticus * | 221 | 2829 | 0 | 79 | 27 | 0 | 2550 | 40 | 100 | 33 | 0 | 0 | 0 | 0 |
| Aspergillus flavus | 15 | 17 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus fumigatus | 40 | 37 | 0 | 0 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus glaucus * | 1 | 2111 | 0 | 1980 | 0 | 0 | 83 | 1 | 47 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus hiratsukae | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Aspergillus niger | 0 | 15 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Aspergillus penicillioides clade * | 0 | 91 | 0 | 0 | 71 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus salinarum | 11 | 80 | 0 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus series Versicolores * | 0 | 428 | 0 | 127 | 119 | 0 | 139 | 0 | 38 | 5 | 0 | 0 | 1620 | 4108 |
| Aspergillus vitricola | 4 | 813 | 0 | 230 | 136 | 369 | 0 | 60 | 1 | 17 | 0 | 0 | 0 | 1 |
| Aspergillus westerdijkiae | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Botryotrichum murorum * | 11 | 269 | 0 | 233 | 0 | 0 | 0 | 0 | 36 | 0 | 0 | 0 | 0 | 0 |
| Chaetomium globosum | 19 | 69 | 0 | 60 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladosporium allicinum * | 2700 | 6909 | 588 | 1636 | 655 | 982 | 115 | 56 | 893 | 1984 | 0 | 110 | 0 | 0 |
| Cladosporium cladosporioides complex * | 1110 | 3222 | 522 | 182 | 589 | 835 | 58 | 1 | 353 | 618 | 64 | 0 | 0 | 0 |
| Cladosporium dominicanum * | 264 | 140 | 0 | 100 | 1 | 38 | 0 | 0 | 0 | 1 | 0 | 0 | 476 | 2 |
| Cladosporium halotolerans * | 805 | 72 | 0 | 24 | 0 | 0 | 0 | 0 | 48 | 0 | 0 | 33 | 14 | 0 |
| Cladosporium sphaerospermum * | 29 | 185 | 0 | 0 | 38 | 139 | 0 | 0 | 0 | 8 | 0 | 0 | 8172 | 5 |
| Debaryomyces hansenii | 203 | 704 | 125 | 116 | 27 | 0 | 395 | 0 | 27 | 14 | 0 | 94 | 1386 | 1536 |
| Monocillium tenue | 12 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 0 | 8540 |
| Penicillium adametzioides | 0 | 112 | 0 | 0 | 0 | 112 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillium bialowiezense | 10 | 21 | 0 | 0 | 7 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Penicillium brevicompactum * | 103 | 1116 | 233 | 0 | 48 | 0 | 744 | 0 | 81 | 10 | 0 | 0 | 0 | 7 |
| Penicillium chrysogenum * | 44 | 909 | 16 | 230 | 67 | 45 | 409 | 0 | 138 | 4 | 0 | 0 | 1 | 1 |
| Penicillium citreonigrum | 23 | 138 | 0 | 0 | 0 | 13 | 0 | 0 | 36 | 0 | 89 | 0 | 0 | 0 |
| Penicillium citrinum | 0 | 69 | 0 | 0 | 0 | 0 | 69 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillium corylophilum * | 149 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 115 |
| Penicillium digitatum * | 48 | 2114 | 763 | 28 | 190 | 2 | 750 | 0 | 377 | 4 | 0 | 0 | 0 | 0 |
| Penicillium glabrum * | 23 | 511 | 53 | 0 | 113 | 7 | 84 | 0 | 235 | 19 | 0 | 0 | 0 | 0 |
| Penicillium lanosum | 0 | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillium olsonii * | 0 | 62 | 0 | 0 | 55 | 0 | 1 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| Penicillium oxalicum | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillium roqueforti * | 7 | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 0 | 0 | 0 | 0 | 0 |
| Penicillium roseopurpureum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2156 |
| Saccharomyces cerevisiae * | 11 | 1266 | 0 | 0 | 415 | 146 | 252 | 0 | 423 | 30 | 0 | 0 | 0 | 0 |
| Stachybotrys chartarum | 48 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 0 | 0 | 0 | 1 |
| Verrucocladosporium dirinae | 243 | 97 | 0 | 0 | 26 | 0 | 71 | 0 | 0 | 0 | 0 | 0 | 352 | 0 |
| Wallemia ichthyophaga | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 |
| Wallemia muriae | 1 | 722 | 0 | 107 | 0 | 0 | 548 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
2.6. Culture-Dependent Method: Fungal Growth, Identification and Enumeration
3. Results
3.1. Culture-Independent Methods (DNA Sequencing)
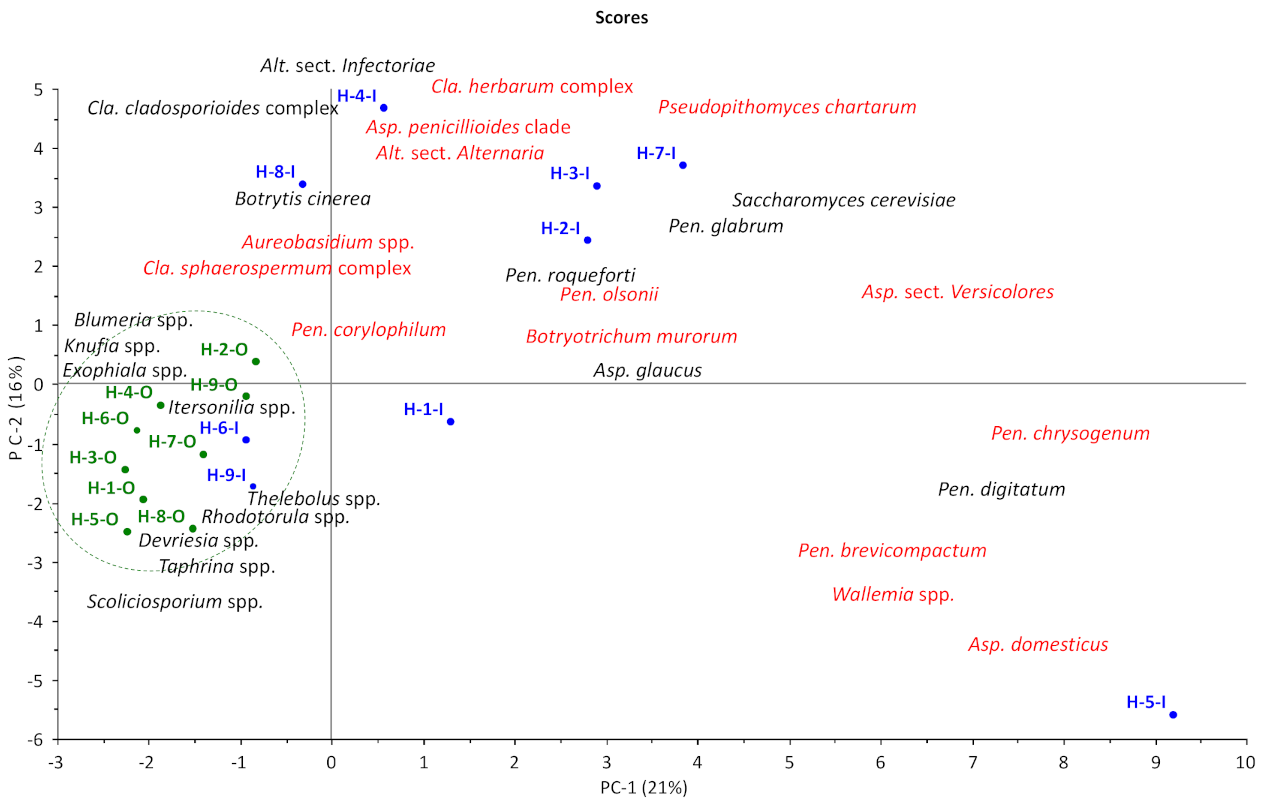
3.1.1. OTU Diversity and Abundance
3.1.2. Genus Diversity and Abundance
3.1.3. Species Diversity and Abundance Indoors
3.2. Culture-Dependent Methods (Growth on DG18 and V8)
3.2.1. Colony Forming Unit (CFU) and Genus Diversity and Abundance
3.2.2. Species Diversity and Abundance Indoors
4. Discussion
4.1. Detection Methods
4.2. Fungal Identification
4.3. Baseline Spora in a Danish Setting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Dosen, I.; Lewinska, A.M.; Nielsen, K.F. Pre-contamination of new gypsum wallboard with potentially harmful fungal species. Indoor Air 2017, 27, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Prezant, B.; Weekes, D.M.; Miller, J.D. (Eds.) Recognition, Evaluation, and Control of Indoor Mold; American Industrial Hygiene Association: Fairfax, VA, USA, 2008. [Google Scholar]
- Adan, O.; Samson, R. Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011. [Google Scholar]
- Nevalainen, A.; Täubel, M.; Hyvärinen, A. Indoor fungi: Companions and contaminants. Indoor Air 2015, 25, 125–156. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; Westerdijk Laboratory Manual Series 2; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019. [Google Scholar]
- Hunter, C.A.; Grant, C.; Flannigan, B.; Bravery, A.F. Mould in buildings: The air spora of domestic dwellings. Int. Biodeterior. 1988, 24, 81–101. [Google Scholar] [CrossRef]
- Horner, W.E.; Worthan, A.G.; Morey, P.R. Air- and dust-borne mycoflora in houses free of water damage and fungal growth. Appl. Environ. Microbiol. 2004, 70, 6394–6400. [Google Scholar] [CrossRef]
- Ara, K.; Aihara, M.; Ojima, M.; Toshima, Y.; Yabune, C.; Tokuda, H.; Kawai, S.; Ueda, N.; Tanaka, T.; Akiyama, K.; et al. Survey of fungal contamination in ordinary houses in Japan. Allergol. Int. 2004, 53, 369–377. [Google Scholar] [CrossRef][Green Version]
- Ren, P.; Jankun, T.M.; Belanger, K.; Bracken, M.B.; Leaderer, B.P. The relation between fungal propagules in indoor air and home characteristics. Allergy 2001, 56, 419–424. [Google Scholar] [CrossRef]
- Gent, J.F.; Ren, P.; Belanger, K.; Triche, E.; Bracken, M.B.; Holford, T.R.; Leaderer, B.P. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect. 2002, 110, A781–A786. [Google Scholar] [CrossRef]
- Chew, G.L.; Rogers, C.; Burge, H.A.; Muilenberg, M.L.; Gold, D.R. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy 2003, 58, 13–20. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. The diversity and distribution of fungi on residential surfaces. PLoS ONE 2013, 8, e78866. [Google Scholar] [CrossRef] [PubMed]
- Fouquier, J.; Schwartz, T.; Kelley, S.T. Rapid assemblage of diverse environmental fungal communities on public restroom floors. Indoor Air 2016, 26, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Balolong, M.P.; Dalmacio, L.M.M.; Magabo, M.L.V.; Sy, D.N.L.; Hallare, A.V. Next-generation sequencing revealed dominant fungal populations in collected dust from selected public-school classrooms in Metro Manila. Aerobiologia 2017, 33, 127–135. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Sassi, M.; Lemire, P.; Le Cann, P. Metagenomic characterization of indoor dust bacterial and fungal microbiota in homes of asthma and non asthma patients using next generation sequencing. Front. Microbiol. 2020, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. In Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar] [CrossRef]
- Amend, A.S.; Seifert, K.A.; Samson, R.A.; Bruns, T.D. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 13748–13753. [Google Scholar] [CrossRef]
- Larsen, L.; Gravesen, S. Seasonal variation of outdoor airborne viable microfungi in Copenhagen, Denmark. Grana 1991, 30, 467–471. [Google Scholar] [CrossRef]
- Frankel, M.; Bekö, G.; Timm, M.; Gustavsen, S.; Hansen, E.W.; Madsen, A.M. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl. Environ. Microbiol. 2012, 78, 8289–8297. [Google Scholar] [CrossRef]
- Dunn, R.R.; Fierer, N.; Henley, J.B.; Leff, J.W.; Menninger, H.L. Home life: Factors structuring the bacterial diversity found within and between homes. PLoS ONE 2013, 8, e64133. [Google Scholar] [CrossRef]
- Barberán, A.; Dunn, R.R.; Reich, B.J.; Pacifici, K.; Laber, E.B.; Menninger, H.L.; Morton, J.M.; Henley, J.B.; Leff, J.W.; Miller, S.L.; et al. The ecology of microscopic life in household dust. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151139. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007. [Google Scholar]
- Valdez, J.G.; Makuch, M.A.; Ordovini, A.F.; Frisvad, J.C.; Overy, D.P.; Masuelli, R.W.; Piccolo, R.J. Identification, pathogenicity and distribution of Penicillium spp. isolated from garlic in two regions in Argentina. Plant Pathol. 2009, 58, 352–361. [Google Scholar] [CrossRef]
- Chen, A.J.; Hubka, V.; Frisvad, J.C.; Visagie, C.M.; Houbraken, J.; Meijer, M.; Varga, J.; Demirel, R.; Jurjević, Ž.; Kubátová, A.; et al. Polyphasic taxonomy of Aspergillus section Aspergillus (formerly Eurotium), and its occurrence in indoor environments and food. Stud. Mycol. 2017, 88, 37–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Houbraken, J.; Groenewald, J.Z.; Meijer, M.; Andersen, B.; Nielsen, K.F.; Crous, P.W.; Samson, R.A. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud. Mycol. 2016, 84, 145–224. [Google Scholar] [CrossRef] [PubMed]
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Houbraken, J.; Crous, P.W.; Samson, R.A. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, A.; Reponen THusman, T.; Ruuskanen, J.; Nevalainen, A. Characterizing mold problem buildings–concentrations and flora of viable fungi. Indoor Air 1993, 3, 337–343. [Google Scholar] [CrossRef]
- Tonge, D.P.; Pashley, C.H.; Gant, T.W. Amplicon–based metagenomic analysis of mixed fungal samples using proton release amplicon sequencing. PLoS ONE 2014, 9, e93849. [Google Scholar] [CrossRef]
- Page, W.M. Viability of spores of some coprophilous species of Sordaria and Chaetomium. Trans. Br. Mycol Soc. 1951, 34, 539. [Google Scholar] [CrossRef]
- Valero, A.; Begum, M.; Leong, S.L.; Hocking, A.D.; Ramos, A.J.; Sanchis, V.; Marin, S. Effect of germicidal UVC light on fungi isolated from grapes and raisins. Lett. Appl. Microbiol. 2007, 45, 238–243. [Google Scholar] [CrossRef]
- Latorre, B.A.; Rojas, S.; Díaz, G.A.; Chuaqui, H. Germicidal effect of UV light on epiphytic fungi isolated from blueberry. Int. J. Agric. Nat. Resour. 2012, 39, 473–480. [Google Scholar]
- Martiny, A.C. High proportions of bacteria are culturable across major biomes. ISME J. 2019, 13, 2125–2128. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Ryberg, M.; Kristiansson, E.; Abarenkov, K.; Larsson, K.H.; Kõljalg, U. Taxonomic reliability of DNA sequences in public sequence databases: A fungal perspective. PLoS ONE 2006, 1, e59. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Crous, P.W.; Redhead, S.A.; Reynolds, D.R.; Samson, R.A.; Seifert, K.A.; Taylor, J.W.; Wingfield, M.J.; Abaci, Ö.; Aime, C.; et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011, 2, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Taylor, J.W. Aspergillus, its sexual states and the new International Code of Nomenclature. Mycologia 2014, 106, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Seifert, K.A.; Overy, D.P.; Tuthill, D.M.; Valdez, J.G.; Samson, R.A. New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia 2012, 29, 78. [Google Scholar] [CrossRef]
- Reboux, G.; Rocchi, S.; Vacheyrou, M.; Millon, L. Identifying indoor air Penicillium species: A challenge for allergic patients. J. Med. Microbiol. 2019, 68, 812–821. [Google Scholar] [CrossRef]
- Vesper, S.; McKinstry, C.; Haugland, R.; Wymer, L.; Bradham, K.; Ashley, P.; Cox, D.; Dewalt, G.; Friedman, W. Development of an environmental relative moldiness index for US homes. J. Occup. Environ. Med. 2007, 49, 829–833. [Google Scholar] [CrossRef]
- Pitkäranta, M.; Meklin, T.; Hyvärinen, A. Molecular profiling of fungal communities in moisture damaged buildings before and after remediation-a comparison of culture-dependent and culture-independent methods. BMC Microbiol. 2011, 11, 235. [Google Scholar] [CrossRef]
- Miller, J.D.; McMullin, D.R. Fungal secondary metabolites as harmful indoor air contaminants: 10 years on. Appl. Microbiol. Biotechnol. 2014, 98, 9953–9966. [Google Scholar] [CrossRef]
- Prahl, P. Reduction of indoor airborne mould spores. Allergy 1992, 47, 362–365. [Google Scholar] [CrossRef]
- Cheong, C.D.; Neumeister-Kemp, H.G.; Dingle, P.W.; Hardy, G.S.J. Intervention study of airborne fungal spora in homes with portable HEPA filtration units. J. Environ. Monit. 2004, 6, 866–873. [Google Scholar] [CrossRef]
- Wong, W.C.; Zalesak, S.; Yoets, A.; Capriotti, J.; Smith, M.J.; Castro, V.A.; Pierson, D.L. Engineering case report: Effectiveness of HEPA filter vacuum in removing transient microbial contaminants on cargo bags destined for the International Space Station. J. Occup. Environ. Hyg. 2013, 10, D71–D75. [Google Scholar] [CrossRef] [PubMed]

| Home | Location | Type of Residence | Previous Water Damage | Fungal Growth Detected in 2015 |
|---|---|---|---|---|
| H-1 | 3460 Birkerød | Flat, mezzanine | None | None |
| H-2 | 2800 Kgs. Lyngby | 2-storey house | In basement, 2011 | None |
| H-3 | 2840 Holte | Terraced house | None | Inner wall in bathroom |
| H-4 | 3520 Farum | Bungalow | None | Outer wall in bedroom |
| H-5 | 2860 Søborg | 2-storey house | None | None—dampness in basement |
| H-6 | 3200 Helsinge | Bungalow | None | None |
| H-7 | 2200 København | Flat, fifth floor | In attic, 2013 | None |
| H-8 | 2500 Valby | 3-storey house | In attic, 2014 | None |
| H-9 | 2500 Valby | Flat, third floor | None | None |
| Species * | Total DNA Reads | Gypsum Wallboard (GW) | Painted Brick Wall § | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Out | In | Ua † | Ub † | Pa ‡ | Pb ‡ | H-3-G | H-4-Ga | H-4-Gb | |
| Acr. charticola | 0 | 0 | 0 | 0 | 856 | 0 | 0 | 3483 | 50 |
| Aka. lecanii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1289 |
| Alt. sect. Ulocladium | 0 | 59 | 0 | 0 | 2077 | 0 | 0 | 0 | 0 |
| Asp. canadensis | 64 | 361 | 0 | 0 | 0 | 0 | 1 | 1856 | 0 |
| Asp. hiratsukae | 1 | 2 | 8123 | 8824 | 0 | 0 | 1 | 0 | 0 |
| Asp. penicillioides clade | 0 | 20 | 0 | 0 | 0 | 98 | 0 | 0 | 0 |
| Asp. sect. Versicolores | 0 | 428 | 0 | 0 | 334 | 80 | 0 | 1620 | 4108 |
| Asp. vitricola | 4 | 813 | 0 | 0 | 0 | 688 | 0 | 0 | 1 |
| Can. parapsilosis | 1 | 1 | 1 | 0 | 0 | 16,045 | 1 | 0 | 0 |
| Cha. globosum | 19 | 69 | 0 | 235 | 0 | 0 | 0 | 0 | 0 |
| Cla. allicinum | 2700 | 6909 | 0 | 0 | 0 | 77 | 110 | 0 | 0 |
| Cla. dominicanum | 264 | 140 | 0 | 0 | 6 | 101 | 0 | 476 | 2 |
| Cla. halotolerans | 805 | 72 | 0 | 0 | 124 | 317 | 33 | 14 | 0 |
| Cla. sphaerospermum | 29 | 185 | 0 | 0 | 4447 | 828 | 0 | 8172 | 5 |
| Deb. hansenii | 203 | 704 | 0 | 0 | 0 | 0 | 94 | 1386 | 1536 |
| Exo. lecanii-corni | 0 | 0 | 0 | 0 | 0 | 162 | 0 | 0 | 0 |
| Gib. nigrescens | 10 | 0 | 0 | 0 | 5105 | 0 | 0 | 0 | 0 |
| Mey. guilliermondii | 0 | 221 | 0 | 0 | 0 | 359 | 0 | 0 | 0 |
| Mon. tenue | 12 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 8540 |
| Mor. alpina | 214 | 0 | 0 | 0 | 0 | 129 | 0 | 0 | 0 |
| Pen. chrysogenum | 44 | 909 | 8718 | 0 | 41 | 0 | 0 | 1 | 1 |
| Pen. corylophilum | 149 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 115 |
| Pen. roseopurpureum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2156 |
| Sta. chartarum | 48 | 38 | 0 | 4655 | 4477 | 0 | 0 | 0 | 1 |
| Tau. pullulans | 294 | 87 | 0 | 0 | 0 | 108 | 0 | 0 | 0 |
| Wal. muriae | 1 | 0 | 0 | 0 | 0 | 90 | 0 | 0 | 0 |
| Species * | Total CFUs | Outdoors | Indoors | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Out | In | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | H-9 | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 | H-8 | H-9 | |
| Alt. arborescens | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Alt. infectoria | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arthrinium spp. | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Asp. fumigatus | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Asp. glaucus | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asp. niger | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asp. sydowii | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Asp. versicolor | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 |
| Aureobasidium spp. | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Botrytis spp. | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cha. globosum | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cla. allicinum | 28 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cla. cladosporioides | 23 | 4 | 1 | 1 | 9 | 0 | 1 | 6 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 |
| Cla. sphaerospermum | 36 | 3 | 0 | 2 | 6 | 13 | 0 | 0 | 1 | 2 | 12 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 |
| Didymella spp. | 20 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epi. nigrum | 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fusarium sp. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pen. allii | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 10 | 0 | 0 | 0 | 0 |
| Pen. atramentosum | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pen. brevicompactum | 13 | 16 | 1 | 0 | 1 | 0 | 0 | 10 | 0 | 0 | 1 | 2 | 0 | 2 | 1 | 6 | 0 | 0 | 5 | 0 |
| Pen. chrysogenum | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 |
| Pen. citreonigrum | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pen. crustosum | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pen. decumbens | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pen. digitatum | 2 | 69 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 9 | 0 | 29 | 0 | 31 | 0 | 0 | 0 | 0 |
| Pen. echinulatum | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pen. glabrum | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 10 | 0 | 1 | 0 | 1 |
| Pen. olsonii | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 |
| Pen. phoeniceum | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pen. polonicum | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pen. spatulatum | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Scopulariopsis sp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sta. chartarum | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichoderma sp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Mycelia sterilia | 45 | 25 | 0 | 2 | 5 | 4 | 1 | 2 | 1 | 26 | 4 | 10 | 0 | 4 | 0 | 2 | 0 | 1 | 4 | 4 |
| Yeast | 64 | 2 | 5 | 0 | 11 | 3 | 12 | 1 | 2 | 16 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Total CFUs | 262 | 205 | 9 | 7 | 38 | 27 | 15 | 23 | 8 | 63 | 72 | 27 | 4 | 62 | 9 | 61 | 2 | 15 | 19 | 6 |
| Previous Name | Current Name(s) | Detection Method | Substratum/Origin |
|---|---|---|---|
| Baseline fungi | |||
| Alternaria infectoria | Alt. infectoria | V8/ITS | Cereal plants, grasses/outside |
| Aspergillus fumigatus | Asp. fumigatus | DG18/ITS | Wood chip, compost/outside |
| Botrytis cinerea | Bot. cinerea | V8/ITS | Onions, cabbage, soft fruit |
| Eurotium herbariorum | Asp. glaucus | DG18/ITS | Dried foods, herbs and spices |
| Epicoccum nigrum | Epi. nigrum | V8/ITS | Soil, dead plant debris/outside |
| Penicillium digitatum | Pen. digitatum | DG18/ITS | Lemons, oranges |
| Pen. glabrum | Pen. glabrum | DG18/ITS | Onions |
| Pen. roqueforti | Pen. roqueforti | DG18/ITS | Rye bread, blue cheese |
| Moisture indicator fungi | |||
| Asp. versicolor | Asp. creber, Asp. jensenii, Asp. versicolor | V8, DG18/ITS | Most building materials |
| Asp. penicillioides | Asp. penicillioides, Asp. tardicrescens, Asp. vitricola | DG18/ITS | Textiles, leather, paintings, dried grain |
| Chaetomium globosum | Cha. globosum | V8/ITS | Gypsum wallboard, plywood |
| Cha. murorum | Botryotrichum murorum | V8/ITS | Ceiling tile |
| Cladosporium bruhnei | Cla. allicinum | V8, DG18/ITS | Woodwork, plaster, grain |
| Cla. sphaerospermum | Cla. dominicanum, Cla. halotolerans, Cla. sphaerospermum | V8, DG18/ITS | Woodwork, plaster, paint, plywood, textiles |
| Pen. chrysogenum | Pen. chrysogenum, Pen. rubens | DG18/ITS | Most building materials |
| Stachybotrys chartarum | Sta. chartarum | V8/ITS | Gypsum wallboard, cardboard |
| Ulocladium alternariae | Alt. alternariae | V8/ITS | Wallpaper, textiles, plant debris |
| Wallemia sebi | Wal. ichthyophaga, Wal. muriae, Wal. sebi | DG18/ITS | Plaster, brickwork, hay, dried food |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, B.; Frisvad, J.C.; Dunn, R.R.; Thrane, U. A Pilot Study on Baseline Fungi and Moisture Indicator Fungi in Danish Homes. J. Fungi 2021, 7, 71. https://doi.org/10.3390/jof7020071
Andersen B, Frisvad JC, Dunn RR, Thrane U. A Pilot Study on Baseline Fungi and Moisture Indicator Fungi in Danish Homes. Journal of Fungi. 2021; 7(2):71. https://doi.org/10.3390/jof7020071
Chicago/Turabian StyleAndersen, Birgitte, Jens C. Frisvad, Robert R. Dunn, and Ulf Thrane. 2021. "A Pilot Study on Baseline Fungi and Moisture Indicator Fungi in Danish Homes" Journal of Fungi 7, no. 2: 71. https://doi.org/10.3390/jof7020071
APA StyleAndersen, B., Frisvad, J. C., Dunn, R. R., & Thrane, U. (2021). A Pilot Study on Baseline Fungi and Moisture Indicator Fungi in Danish Homes. Journal of Fungi, 7(2), 71. https://doi.org/10.3390/jof7020071






