In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Antifungal Agents
2.3. Minimum Inhibitory Concentration (MIC) Determination
2.4. Confirming Echinocandin Resistance
2.5. Checkerboard Assay
2.6. Disk Diffusion Assay
2.7. Time-Killing Assay
2.8. Statistical Analysis
3. Results
3.1. Antifungal Susceptibility Profiling of the Tested Isolates
3.2. Checkerboard Assay
3.3. Disk Diffusion Assay
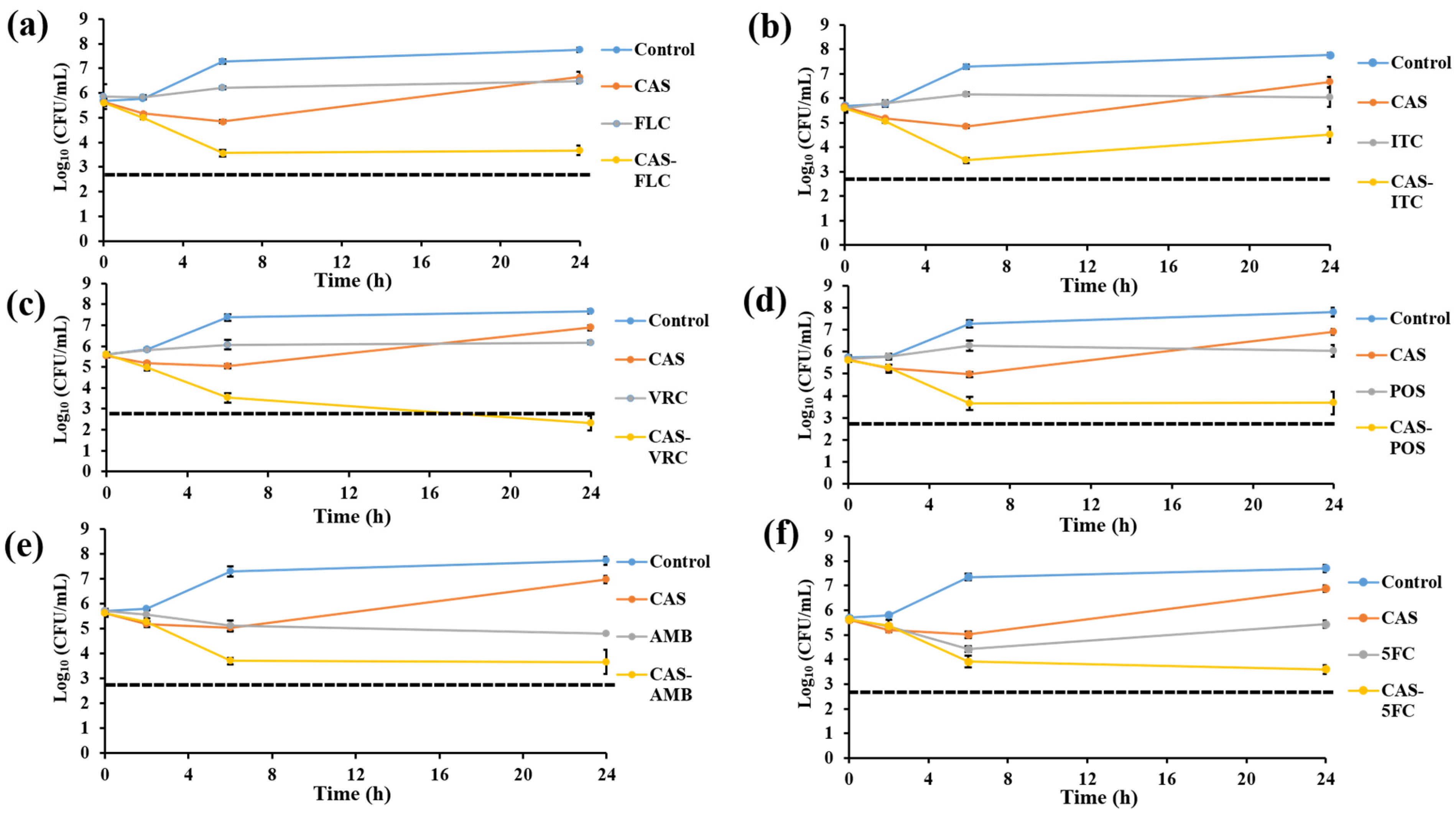
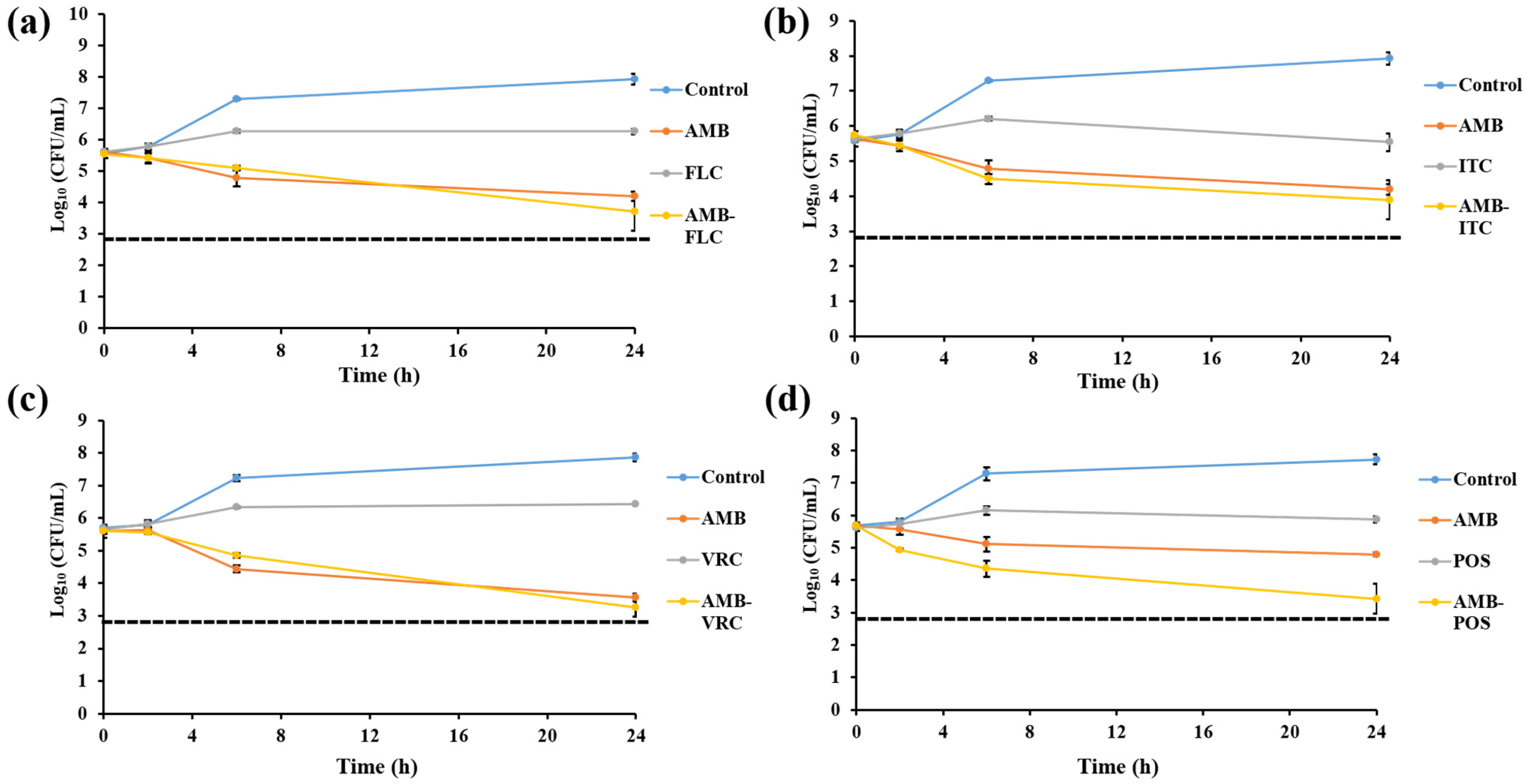
3.4. Killing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, D.C.; Govender, N.P.; Chakrabarti, A.; Sacarlal, J.; Denning, D.W. Improvement of fungal disease identification and management: Combined health systems and public health approaches. Lancet Infect Dis. 2017, 17, e412–e419. [Google Scholar] [CrossRef]
- Yousfi, H.; Cassagne, C.; Ranque, S.; Rolain, J.M.; Bittar, F. Repurposing of ribavirin as an adjunct therapy against invasive Candida strains in an in vitro study. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1, 3-β-d-glucan synthase: Implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.; Grussemeyer, C.A.; Spalding, J.R.; Benjamin, D.K., Jr.; Reed, S.D. Comparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infections. Am. J. Infect. Control 2010, 38, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.R.; Zhao, Y.; Perez, W.B.; Lockhart, S.R.; Sobel, J.D.; Farmakiotis, D.; Kontoyiannis, D.P.; Sanglard, D.; Taj-Aldeen, S.J.; Alexander, B.D.; et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 2016, 7, 11128. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Arai, T.; Majima, H.; Watanabe, A.; Kamei, K. Genetic basis of azole and echinocandin resistance in clinical Candida glabrata in Japan. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Barchiesi, F.; Spreghini, E.; Maracci, M.; Fothergill, A.W.; Baldassarri, I.; Rinaldi, M.G.; Scalise, G. In vitro activities of voriconazole in combination with three other antifungal agents against Candida glabrata. Antimicrob. Agents Chemother. 2004, 48, 3317–3322. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Pham, C.D.; Iqbal, N.; Bolden, C.B.; Kuykendall, R.J.; Harrison, L.H.; Farley, M.M.; Schaffner, W.; Beldavs, Z.G.; Chiller, T.M.; Park, B.J.; et al. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob. Agents Chemother. 2014, 58, 4690–4696. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Ramani, R.; Andes, D.; Diekema, D.J.; Pfaller, M.A.; Ghannoum, M.A.; Knapp, C.; Lockhart, S.R.; Ostrosky-Zeichner, L.; Walsh, T.J.; et al. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob. Agents Chemother. 2011, 55, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Oz, Y. Synergistic activities of three triazoles with caspofungin against Candida glabrata isolates determined by time-kill, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 2010, 54, 2244–2247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saraya, T.; Tanabe, K.; Araki, K.; Yonetani, S.; Makino, H.; Watanabe, T.; Tsujimoto, N.; Takata, S.; Kurai, D.; Ishii, H.; et al. Breakthrough invasive Candida glabrata in patients on micafungin: A novel FKS gene conversion correlated with sequential elevation of MIC. J. Clin. Microbiol. 2014, 52, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Burns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfland, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; p. 315332. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. CLSI Stand m27, 4th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI Suppl m60, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Beyda, N.D.; John, J.; Kilic, A.; Alam, M.J.; Lasco, T.M.; Garey, K.W. FKS mutant Candida glabrata: Risk factors and outcomes in patients with candidemia. Clin. Infect. Dis. 2014, 59, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Arendrup, M.C.; Pfaller, M.A.; Bonfietti, L.X.; Bustamante, B.; Canton, E.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Fothergill, A.; et al. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: Should the clinical laboratory be testing this agent? Antimicrob. Agents Chemother. 2013, 57, 5836–5842. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergistic, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. CLSI Suppl M44, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Barchiesi, F.; Spreghini, E.; Tomassetti, S.; Giannini, D.; Scalise, G. Caspofungin in combination with amphotericin B against Candida parapsilosis. Antimicrob. Agents Chemother. 2007, 51, 941–945. [Google Scholar] [CrossRef][Green Version]
- Eliopoulos, G.M.; Moellering, R.C. Antimicrobial combinations. In Antibiotics in Laboratory Medicine, 4th ed.; Lorian, V., Ed.; The Williams and Wilkins Co.: Baltimore, MD, USA, 1996; pp. 330–396. [Google Scholar]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef]
- Mattiuzzi, G.N.; Estey, E.; Raad, I.; Giles, F.; Cortes, J.; Shen, Y.; Kontoyiannis, D.; Koller, C.; Munsell, M.; Beran, M.; et al. Liposomal amphotericin B versus the combination of fluconazole and itraconazole as prophylaxis for invasive fungal infections during induction chemotherapy for patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer 2003, 97, 450–456. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Fu, Y.; Ibrahim, A.S.; Mortara, L.A.; Shafiq, M.C.; Edwards, J.E.; Criddle, R.S. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob. Agents Chemother. 1995, 39, 2459–2465. [Google Scholar] [CrossRef][Green Version]
- Louie, A.; Banerjee, P.; Drusano, G.L.; Shayegani, M.; Miller, M.H. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob. Agents Chemother. 1999, 43, 2841–2847. [Google Scholar] [CrossRef]
- Louie, A.; Kaw, P.; Banerjee, P.; Liu, W.; Chen, G.; Miller, M.H. Impact of the order of initiation of fluconazole and amphotericin B in sequential or combination therapy on killing of Candida albicans in vitro and in a rabbit model of endocarditis and pyelonephritis. Antimicrob. Agents Chemother. 2001, 45, 485–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rex, J.H.; Pappas, P.G.; Karchmer, A.W.; Sobel, J.D.; Edwards, J.E.; Hadley, S.; Brass, C.; Vazquez, J.A.; Chapman, S.W.; Horowitz, H.W.; et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 2003, 36, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.M.; Hitchcock, C.A.; Troke, P.F.; Picard, M. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob. Agents Chemother. 1995, 39, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Bennett, J.E.; Sugar, A.M.; Pappas, P.G.; Van Der Horst, C.M.; Edwards, J.E.; Washburn, R.G.; Scheld, W.M.; Karchmer, A.W.; Dine, A.P.; et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 1994, 331, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Te Dorsthorst, D.T.A.; Verweij, P.E.; Meletiadis, J.; Bergervoet, M.; Punt, N.C.; Meis, J.F.G.M.; Mouton, J.W. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 2002, 46, 2982–2989. [Google Scholar] [CrossRef]
- Barchiesi, F.; Schimizzi, A.M.; Najvar, L.K.; Bocanegra, R.; Caselli, F.; Di Cesare, S.; Giannini, D.; Di Francesco, L.F.; Giacometti, A.; Carle, F.; et al. Interactions of posaconazole and flucytosine against Cryptococcus neoformans. Antimicrob. Agents Chemother. 2001, 45, 1355–1359. [Google Scholar] [CrossRef][Green Version]
- Barchiesi, F.; Gallo, D.; Caselli, F.; Di Francesco, L.F.; Arzeni, D.; Giacometti, A.; Scalise, G. In-vitro interactions of itraconazole with flucytosine against clinical isolates of Cryptococcus neoformans. J. Antimicrob. Chemother. 1999, 44, 65–70. [Google Scholar] [CrossRef][Green Version]
- Nguyen, M.H.; Najvar, L.K.; Yu, C.Y.; Graybill, J.R. Combination therapy with fluconazole and flucytosine in the murine model of cryptococcal meningitis. Antimicrob. Agents Chemother. 1997, 41, 1120–1123. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Tao, R.; Wu, J.; Dai, X.; Hu, C.; Huang, Y.; Chen, Y.; Zhu, B.; He, J. Short-course rather than low-dose amphotericin B may exert potential influence on mortality in cryptococcal meningitis patients treated with amphotericin B plus flucytosine alone or in combination with fluconazole. Front. Microbiol. 2019, 10, 2082. [Google Scholar] [CrossRef]
- Antachopoulos, C.; Papakonstantinou, E.; Dotis, J.; Bibashi, E.; Tamiolaki, M.; Koliouskas, D.; Roilides, E. Fungemia due to Trichosporon asahii in a neutropenic child refractory to amphotericin B: Clearance with voriconazole. J. Pediatr. Hematol. Oncol. 2005, 27, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J.H. Combination antifungal therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 2002, 49, 345–351. [Google Scholar] [CrossRef] [PubMed]




| Primer Name | Sequence | Target Gene/Purpose | PCR Product Sizes | Reference |
|---|---|---|---|---|
| FKS1-HS1,2,3-FW | GTCGCTACATTGCTATTTTTCTCAGTCATGCC | CgFKS1 (HS1, HS2, HS3)/PCR and sequencing | 2641 bp | [6] |
| FKS1-HS1,2,3-RV | CCATATAAATGGCAGAGCCTGCAAATCTGG | CgFKS1 (HS1, HS2, HS3)/PCR and sequencing | ||
| FKS1-HS1,3-RV1 | GAGATAATGATAGCGTTCCAGACTTGGG | CgFKS1 (HS1, HS3)/sequencing | [6] | |
| FKS1-HS2-FW1 | AAGATTGGTGCTGGTATGG | CgFKS1 (HS2)/sequencing | [6] | |
| FKS2-HS1,2-FW | CCATTAGGTGGTCTTTTCACCTCATATATGC | CgFKS2 (HS1, HS2)/PCR and sequencing | 2726 bp | [6] |
| FKS2-HS1,2-RV | GGATTAAATATGAATGGAGAGAACAGTAAAGCAG | CgFKS2 (HS1, HS2)/PCR and sequencing | ||
| FKS2-HS1-RV1 | GCAAGTAAATGTTCTCTGTACATGG | CgFKS2 (HS1)/sequencing | [6] | |
| FKS2-HS2-FW2 | TACTATGCGCATCCTGGTTTCCAT | CgFKS2 (HS2)/sequencing | [6] |
| Drug 1 | No. of Isolates at Each Determined MIC Value (µg/mL) | MIC Range (µg/mL) | GM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | |||
| CAS | 11 | 2 | 3 | 1 | 0.5–64 | 1.18 | ||||||||||
| FLC | 5 | 3 | 5 | 2 | 1 | 1 | 2–64 | 6.26 | ||||||||
| ITC | 10 | 3 | 4 | 0.5–2 | 0.78 | |||||||||||
| VRC | 10 | 3 | 1 | 1 | 1 | 1 | 0.06–8 | 0.13 | ||||||||
| POS | 1 | 10 | 4 | 1 | 1 | 0.25–4 | 0.69 | |||||||||
| AMB | 7 | 10 | 1–2 | 1.5 | ||||||||||||
| 5FC | 2 | 13 | 2 | 0.008–0.03 | 0.015 | |||||||||||
| Combinations 1 | ΣFIC | % Isolates Showing the Following Interactions: | |||
|---|---|---|---|---|---|
| ΣFIC Range | Median ΣFIC | Synergism (Number/Total) | Indifference (Number/Total) | Antagonism (Number/Total) | |
| 1—Combinations including CAS | |||||
| CAS-FLC | 0.38–1.5 | 0.76 | 23.53 (4/17) | 76.47(13/17) | 0 (0/17) |
| CAS-ITC | 0.38–1 | 0.68 | 29.41 (5/17) | 70.59 (12/17) | 0 (0/17) |
| CAS-VRC | 0.38–1.24 | 0.81 | 17.65 (3/17) | 82.35 (14/17) | 0 (0/17) |
| CAS-POS | 0.28–0.75 | 0.61 | 17.65 (3/17) | 82.35 (14/17) | 0 (0/17) |
| CAS-AMB | 0.25–0.75 | 0.56 | 23.53 (4/17) | 76.47(13/17) | 0 (0/17) |
| CAS-5FC | 0.73–2 | 1.14 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| 2—Azoles combinations | |||||
| FLC-ITC | 0.56–1.13 | 0.90 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| FLC-VRC | 0.61–1.25 | 0.82 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| FLC-POS | 0.56–1.5 | 0.80 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| ITC-VRC | 0.74–1.25 | 0.87 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| ITC-POS | 0.56–1.5 | 0.79 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| VRC-POS | 0.57–1.25 | 0.84 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| 3—AMB with azoles | |||||
| AMB-FLC | 0.25–1.02 | 0.93 | 11.76 (2/17) | 88.24 (15/17) | 0 (0/17) |
| AMB-ITC | 0.19–1.02 | 0.84 | 11.76 (2/17) | 88.24 (15/17) | 0 (0/17) |
| AMB-VRC | 0.09–1.03 | 0.80 | 11.76 (2/17) | 88.24 (1517) | 0 (0/17) |
| AMB-POS | 0.31–1.24 | 0.64 | 29.41 (5/17) | 70.59 (12/17) | 0 (0/17) |
| 4—5FC with azoles | |||||
| 5FC-FLC | 0.55–1.11 | 1.03 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| 5FC-ITC | 0.72–1.25 | 1.09 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| 5FC-VRC | 1.03–1.50 | 1.20 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| 5FC-POS | 1.05–1.12 | 1.07 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| 5—AMB with 5FC | 0.51–1.13 | 0.78 | 0 (0/17) | 100 (17/17) | 0 (0/17) |
| No. | IFM | FKS Mutations | Echinocandin Resistance d | Combinations a,b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAS-FLC | CAS-ITC | CAS-VRC | CAS-POS | CAS-AMB | AMB-FLC | AMB-ITC | AMB-VRC | AMB-POS | ||||
| 1 | 60089 | FKS1 S629P | R | + (0.38) | + (0.38) | + (0.49) | + (0.28) | + (0.38) | - | - | - | - |
| 2 | 61000 | S | - | - | - | - | - | - | - | - | - | |
| 3 | 61017 | S | - | - | - | - | - | - | - | - | - | |
| 4 | 61169 | S | - | - | - | - | - | - | - | - | - | |
| 5 | 61186 | S | - | - | - | - | - | - | - | - | - | |
| 6 | 61193 | S | - | - | - | - | - | - | - | - | - | |
| 7 | 61743 | S | - | - | - | - | - | - | - | - | - | |
| 8 | 61756 | S | - | - | - | - | - | - | - | - | + (0.38) | |
| 9 | 62339 | S | - | - | - | - | - | - | - | - | + (0.50) | |
| 10 | 64652 | S | - | - | - | - | - | - | - | - | + (0.50) | |
| 11 | 64679 | FKS2 S663P c | R | + (0.50) | + (0.50) | - | - | - | - | - | - | - |
| 12 | 64684 | FKS2 S663P c | R | - | - | - | - | - | - | - | - | - |
| 13 | 64686 | FKS2 S663P c | R | + (0.38) | + (0.50) | + (0.38) | + (0.5) | + (0.38) | + (0.25) | + (0.19) | + (0.09) | + (0.31) |
| 14 | 64689 | FKS2 S663P c | R | + (0.50) | + (0.38) | + (0.38) | + (0.38) | + (0.25) | + (0.31) | + (0.38) | + (0.50) | + (0.50) |
| 15 | 64903 | S | - | - | - | - | - | - | - | - | - | |
| 16 | 64905 | S | - | - | - | - | - | - | - | - | - | |
| 17 | 58273 | FKS2 F659del | R | - | + (0.50) | - | - | + (0.38) | - | - | - | - |
| Antifungal Combination Showed Synergistic Action 1 | Echinocandin-Resistant Isolates (n = 6) | Echinocandin-Susceptible Isolates (n = 11) | Total (n = 17) |
|---|---|---|---|
| CAS-FLC | 4/6 (66.66%) | 0/11 (0%) | 4/17 (23.53%) |
| CAS-ITC | 5/6 (83.33%) | 0/11 (0%) | 5/17 (29.41%) |
| CAS-VRC | 3/6 (50%) | 0/11 (0%) | 3/17 (17.65%) |
| CAS-POS | 3/6 (50%) | 0/11 (0%) | 3/17 (17.65%) |
| CAS-AMB | 4/6 (66.66%) | 0/11 (0%) | 4/17 (23.53%) |
| AMB-FLC | 2/6 (33.33%) | 0/11 (0%) | 2/17 (11.76%) |
| AMB-ITC | 2/6 (33.33%) | 0/11 (0%) | 2/17 (11.76%) |
| AMB-VRC | 2/6 (33.33%) | 0/11 (0%) | 2/17 (11.76%) |
| AMB-POS | 2/6 (33.33%) | 3/11 (27.27%) | 5/17 (29.41%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, H.O.; Majima, H.; Watanabe, A.; Kamei, K. In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata. J. Fungi 2021, 7, 108. https://doi.org/10.3390/jof7020108
Khalifa HO, Majima H, Watanabe A, Kamei K. In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata. Journal of Fungi. 2021; 7(2):108. https://doi.org/10.3390/jof7020108
Chicago/Turabian StyleKhalifa, Hazim O., Hidetaka Majima, Akira Watanabe, and Katsuhiko Kamei. 2021. "In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata" Journal of Fungi 7, no. 2: 108. https://doi.org/10.3390/jof7020108
APA StyleKhalifa, H. O., Majima, H., Watanabe, A., & Kamei, K. (2021). In Vitro Characterization of Twenty-One Antifungal Combinations against Echinocandin-Resistant and -Susceptible Candida glabrata. Journal of Fungi, 7(2), 108. https://doi.org/10.3390/jof7020108








