Abstract
Penicillium expansum is a major postharvest pathogen that mainly threatens the global pome fruit industry and causes great economic losses annually. In the present study, the antifungal effects and potential mechanism of cinnamon oil against P. expansum were investigated. Results indicated that 0.25 mg L−1 cinnamon oil could efficiently inhibit the spore germination, conidial production, mycelial accumulation, and expansion of P. expansum. In addition, it could effectively control blue mold rots induced by P. expansum in apples. Cinnamon oil could also reduce the expression of genes involved in patulin biosynthesis. Through a proteomic quantitative analysis, a total of 146 differentially expressed proteins (DEPs) involved in the carbohydrate metabolic process, most of which were down-regulated, were noticed for their large number and functional significance. Meanwhile, the expressions of 14 candidate genes corresponding to DEPs and the activities of six key regulatory enzymes (involving in cellulose hydrolyzation, Krebs circle, glycolysis, and pentose phosphate pathway) showed a similar trend in protein levels. In addition, extracellular carbohydrate consumption, intracellular carbohydrate accumulation, and ATP production of P. expansum under cinnamon oil stress were significantly decreased. Basing on the correlated and mutually authenticated results, we speculated that disturbing the fungal carbohydrate metabolic process would be partly responsible for the inhibitory effects of cinnamon oil on P. expansum growth. The findings would provide new insights into the antimicrobial mode of cinnamon oil.
1. Introduction
Food loss and waste by fungal contamination are critical problems in both developing and developed countries. Among the fungi most commonly found, Penicillium expansum is the causal agent of blue mold rot disease in a wide range of fresh fruits and vegetables during transport, handling, or postharvest storage. It leads to huge economic losses around the world annually. Additionally, P. expansum can produce an array of secondary metabolites such as patulin, citrinin, roquefortine C, chaetoglobosins A and C, and communesins [1,2]. Direct exposure to these mycotoxins will result in serious health problems in humans due to their carcinogenic, immunosuppressive, nephrotoxic, teratogenic, and mutagenic attributes [3,4]. In addition to screening resistant cultivars, blue mold rots can be prevented by careful harvest and handling practices, stringent sanitation, and proper storage conditions. Furthermore, many advances have been made toward chemical and biological control [5,6]. However, so far, the control of P. expansum mainly relies on synthetic fungicides containing fludioxonil, pyrimethanil, or difenoconazole as specific active ingredients [7,8]. Nevertheless, the excessive application of fungicides is prone to aggravate chemical residue and develop fungicide-resistant strains. Therefore, with the increased concerns for food safety and environmental contamination, investigations of safe, novel, and efficient antifungal substances or alternative strategies are necessary and urgent.
Essential oils extracted from various aromatic herbs are volatile liquids and generally exhibit potent antioxidative and antimicrobial activities. They are generally regarded as safe substances due to their very low toxicity, which makes them interesting additives in the food industry ([9]. Cinnamon (Cinnamomum zeylanicum L.) is a traditional herbal medicine that is grown in almost all tropical regions of the world. Cinnamon oil as an important essential oil contains a large number of aromatic compounds, fatty groups, and terpenoids. A total of 37 components have been detected in cinnamon oil, and the main component is trans-cinnamaldehyde [10]. Currently, cinnamon oil has been applied in many fields due to its multiple functions including anti-inflammatory, anti-cancer, anti-oxidative, insecticidal, and antimicrobial actions [11,12,13,14,15,16]. Cinnamon oil could also maintain or improve the quality of postharvest vegetables and fruits such as mangoes, apples, cherries, jujubes, guavas, and cucumbers [17,18,19,20,21,22]. Cinnamon oil has been used to control postharvest fungi Penicillium spp., Aspergillus spp., Rhizopus spp., Colletotrichum spp., Botrytis cinerea, Fusarium verticillioides, and Alternaria alternata in film components, in a direct application or in collaboration with other materials [23,24,25,26,27,28,29]. However, the possible antifungal mechanism of cinnamon oil was not fully understood. Effects of cinnamon oil on the global protein level of P. expansum had not been reported.
Therefore, this study was undertaken to examine the effects of cinnamon oil on P. expansum. Proteomic changes in P. expansum spores induced by cinnamon oil were investigated. Combining with the biochemical and physiological detections, the potential antifungal mechanisms of cinnamon oil against P. expansum were subsequently discussed. The findings will provide a good guiding significance in the postharvest utilization of cinnamon oil.
2. Materials and Methods
2.1. Antifungal Assays of Cinnamon Oil
P. expansum Link (CGMDD3.3703) was cultured on potato dextrose agar (PDA) medium at 25 oC for 10 days. Fresh spores were harvested using sterile water and added to potato dextrose broth (PDB) medium with a final concentration of 1.0 × 106 spores mL−1. Cinnamon oil (Product NO. A501966, EINECS NO. 283-479-0, CAS NO. 8015-91-6, EC NO. 616-967-2, Sangon, Shanghai, China) was added to the medium with final concentrations 0, 0.05, 0.15, 0.25, and 0.35 mg L−1. Tween-80 with a final concentration of 0.2% (v/v) was used to promote cinnamon oil dissolving [30]. The spore germination ratio was measured by a Nikon DS-Fi1 microscope after 12 h of culturing at 25 °C under 200 rpm shaking condition. After 24, 48, and 72 h of culturing, the fungal mycelia in a 100 mL system were separated by centrifugation. The mycelia were weighed after drying at 60 °C in an oven (Modell 100–800 m, Memmert, Schwabach, Germany). To assess the effect of cinnamon oil on mycelial expansion, a mycelial agar disk was put on the center of a PDA plate containing 0 or 0.25 mg L−1 cinnamon oil. The colony sizes were recorded daily, and the morphologies were photographed by a Nikon Coolpix P7100 digital camera. To evaluate the effect of cinnamon oil on the sporulation of P. expansum, 100 μL of spore suspension (1.0 × 106 spores mL−1) was evenly spread on a 9 cm Petri dish containing 25 mL of PDA with or without 0.25 mg L−1 cinnamon oil. It should be aware that cinnamon oil was added into PDA only when the temperature of PDA was lower than 60 °C. After 3 to 12 days of culturing at 25 °C, the Petri dish was washed by 10 mL of sterile water with 0.2% Tween-20. The spore concentration was determined utilizing a Nikon DS-Fi1 microscope and a hemocytometer.
Apples (Malus domestica Borkh cv. Red Fuji) without mechanical injury were bought from the local market of Yuhang district, Hangzhou, China. The fruit were soaked in 2% sodium hypochlorite for 2 min, washed by distilled water twice, and air-dried at room temperature [31]. A round hole (0.3 cm in width and depth) was made at the fruit equator by a sterile nail. Then, 10 μL of the spore suspension at 1.0 × 105 spores mL−1 with 0 or 0.25 mg L−1 cinnamon oil was inoculated into the wound. Apples were stored at 25 °C and 80% humidity, and disease incidence and lesion diameters were measured daily. Each experiment contained three replicates, and each treatment consisted of 10 apples. The experiment was performed twice.
2.2. Fluorescent Staining of Spores
Fresh spores were incubated in PDB medium (1.0 × 106 spores mL−1) with or without 0.25 mg L−1 cinnamon oil for 6 h at 25 °C under 200 rpm shaking condition. The spores were collected by centrifugation, washed by phosphate buffer solution (PBS, 20 mmoL L−1, pH 7.4), and stained by 5μmoL L−1 fluorescein diacetate (No. A600202, Sangon, Shanghai, China), 5 μmoL L−1 MitoTraker Orange (Invitrogen, Carlsbad, CA, USA), 50 mg L−1 4′, 6′-diamidino-2-phenylindole dihydrochloride (DAPI, No. A606584, Sangon, Shanghai, China), and 20 mg L−1 propidium iodide (PI, NO. E607306, Sangon, Shanghai, China). For PI staining, half of the cinnamon oil-treated spores were incubated in boiling water for 10 min, which was as a positive control. The staining was performed following the product instruction. Then, stained spores were observed microscopically and photographed by a Nikon Eclipse Ni-U microscope with individual filter sets.
2.3. Quantitative Proteomics Analysis Based on iTRAQ
Fresh spores were incubated in PDB medium (1.0 × 107 spores mL−1) containing 0 or 0.25 mg L−1 cinnamon oil for 6 h at 25 °C under 200 rpm shaking condition. The spores were collected by centrifugation, washed by PBS twice, and quickly frozen in liquid nitrogen. Each treatment contained three replicates, and the whole experiment was repeated. The equal amounts of spores from three replicas were pooled into one sample. Then, the protein extraction and iTRAQ (Isobaric Tags for Relative and Absolute Quantitation) analysis were performed by LC-Bio Technologies (Hangzhou) CO., LTD following the standard operating procedure of technical service. Raw data files were converted into MGF files by Thermo Proteome Discoverer 1.2 (Thermo, Waltham, MA, USA). Proteins identification was conducted by Mascot search engine (Matrix Science, London, UK) against the reference sequence of P. expansum (NCBI: txid27334). Fragment and peptide mass tolerance were ± 0.1Da and ± 0.05Da respectively. Mass values and max missed cleavages were set as monoisotopic and 1. iTRAQ8plex (Y), Gln->pyro-Glu (N-term Q) and oxidation (M) were set as acceptable variable modifications. Carbamidomethyl (C), iTRAQ8plex (N-term), and iTRAQ8plex (K) were set as acceptable fixed modifications. Each confident protein contained one or more unique peptides. The differentially expressed proteins (DEPs) were confirmed by the standard that the ration with p-value < 0.05 and fold changes of >1.2 or <0.83 (control versus cinnamon oil-treated spores) in both two biological repeats. The Blast2GO program was used for protein functional annotation against the non-redundant protein database (https://www.ncbi.nlm.nih.gov/protein/ (accessed on 1 January 2021)). DEPs were annotated, classified, and grouped by Clusters of Orthologous Groups of proteins (COGs), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Ontology Consortium databases. The detailed information of DEPs is shown in Table S1.
2.4. Expression Analysis of Related Genes by qRT-PCR
Fresh spores were cultured in PDB medium (1.0 × 107 spores mL−1) containing 0 or 0.25 mg L−1 cinnamon oil for 6, 12, and 18 h at 25 °C under 200 rpm shaking condition. The spores and mycelia were collected by centrifugation, and total RNAs extraction of samples was performed using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA synthesis was conducted by a FastQuant RT Kit (Tiangen Biotech, Beijing, China) following the product instruction. Relative expression levels of genes were determined by quantitative real-time PCR (qRT-PCR) utilizing a CFX96-real Time System (Bio-Rad, Hercules, CA, USA) and 2 × Ultro SYBr mixture (CW Bio, Beijing, China). Specific primer pairs for genes (PatA-PatO) involved in the patulin biosynthesis were the same as described by Tannous et al. [32]. Specific primer pairs for genes (PeCO1-PeCO15) involved in the carbohydrate metabolic process were designed and shown in Table S2. The SYBR Green fluorescence intensity change and the threshold cycle (Ct) over the background were assessed for each reaction. Expression changes of targeted genes were normalized by reference gene β-tubulin and measured using the 2(−△Ct) analysis method.
2.5. Measurement of Enzymatic Activities
Fresh spores were cultured in PDB medium (1.0 × 107 spores L−1) containing 0 or 0.25 mg L−1 cinnamon oil for 6, 12, and 18 h at 25 °C under 200 rpm shaking condition. Assay kits from Solarbio Life Science were used for measuring enzymatic activities of collected spores and mycelia. The product numbers are BC2560 for β-Glucosidase, BC0710 for α-ketoglutarate dehydrogenase, BC0740 for hexokinase, BC0540 for pyruvate kinase, BC0530 for 6-phosphofructokinase, and BC0260 for glucose 6-phosphatedehydrogenase. The operating strictly followed the product instructions.
2.6. Measurement of Carbohydrate, ATP and Patulin Content
Fresh spores were cultured in PDB medium (1.0 × 107 spores mL−1) containing 0 or 0.25 mg L−1 cinnamon oil for 6, 12, and 18 h at 25 °C under 200 rpm shaking condition. The spores and mycelia were collected by centrifugation, and the carbohydrate content of the supernatant was measured utilizing a portable refractometer (LB20T, Suwei, Taiwan, China). The total carbohydrate content of spores and mycelia was determined by a Total Carbohydrate Content Assay kit (BC2710, Solarbio, Beijing, China). The ATP content was measured by an ATP Content Assay Kit (BC0300, Solarbio, Beijing, China) in strict accordance with the product manual.
Fresh spores were cultured in PDB medium (1.0 × 107 spores mL−1) containing 0 or 0.25 mg L−1 cinnamon oil for 2, 4, and 6 days at 25 °C under static condition. After centrifugation, the supernatant was filtered through a 0.22 μm syringe filter (NO. F513152, Sangon, Shanghai, China). Patulin content was evaluated by a reversed-phase HPLC (high-performance liquid chromatography) with UV detection (Waters, Milford, MA, USA). A Waters XTerra RP18 column was used with mobile phases (95% water and 5% acetonitrile). The operational parameters were descripted as Zhou et al. [33].
2.7. Statistical Analysis
Unless otherwise specified, data were pooled across three independent repeated experiments. Statistical analysis was conducted by Microsoft Excel software. Analysis of variance was used for comparing more than two means. Mean separations were analyzed by Duncan’s multiple range test. Differences at p < 0.05 were considered to be significant.
3. Results
3.1. Antifungal Activity of Cinnamon Oil
The antifungal ability of cinnamon oil on P. expansum showed a dose-dependent manner. It did not affect the spore germination of P. expansum with a concentration of 0.05 mg L−1. When the concentration reached 0.25 mg L−1, cinnamon oil showed a stable inhibitory effect. After 12 h of treatment, the spore germination rate was below 20%, while it was over 75% in control. With concentration increasing, the inhibitory effect was more positive (Figure 1A). With the extension of culturing, mycelial biomass production of P. expansum was significantly lower than that of control in PDB supplemented with 0.25 mg L−1 cinnamon oil (Figure 1B), and the mycelial expansion and sporulation were significantly inhibited on PDA under cinnamon oil stress (Figure 1C,D and Figure S1B). For inoculation tests in vivo, decay symptoms were found in all inoculated apples, while lesion diameters in treated apples were significantly smaller than those in the control group (Figure S2). Therefore, 0.25 mg L−1 was considered as a minimum effective concentration of cinnamon oil on P. expansum and used in the subsequent experiments.
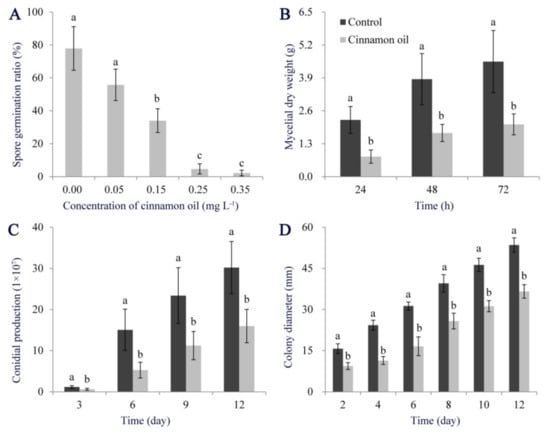
Figure 1.
Effects of cinnamon oil on the development of P. expansum. Effects of cinnamon oil with different concentrations on spore germination ratio of P. expansum after 12 h of culturing (A). Effects of cinnamon oil with a concentration of 0.25 mg L−1 on hyphae production (B), sporulation (C), and colony expansion of P. expansum (D). Bar represents the standard deviation of the means of three independent experiments. Lower-case letters indicate significant differences at p < 0.05 at each time point.
To confirm the inhibitory effect of cinnamon oil on P. expansum, DAPI staining was used for microscopic observation. After 6 h of culturing, the average volume of control spores was significantly larger than that of cinnamon oil-treated spores. The nuclei in control spores were also bigger, visually clearer, and more regular in shape (Figure S1A). Fluorescein diacetate (FDA) is a cell-permeant esterase substrate that can serve as a viability probe. Upon hydrolysis by intracellular esterases, the product yields fluorescein. After FDA staining, compared with the hypofluorescence in treated spores, the control spores emitted a strong and stable green fluorescence (Figure 2A). That indicated that the cellular viability of P. expansum was weakened under cinnamon oil stress. The cell-permeant MitoTracker Orange probe containing a mildly thiol-reactive chloromethyl moiety was used for labeling mitochondria. In cinnamon oil-treated spores, the fluorescent intensity was weaker, which indicated that the number of mitochondria decreased or the mitochondria experienced a loss in membrane potential (Figure 2B). These were consistent with the finding that most of the DEPs involved in energy metabolism such as oxidative phosphorylation were remarkably down-regulated under cinnamon oil stress.
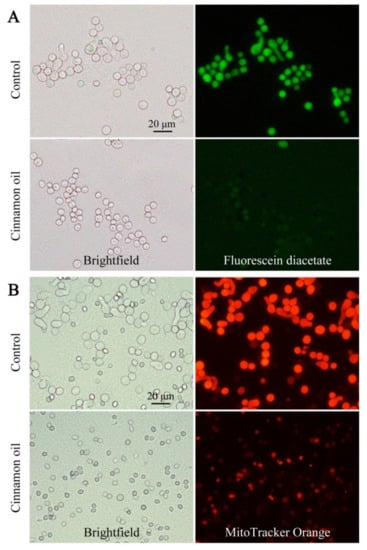
Figure 2.
Microscopic observations of spores with fluorescein diacetate (A) and MitoTracker Orange staining (B) after 6 h of incubation under 0.25 mg L−1 cinnamon oil treatment.
The expression changes of 15 genes involved in patulin production in P. expansum under cinnamon oil stress were determined. Compared with the control, after 6 h of culturing under cinnamon oil stress, the expression of PatA, PatD, PatE, and PatL were up-regulated, and the others were down-regulated or unchanged. With treatment time increasing, all the genes showed a gradually decreasing trend. After 18 h of treatment, the expression levels of all the genes, except for PatD, were significantly lower than those in the control (Figure S3). The effect of cinnamon oil on patulin production was measured by HPLC method. Along with the extension of culturing time, patulin accumulation significantly increased in the control group. After 6 days, the content of patulin reached about 450 μg L−1. However, the production of patulin was significantly inhibited by cinnamon oil treatment, and the patulin content was less than one-third of the control (Figure S4). These results indicated that cinnamon oil can weaken the patulin production capability of P. expansum, and it was positively correlated with treatment intensity.
3.2. A Global View of iTRAQ Analysis
After 6 h of treatment, the viability of treated spores was different from control spores. However, except for size, the morphologies of control and treated spores were similar. At this time point, the differential expression proteins between control and treatment samples could more exactly reflect the proteomic changes induced by cinnamon oil, which would be helpful for finding cinnamon oil action sites. Therefore, an iTRAQ-based quantitative proteomic analysis was performed focusing on the proteome alteration of samples after 6 h of culturing under cinnamon oil stress. A total of 22,514 unique peptides and 3598 proteins were identified and annotated in all samples. According to the screening threshold, 873 proteins (about 24.3% of the total) were considered as differentially expressed proteins. Among them, 343 DEPs were up-regulated and 530 DEPs were down-regulated. The ratio range of DEPs in expression level was 0.203 to 6.941 (Figure 3). A sum of 159 DEPs that were hypothetical, uncharacterized, or putative proteins were functionally unknown (Table S1). According to the GO enrichment statistics, most of the functional DEPs with peptidase and organic substance catabolic activities were involved in intracellular components biosynthesis. Through KEGG enrichment analysis, the majority of DEPs participated in butanoate metabolism, fructose and mannose metabolism, ribosome biogenesis, proteasome functioning, linoleic acid metabolism, and glutathione metabolism. The groups involved in the synthesis and degradation of ketone bodies, proteasome, and glutathione metabolism had a higher rich factor value (Figure 4).
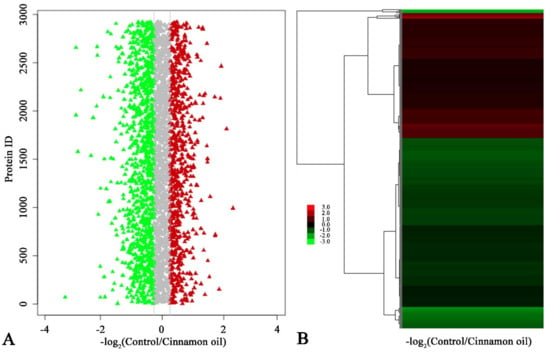
Figure 3.
(A) Ratio distribution of identified proteins in control and cinnamon oil-treated spores after 6 h of culture. Red, gray, and green triangles represent up-regulated, non-regulated, and down-regulated proteins, respectively. (B) Heatmap of the ratio of identified proteins in control and cinnamon oil-treated spores after 6 h of culture. Gradient color barcode (red to green) indicates the up-regulation to down-regulation of protein expression. Each row represents a protein. Proteins with similar fold change values are clustered at the column level. The detailed original data for (A,B) are listed in Table S2.
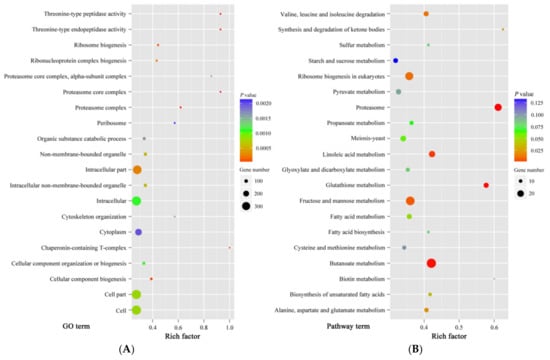
Figure 4.
Scatter plots of the top 20 Gene Ontology (GO) (A) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (B) enrichment of differentially expressed proteins (DEPs) in P. expansum spores under 0.25 mg L−1 cinnamon oil stress after 6 h of culture. The deeper color in the color code represents the highest confidence of the biological process. Rich factor: the number of DEPs in one GO or KEGG/the number of total identified proteins in the same GO or KEGG. A higher value indicates a higher enrichment level. The size of dot represents the number of DEPs. A larger dot indicates more DEPs.
It was noteworthy that a total of 146 DEPs were involved in the carbohydrate metabolic process. Specifically, there were 29 for butanoate metabolism, 27 for fructose and mannose metabolism, 12 for propanoate metabolism, 12 for glyoxylate and dicarboxylate metabolism, 26 for pyruvate metabolism, 14 for starch and sucrose metabolism, 9 for pentose and glucuronate interconversions, 17 for glycolysis and gluconeogenesis, 6 for pentose phosphate pathway, and 4 for galactose metabolism. Most of them were down-regulated with the ratio range (control/cinnamon oil) of 0.3 to 6.65 (Figure 5).
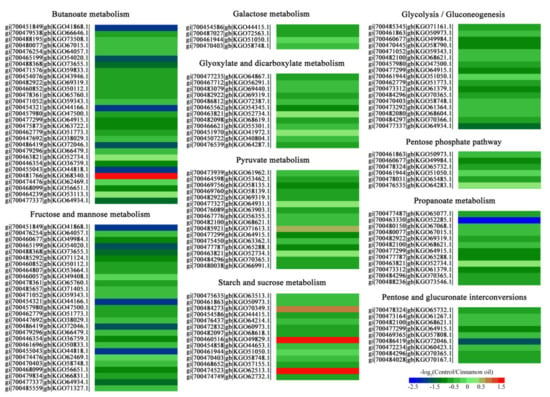
Figure 5.
Heatmap of expression changes of DEGs involved in the carbohydrate metabolic process in P. expansum spores under 0.25 mg L−1 cinnamon oil stress after 6 h of culture. Each row represents a DEP. The annotation and involved metabolic pathway of each DEP are listed. Red to blue in the color code indicates up- to down-regulated expression of the DEPs.
3.3. Effects of CO on Genes Expression Involved in Carbohydrate Metabolic Process
The expression changes of 14 genes in accordance with DEPs involved in the carbohydrate metabolic process were assessed by qRT-PCR. After 6 h of treatment, compared with a significant decrease of all candidates in the protein level, the expression of four genes (PeCO3, PeCO5, PeCO6 and PeCO7) were unchanged, three genes (PeCO1, PeCO9 and PeCO11) were up-regulated, and another seven genes were down-regulated. After 18 h of treatment, the expression of PeCO13 changed little, the expressions of PeCO2 and PeCO11 were respectively up-regulated more than nine and three times, and all the others were down-regulated remarkably (Table 1). These results indicated that the expression trends of candidates in mRNA levels were similar to those in protein levels with culture time growing. The increase in mRNA levels for a few genes may be due to a cellular stress response under an adverse ambient condition or a compensatory reaction. In addition, CreA is a C2H2 finger domain DNA-binding protein which plays critical roles in carbon catabolite repression and patulin synthesis [34,35]. The expression of CreA (PeCO15) was first up-regulated after 6 h of treatment. With prolongation of treatment time, the expression was down-regulated to one-third of the control (Table 1).

Table 1.
Effects of cinnamon oil on the expression of genes involved in the carbohydrate metabolic process in P. expansum.
3.4. Effects of Cinnamon Oil on Enzymatic Activities Involving in Carbohydrate Metabolic Process
The effects of cinnamon oil on the activities of six enzymes involves in the carbohydrate metabolic process were evaluated. Among them, the β-glucosidase (cellobiase) hydrolyzes maltose and cellobiose, and it acts in the last phase of cellulose degradation process [36]. The α-ketoglutarate dehydrogenase is the fourth enzyme of the Krebs cycle performing the decarboxylation of α-ketoglutarate to succinyl-CoA [37]. The hexokinase catalyzes the first step of glucose metabolism, phosphorylating glucose to glucose 6-phosphate [38]. The 6-phosphofructokinase catalyzes one of the rate-limiting steps of the glycolysis, the phosphorylation of fructose 6-phosphate [39]. Pyruvate kinase catalyzes the irreversible conversion of ADP and phosphoenolpyruvate to ATP and pyruvic acid in the last step of glycolysis [40]. Glucose 6-phosphate dehydrogenase is the rate-limiting enzyme in the first step of the pentose phosphate pathway which oxidizes glucose-6-phosphate to 6-phosphogluconate in the presence of NADP+ [41]. In control spores, the enzymatic activities of α-ketoglutarate dehydrogenase, hexokinase, pyruvate kinase, and glucose 6-phosphatedehydrogenase showed a gradually increasing trend with the extension of culturing time, while enzymatic activities of β-glucosidase and 6-phosphofructokinase did not show substantial changes (Figure 6). Under cinnamon oil stress, enzymatic activities of hexokinase, pyruvate kinase, and 6-phosphofructokinase were significantly lower than those in control during 24 h of culture (Figure 6C–E). Compared with the control, enzymatic activities of α-ketoglutarate dehydrogenase and glucose 6-phosphatedehydrogenase did not show obvious changes after 6 h of cinnamon oil treatment, while their activities were significantly lower than those in control along with the increase of treatment time (Figure 6B,F). In addition, enzymatic activities of β-Glucosidase increased remarkably at first, and they presented a gradually decreasing trend in cinnamon oil-treated spores (Figure 6A). The results indicated that cinnamon oil showed an inhibitory effect on enzymes involved in the carbohydrate metabolic process.
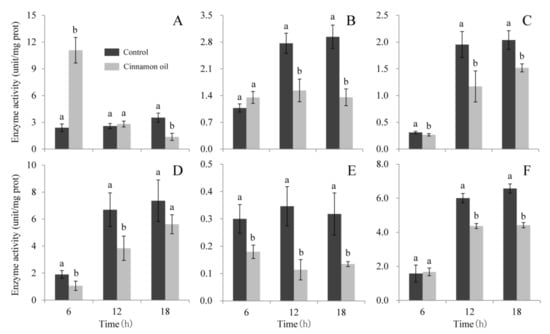
Figure 6.
Effects of 0.25 mg L−1cinnamon oil on β-glucosidase (A), α-ketoglutarate dehydrogenase (B), hexokinase (C), pyruvate kinase (D), 6-phosphofructokinase (E), and glucose 6-phosphate dehydrogenase (F) activities in P. expansum. One unit of β-glucosidase activity was defined as the amount of enzyme capable of producing 1 nmoL p-nitrophenol per milligram of protein in one hour. One unit of α-ketoglutarate dehydrogenase activity was defined as the amount of enzyme capable of producing 1 nmoL NADH (nicotinamide adenine dinucleotide) per min per milligram of protein. One unit of hexokinase activity was defined as the amount of enzyme capable of producing 1 nmoL NADPH (nicotinamide adenine dinucleotide phosphate) per min per milligram of protein. One unit of pyruvate kinase activity was defined as the amount of enzyme capable of consuming 1 nmoL NADH (nicotinamide adenine dinucleotide) per min per milligram of protein. One unit of 6-phosphofructokinase was defined as the amount of enzyme capable of converting 1 nmoL fructose-6-phosphate and 1 nmoL ATP to 1 nmoL fructose-1, 6-diphosphate and 1 ADP per min per milligram of protein. One unit of 6-phosphatedehydrogenase activity was defined as the amount of enzyme capable of producing 1 nmoL NADPH (nicotinamide adenine dinucleotide phosphate) per min per milligram of protein. Bar represents the standard deviation of the means of three independent experiments. Lower-case letters indicate significant differences at p < 0.05 at each time point.
3.5. Effects of Cinnamon Oil on Carbohydrate Consumption and ATP Synthesis
Under cinnamon oil stress, the carbohydrate consumption of P. expansum was significantly lower than control (Figure 7A). Meanwhile, the carbohydrate production in spores and mycelia was inhibited by cinnamon oil as well. After 18 h of treatment, the mycelial carbohydrate content in cinnamon oil-treated samples was less half than that in control samples (Figure 7B). The crucial function of carbohydrate metabolism is to produce energy by respiration within the inner membrane of mitochondria. The Mito Tracker Orange staining indicated that the mitochondrial number and membrane potential in cinnamon oil-treated spores decreased remarkably. Showing a decreasing trend with time extension in both control and cinnamon oil-treated groups, the ATP content in cinnamon oil-treated samples was significantly lower than that in control at each time point (Figure 7C). These results indicated that cinnamon oil had a notable inhibitory effect on the carbohydrate metabolic process in P. expansum.
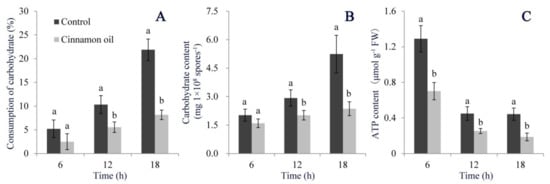
Figure 7.
Effects of 0.25 mg L−1 cinnamon oil on carbohydrate consumption in potato dextrose broth (PDB) (A), intracellular carbohydrate production (B), and intracellular ATP content (C). Bar represents the standard deviation of the means of three independent experiments. Lower-case letters indicate significant differences at p < 0.05 at each time point.
4. Discussion
P. expansum is the most popular and economically significant postharvest pathogen, which mainly threatens the pome fruits and derived products. To control this pathogen, apart from an excessive application of fungicides, great advances have been made toward obtaining alternative antifungal strategies. Among them, the use of essential oils and extracts is one of the optional approaches that meets the requirement of high efficiency, environmental protection, and safety. Due to being less toxic, fat soluble, permeable across living cell membranes, easily undergoing degradation, and its acceptance as biocompatible, cinnamon oil is known as a Generally Recognized as Safe (GRAS) compound and is suggested as a potential candidate for chemoprevention against pathogenic problems. It shows broad-spectrum and potent antifungal, antiviral, and antibacterial activities because of containing many active ingredients such as trans-cinnamaldehyde, eugenol, and linalool [42]. Meanwhile, it has a limited impact on the growth of endogenous strains, which avoids an ecological imbalance that could favor the appearance of new fungal strains or other microorganisms producing other toxic compounds [10,43]. These make cinnamon oil ideal for utilization in various postharvest products.
Particularly for Penicillium spp., Ryu and Holt found that cinnamon oil could inhibit P. expansum growth and patulin accumulation on apples and in apple juice, and the effectiveness was dependent on the application concentration and the apple cultivar treated [44]. Xing et al. determined the minimum inhibitory concentration of cinnamon oil against P. expansum was 0.16% (v/v). Cinnamon oil with a concentration of 2.0% (v/v) showed complete control of P. expansum in Lingwu Long Jujube and Sand Sugar Orange fruits [45]. Jeong et al. found that cinnamon oil had a wide antifungal spectrum against different Penicillium fungal contaminants isolated from cheese. Both essential oils extracted from cinnamon leaf and bark with a concentration of 4000 ppm/mm2 exerted powerful antifungal activities against Penicillium spp. on Appenzeller cheese [46]. Meanwhile, with the development of active food packaging film, the encapsulation of cinnamon oil in Ag+/Zn2+-permutite can effectively control decay induced by Penicillium citrinum in fresh Chinese bayberry fruit [47]. Moreover, cinnamaldehyde and citral combination led to wrinkles and depressions of spores and mycelia, and lower levels of patulin production in P. expansum [48]. In this study, the effects of cinnamon oil on the growth and development of P. expansum were firstly evaluated. The results indicated that 0.25 mg L−1 cinnamon oil could efficiently inhibit the spore germination, mycelial accumulation and expansion, and conidial production of P. expansum. Meanwhile, the blue mold rots in apples induced by P. expansum were effectively controlled by cinnamon oil. These results were basically consistent with the previous reports [44,45,46]. The minor differences probably derived from different fungal strains and sources of cinnamon oil.
Weakened fungal development is not always associated with the decrease of mycotoxin accumulation, since they might have different action modes or a negative feedback relationship. For cinnamon oil, it can play a decisive role in the inhibition of mycotoxin produced by Fusarium graminearum in rice culture [49] and the ochratoxin A production of Aspergillus carbonaria in apples and pears [50]. Patulin is an important mycotoxin produced by P. expansum, and its biosynthesis pathway has been illuminated [32]. The expressions of most genes (except for PatD and PatL) related to patulin biosynthesis were down-regulated under cinnamon oil stress, which were similar to the previous observation [48]. PatD encoded an alcohol dehydrogenase that catalyzed the oxidation and reduction of a wide variety of alcohols and aldehydes. The up-regulation of PatD probably played a general detoxifying role under the context of this study. PatL encoded C6 transcription factor, which can affect the expression of many genes besides the patulin biosynthetic gene cluster [51]. The previous studies showed that the expression of PatL increased under the stress circumstance [52]. Therefore, according to the qRT-PCR and HPLC results, cinnamon oil created a patulin-restrictive condition to P. expansum.
Cinnamon oil contains a broad-spectrum antimicrobial effect and has been widely applied in medical and food industries for a long time [10]. Many advances about the antimicrobial mechanism of cinnamon oil have been achieved. Xing et al. found that cinnamon oil could interfere enzymatic reactions of cell wall synthesis and induce irreversible deleterious morphological and ultrastructural alterations of Fusarium verticillioides [24]. Zhang et al. elucidated that cinnamon oil led to the leakage of small electrolytes, causing a rapid increase in the electric conductivity of Escherichia coli and Staphylococcus aureus [12]. Lyu et al. found that cinnamon oil could damage the membrane fatty acids and proteins of Shewanella putrefaciens [53]. He et al. suggested that cinnamon oil could destroy the cell membrane integrity and change the structure of cell membrane of Colletotrichum acutatum [26]. The transcriptome analysis indicated that cinnamaldehyde and citral combination probably affected the cellular primary structure and limited the nutrition transport, causing energy metabolism disorder and oxidative stress [48]. Chuesiang et al. found that cinnamon oil could disrupt cell wall structures of pathogens and promote the expulsion of internal cellular material [54]. Huang et al. (2019) revealed that cinnamon oil can damage the macromolecules in cell membranes of fish spoilage bacteria according to a Fourier transform infrared (FT-IR) spectroscopy analysis [55]. Yang et al. discovered that Klebsiella pneumoniae exposed to cinnamon oil underwent oxidative stress that eventually disrupts the bacterial membrane possibly via interaction with the phospholipid bilayer [56]. Lee et al. emphasized the toxicity of cinnamon oil against Raffaelea quercus-mongolicae and Rhizoctonia solani due to reactive oxygen species generation and cell membrane disruption [57]. Lee et al. considered that trans-cinnamaldehyde and salicylaldehyde could lead to the down-regulation of an ATP synthesis-related gene cluster, corrupted iron ion homeostasis, and a corrupted ROS defense mechanism in Agrobacterium tumefaciens [16]. Among them, one of the mechanisms for the antimicrobial activity of cinnamon oil has been attributed to the disruption of membranes and cell walls for the high lipophilicity. However, in the present study, under 0.25 mg L−1 cinnamon oil stress, P. expansum spores could keep the membrane integrity after 6 h of culture, and the cell wall did not noticeably change yet (Figure S5). The difference from previous reports might derive from the lower working concentration and the variety of pathogens. Therefore, more explorations were performed to uncover the potential antifungal mechanism of cinnamon oil.
A global proteomic analysis was performed to illuminate the biological changes of P. expansum spores under 6 h of cinnamon oil stress. Through GO and pathway enrichment statistics, DEPs involved in the carbohydrate metabolic process were noticed by the amount and significance. Carbohydrates including monosaccharides, disaccharides, oligosaccharides, and polysaccharides are known as hydrates of carbon or polyhydroxy aldehydes and ketones. They are composed of a six-membered ring (containing carbon and oxygen) either alone or joined together. Carbohydrates and their derivatives have various important biological roles including major energy storage, protein modification and regulation, the backbone of the DNA or RNA, and essential structural components. The carbohydrate metabolism contains various biochemical pathways for biosynthesis, degradation, interconversion, and transport [58]. In the present study, a total of 146 DEPs (about 16.72% of total) related to carbohydrate metabolism were detected in P. expansum spores under cinnamon oil treatment. Most of them were down-regulated except for hydroxymethylglutaryl-coenzyme A synthase, glycosyl transferase, malic enzyme, acetolactate synthase, ribose-phosphate diphosphokinase, formyl transferase, thiolase-like and aldolase-type TIM barrel (DEP3, DEP4, DEP5, DEP47, DEP103, DEP256, DEP263, DEP270, DEP300 and DEP327). That indicated that the carbohydrate metabolic capability of P. expansum spores was seriously impaired by cinnamon oil. To verify the results at the protein level, the expressions of 14 candidate genes corresponding to DEPs involved in butanoate metabolism, propanoate metabolism, fructose and mannose metabolism, pyruvate metabolism, pentose phosphate pathway, starch and sucrose metabolism, and glycolysis/gluconeogenesis were determined. The expression trends of candidates in mRNA levels were similar to those in protein levels with treatment time growing. In addition, selecting the most energetically favorable carbon source, known as carbon catabolite repression (CCR), is a survival strategy for microorganisms because it supports the rapid growth and development. In Aspergillus nidulans, the CCR pathway was mediated by CreA [59]. CreA was essential for growth on various carbon, nitrogen, and lipid sources, and it played a role in amino acid transport and nitrogen assimilation [60]. In P. expansum, the deletion of CreA led to a decreased ability to produce patulin and proteolytic enzymes, and to acidify the environment [35]. In the present study, the expression of CreA was significantly down-regulated by cinnamon oil with treatment time increasing. The patulin production was inhibited as well. These results indicated that cinnamon oil represented the potential to impair the secondary metabolites biosynthesis and carbon/nitrogen metabolism of P. expansum. Furthermore, the activities of six key regulatory enzymes (β-Glucosidase for cellulose hydrolyzation, α-ketoglutarate dehydrogenase for the tricarboxylic acid circle, 6-phosphofructokinase and pyruvate kinase for glycolysis, hexokinase and glucose 6-phosphate dehydrogenase for pentose phosphate pathway) in carbohydrate metabolism decreased significantly. The changes in intracellular macromolecules were ultimately embodied in the decreasing of extracellular carbohydrate consumption, intracellular carbohydrate accumulation, and ATP production. Under cinnamon oil treatment, the declining fluorescent intensity of MitoTracker Orange probe for labeling mitochondria indicated that the number of mitochondria decreased or the mitochondria experienced a loss in membrane potential. That provided more direct evidence for compromised energy metabolism induced by cinnamon oil.
Data acquired from evaluations at the protein, mRNA, biochemical, and physiological levels are correlated and mutually authenticated. Therefore, we speculated that disturbing the fungal carbohydrate metabolic process would partly be responsible for the antifungal action of cinnamon oil. Further exploration will focus on determining the exact components of cinnamon oil and the integrative information for each active component that should be responsible for the inhibitory effect, screening out the detailed action targets and identifying the direct action mechanism of cinnamon oil on the basis of the present available results. In addition, to facilitate the use and improve the efficacy of cinnamon oil in the practical application, optimizing operation parameters and developing novel synergistic strategies with other active substances or carriers are necessary.
In conclusion, cinnamon oil presents an inhibitory effect on the growth and mycotoxin production of postharvest pathogen P. expansum with a dose-dependent manner. The antifungal activity is partially attributed to cinnamon oil-induced carbohydrate metabolic disorder. The results would provide a fundamental understanding of antimicrobial action mode of cinnamon oil.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/7/2/123/s1, Figure S1: (A) Microscopic observation of P. expansum spores with DAPI staining after 6 h of culturing under 0 or 0.25 mg L−1 cinnamon oil stress. (B) Effects of 0.25 mg L−1 cinnamon oil on colonial morphology of P. expansum. Figure S2: (A) Effects of 0.25 mg L−1 cinnamon oil on blue mold rot caused by P. expansum in apple. Bar represents the standard deviation of the means of three independent experiments. Low case letters indicate significant differences at p < 0.05 at each time point. (B) The apples inoculated by P. expansum spores with 0 or 0.25 mg L−1 cinnamon oil after 7 days of storage at 25 oC. Figure S3: Effects of 0.25 mg L−1 cinnamon oil on the expression of genes involved in patulin biosynthesis in P. expansum. Relative expression levels of PatA to PatO (A to O) are determined by qRT-PCR and the β-tubulin is used as the internal control. Bar represents the standard deviation of the means of three independent experiments. Low case letters indicate significant differences at p < 0.05 at each time point. Figure S4: (A) Effects of 0.25 mg L−1 cinnamon oil on patulin production of P. expansum. Bar represents the standard deviation of the means of three independent experiments. Low case letters indicate significant differences at p < 0.05 at each time point. Figure S5: Effect of 0.25 mg L−1 cinnamon oil on the membrane integrity of P. expansum spores. Spores were cultured at 25 oC in PDB medium supplemented with 0 (negative control), 0.25 mg L−1 cinnamon oil. After 6 h of culture, half of the cinnamon oil-treated spores were incubated in boiling water for ten minutes (as positive control). Then, all the spores were stained with the prodium iodide and observed with a fluorescence microscope. Table S1: Information of the differentially expressed proteins in P. expansum spores under 0.25 mg L−1 cinnamon oil stress after 6 h of culturing by iTRAQ analysis.Table S2: Primer pairs used for qRT-PCR analysis.
Author Contributions
Conceptualization, T.Z. and T.L.; methodology, Y.S. and Y.L.; software, Y.C.; validation, Y.S. and Y.L.; formal analysis, R.L.; investigation, Y.S. and Y.L.; resources, T.Z.; data curation, Y.C.; writing—original draft preparation, T.L. and Y.S.; writing—review and editing, T.Z.; visualization, R.L.; supervision, T.Z.; project administration, T.Z.; funding acquisition, T.Z. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY19C200010 and LY18C150009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, B.; Smedsgaard, J.; Frisvad, J.C. Penicillium expansum: Consistent prodctuion of patulin, chaetoglobosins, and other secondary metabolites in culture and their natural occurrence in fruit products. J. Agric. Food Chem. 2004, 21, 2421–2428. [Google Scholar] [CrossRef]
- Li, B.Q.; Chen, Y.; Zhang, Z.Q.; Qin, G.Z.; Chen, T.; Tian, S.P. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3416–3438. [Google Scholar] [CrossRef] [PubMed]
- Moake, M.; Padilla-Zakour, O.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M., II. Penicillium expansum: Biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef]
- Yu, L.L.; Qiao, N.Z.; Zhao, J.X.; Zhang, H.; Tian, F.W.; Zhai, Q.X.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Morales, H.; Marín, S.; Ramos, A.J.; Sanchis, V. Influence of postharvest technologies applied during cold storage of apples in Penicillium expansum growth and patulin accumulation: A review. Food Control 2010, 21, 953–962. [Google Scholar] [CrossRef]
- Mari, M.; Francesco, A.D.; Bertolini, P. Control of fruit postharvest diseases: Old issues and innovative approaches. Stewart Postharvest Rev. 2014, 10, 1. [Google Scholar]
- Palou, L.; Ali, A.; Fallik, E.; Rmanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Techol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FT-IR spectroscopy. J. Biol. Chem. 2013, 41, 269–278. [Google Scholar]
- Goel, N.; Rohilla, H.; Singh, G.; Punia, P. Antifungal activity of cinnamon oil and olive oil against Candida Spp. isolated from blood stream infections. J. Clin. Diagn. Res. 2016, 10, 9–11. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.Y.; Wang, Y.F.; Jiang, P.P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Boito, J.P.; Silva, A.S.D.; Reis, J.H.D.; Santos, D.S.; Gebert, R.R.; Biazus, A.H.; Santos, R.C.V.; Quatrin, P.M.; Ourique, A.F.; Boligon, A.A.; et al. Insecticidal and repellent effect of cinnamon oil on flies associated with livestock. Rev. MVZ Cordoba 2018, 23, 6628–6636. [Google Scholar]
- Franlyne, J.S.; Ebenazer, L.A.; Mukherjee, A.; Natarajan, C. Cinnamon and clove oil nanoemulsions: Novel therapeutic options against vancomycin intermediate susceptible Staphylococcus aureus. Appl. Nanosci. 2019, 9, 1404–1415. [Google Scholar]
- Mukerjee, A.; Pandey, H.; Tripathi, A.K.; Singh, S.K. Development, characterization and evaluation of cinnamon oil and usnic acid blended nanoemulsion to attenuate skin carcinogenicity in swiss albino mice. Biocatal. Agric. Biotechnol. 2019, 20, 101227. [Google Scholar] [CrossRef]
- Lee, J.E.; Jung, M.; Lee, S.C.; Huh, M.J.; Seo, S.M.; Park, I.K. Antibacterial mode of action of trans-cinnamaldehyde derived from cinnamon bark (Cinnamomum verum) essential oil against Agrobacterium tumefaciens. Pestic. Biochem. Phys. 2020, 165, 104546. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.G.; Lin, H.B.; Cao, D.; Xu, Q.L.; Han, W.F.; Wang, R.R.; Che, Z.M.; Li, X.H. Effect of chitosan coating with cinnamon oil on the quality and physiological attributes of China jujube fruits. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Xing, Y.G.; Xu, Q.L.; Yang, S.X.; Chen, C.K.; Tang, Y.; Sun, S.M.; Zhang, L.; Che, Z.M.; Li, X.H. Preservation mechanism of chitosan-based coating with cinnamon oil for fruits storage based on sensor data. Sensors 2016, 16, 1111. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Ramezanian, A.; Dastjerdi, A.M.; Shamili, M. The potential of gum arabic enriched with cinnamon essential oil for improving the qualitative characteristic and storability of guava (Psidium guajava L.) fruit. Sci. Hortic. 2019, 251, 101–107. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.X.; Wang, J.; Liu, Y.; Lu, P.; Huang, L.J. Effect of chitosan- and alginate-based coatings enriched with cinnamon essential oil microcapsules to improve the postharvest quality of mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Istúriz-Zapata, M.A.; Hernández-López, M.; Correa-Pacheco, Z.N.; Barrera-Necha, L.L. Quality of cold-stored cucumber as affected by nanostructured coatings of chitosan with cinnamon essential oil and cinnamaldehyde. Food Sci. Tech-Brazil 2020, 123, 109089. [Google Scholar] [CrossRef]
- Rashid, Z.; Khan, M.R.; Mubeen, R.; Hassan, A.; Saeed, F.; Afzaal, M. Exploring the effect of cinnamon essential oil to enhance the stability and safety of fresh apples. J. Food Process. Preserv. 2020, 44, e14926. [Google Scholar] [CrossRef]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Xing, F.G.; Hua, H.J.; Selvaraj, J.N.; Zhao, Y.J.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Kosegarten, C.E.; Ramírez-Corona, N.; Mani-López, E.; Palou, E.; López-Malo, A. Description of Aspergillus flavus growth under the influence of different factors (water activity, incubation temperature, protein and fat concentration, pH, and cinnamon essential oil concentration) by kinetic, probability of growth, and time to-detection models. Int. J. Food Microbiol. 2017, 240, 115–123. [Google Scholar]
- He, J.L.; Wu, D.T.; Zhang, Q.; Chen, H.; Li, H.Y.; Han, Q.H.; Lai, X.Y.; Wang, H.; Wu, Y.X.; Yuan, J.G.; et al. Efficacy and mechanism of cinnamon essential oil on inhibition of Colletotrichum acutatum isolated from "Hongyang’ kiwifruit. Front. Microbiol. 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Bafort, F.; Damblon, C.; Lognay, G.C.; Barthelemy, J.P.; Perraudin, J.P.; Jijakli, H. Effect of the nature and relative concentration of substrate, water mineralization, and storage temperature on the oxidants produced by lactoperoxidase and on their antifungal activity against Penicillium expansum and Botrytis cinerea. Appl. Sci. 2019, 9, 197. [Google Scholar] [CrossRef]
- Black-Solis, J.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.; Corona-Rangel, M.L.; Bautista-Baños, S. Preharvest use of biodegradable polyester nets added with cinnamon essential oil and the effect on the storage life of tomatoes and the development of Alternaria alternata. Sci. Hortic. 2019, 245, 65–73. [Google Scholar] [CrossRef]
- Naserzadeh, Y.; Mahmoudi, N.; Pakina, E. Antipathogenic effects of emulsion and nanoemulsion of cinnamon essential oil against Rhizopus rot and grey mold on strawberry fruits. Foods Raw Mater. 2019, 7, 210–216. [Google Scholar] [CrossRef]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason. Sonochem. 2020, 60, 104604. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef]
- Tannous, J.; Khoury, R.E.; Snini, S.P.; Lippi, Y.; Khoury, A.E.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, X.H.; Ye, B.S.; Shi, L.; Bai, X.L.; Lai, T.F. Effect of essential oil decanal on growth and transcriptome of the postharvest fungal pathogen Penicillium expansum. Postharvest Biol. Technol. 2018, 145, 203–212. [Google Scholar] [CrossRef]
- Dowzer, C.E.A.; Kelly, J.M. Cloning of the creA gene from Aspergillus nidulans: A gene involved in carbon catabolite repression. Curr. Genet. 1989, 15, 457–459. [Google Scholar] [CrossRef]
- Tannous, J.; Kumar, D.; Sela, N.; Sionov, E.; Prusky, D.; Keller, N.P. Fungal attack and houst defence pathways unveiled in near-avirulent interactions of Penicillium expansum creA mutants on apples. Mol. Plant Pathol. 2018, 19, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Meixner-Monori, B.; Kubicek, C.P.; Habison, A.; Kubicek-Pranz, E.M.; Röhr, M. Presence and regulation of the α-ketoglutarate dehydrogenase multienzyme complex in the filamentous fungus Aspergillus niger. J. Bacteriol. 1985, 161, 265–271. [Google Scholar] [CrossRef]
- Golshani-Hebroni, S.G.; Bessman, S.P. Hexokinase binding to mitochondria: A basis for proliferative energy metabolism. J. Bioenerg. Biomembr. 1997, 29, 331–338. [Google Scholar] [CrossRef]
- Brüser, A.; Kirchberger, J.; Kloos, M.; Sträter, N.; Schöneberg, T. Functional linkage of adenine nucleotide binding sites in mammalian muscle 6-phosphofructokinase. J. Biol. Chem. 2012, 287, 17546–17553. [Google Scholar] [CrossRef]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. 2019, 28, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef]
- Paudel, S.K.; Bhargava, K.; Kotturi, H. Antimicrobial activity of cinnamon oil nanoemulsion against Listeria monocytogenes and Salmonella spp. on melons. Food Sci. Tech-Brazil 2019, 111, 682–687. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Kiymaci, M.E.; Simsek, D.; Erol, H.B.; Erdem, S.A. Comparison of the contents and antimicrobial activities of commercial and natural cinamon oils. Indian J. Pharm. Sci. 2016, 78, 541–546. [Google Scholar] [CrossRef]
- Ryu, D.; Holt, D.L. Growth inhibition of Penicillium expansum by several commonly used food ingredients. J. Food Prot. 1993, 56, 862–867. [Google Scholar] [CrossRef]
- Xing, Y.G.; Li, X.H.; Xu, Q.L.; Yun, J.; Lu, Y.Q. Antifungal activities of cinnamon oil against Rhizopus nigricans, Aspergillus flavus and Penicillium expansum in vitro and in vivo fruit test. Int. J. Food Sci. Technol. 2010, 45, 1837–1842. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, N.K.; Oh, J.; Jang, S.E.; Lee, J.S.; Bae, I.H.; Oh, H.H.; Jung, H.K.; Jeong, Y.S. Inhibitory effect of cinnamon essential oils on selected cheese-contaminating fungi (Penicillium spp.) during the cheese-ripening process. Food Sci. Biotechnol. 2014, 23, 1193–1198. [Google Scholar] [CrossRef]
- Niu, B.; Yan, Z.P.; Shao, P.; Kang, J.; Chen, H.J. Encapsulation of cinnamon essential oil for active food packaging film with synergistic antimicrobial activity. Nanomaterials 2018, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, K.W.; Yang, H.H.; Zhang, Z.W.; Yuan, Y.H.; Yue, T.L. Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front. Microbiol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Wu, D.H.; Lu, J.; Zhong, S.B.; Schwarz, P.; Chen, B.C.; Rao, J.J. Influence of nonionic and ionic surfactants on the antifungal and mycotoxin inhibitory efficacy of cinnamon oil nanoemulsions. Food Funct. 2019, 10, 2817–2827. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Nestora, S.; Evageliou, V.; Skandamis, P.N. Sodium alginate-cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin a production. Food Res. Int. 2019, 119, 876–885. [Google Scholar] [CrossRef]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.F.; Wang, Y.; Fan, Y.Y.; Zhou, Y.Y.; Bao, Y.; Zhou, T. The response of growth and patulin production of postharvest pathogen Penicillium expansum to exogenous potassium phosphite treatment. Int. J. Food Microbiol. 2017, 244, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Hong, Y.L.; Cai, J.H.; Wei, Q.Q.; Zhou, X.X.; Ding, Y.T.; Liu, Z.F.; Liu, L. Antimicrobial effect and mechanism of cinnamon oil and gamma radiation on Shewanella putrefaciens. J. Food Sci. Technol. 2018, 55, 3353–3361. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; McClements, D.J.; McLandsborough, L. Antimicrobial activity of PIT-fabricated cinnamon oil nanoemulsions: Effect of surfactant concentration on morphology of foodborne pathogens. Food Control 2019, 98, 405–411. [Google Scholar] [CrossRef]
- Huang, Z.; Jia, S.; Zhang, L.T.; Liu, X.C.; Luo, Y.K. Inhibitory effects and membrane damage caused to fish spoilage bacteria by cinnamon bark (Cinnamomum tamala) oil. Food Sci. Tech. Brazil 2019, 112, 10895. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.E.; Lai, K.S. Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS ONE 2019, 14, e0214326. [Google Scholar] [CrossRef]
- Lee, J.E.; Seo, S.M.; Huh, M.J.; Lee, S.C.; Park, I.K. Reactive oxygen species mediated-antifungal activity of cinnamon bark (Cinnamomum verum) and lemongrass (Cymbopogon citratus) essential oils and their constituents against two phytopathogenic fungi. Pestic. Biochem. Phys. 2020, 168, 104644. [Google Scholar] [CrossRef]
- Rademacher, T.W.; Parekh, R.B.; Dwek, R.A. Glycobiology. Ann. Rev. Biochem. 1988, 57, 785–838. [Google Scholar] [CrossRef]
- Dowzer, C.E.A.; Kelly, J.M. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol. Cell. Biol. 1991, 11, 5701–5709. [Google Scholar] [CrossRef]
- Ries, L.N.A.; Beattie, S.R.; Espeso, E.A.; Cramer, R.A.; Goldman, G.H. Divers regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics 2016, 203, 335–352. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).