Population Pharmacokinetic Analysis and Dosing Optimization of Prophylactic Fluconazole in Japanese Patients with Hematological Malignancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients
2.3. Fluconazole Administration and Sample Collection
2.4. Measurement of Fluconazole Concentration
2.5. PPK Analysis
2.6. Probability of Target Attainment (PTA) Analysis
2.7. Verification of the Optimal Dose of Fluconazole
3. Results
3.1. Patient Characteristics
3.2. Validation of Fluconazole Measurement
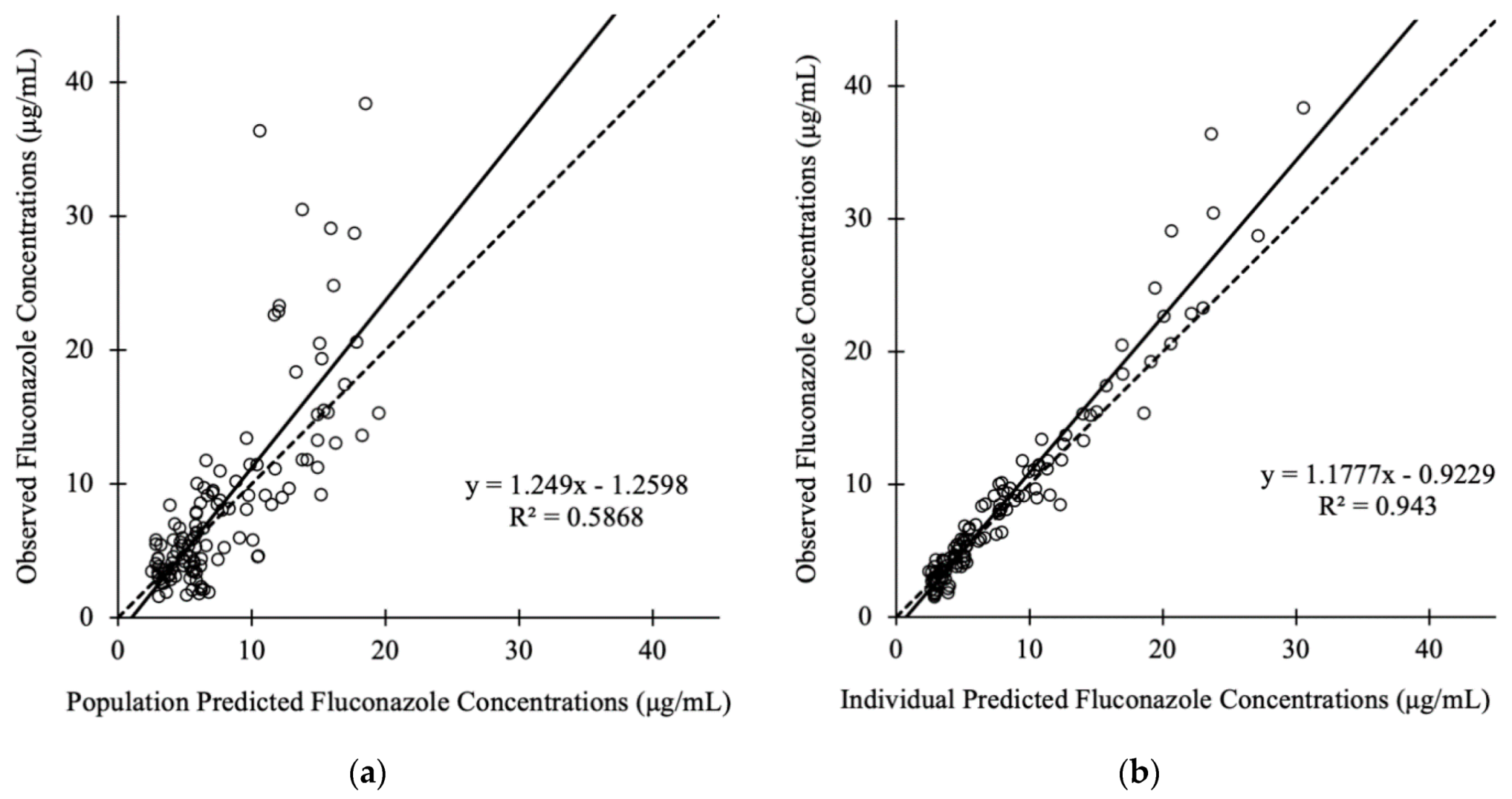
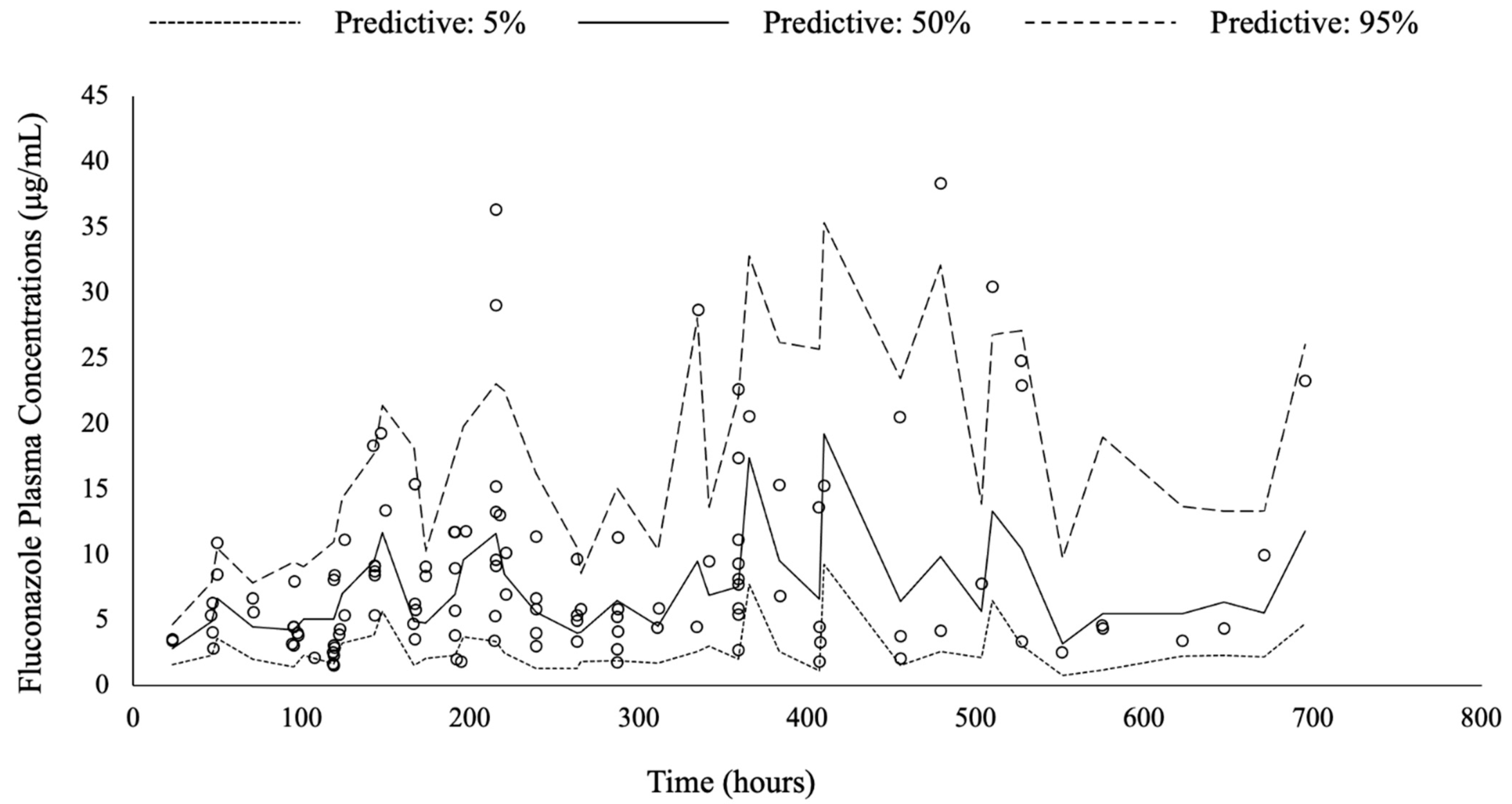
3.3. Pharmacokinetic Model Buiding
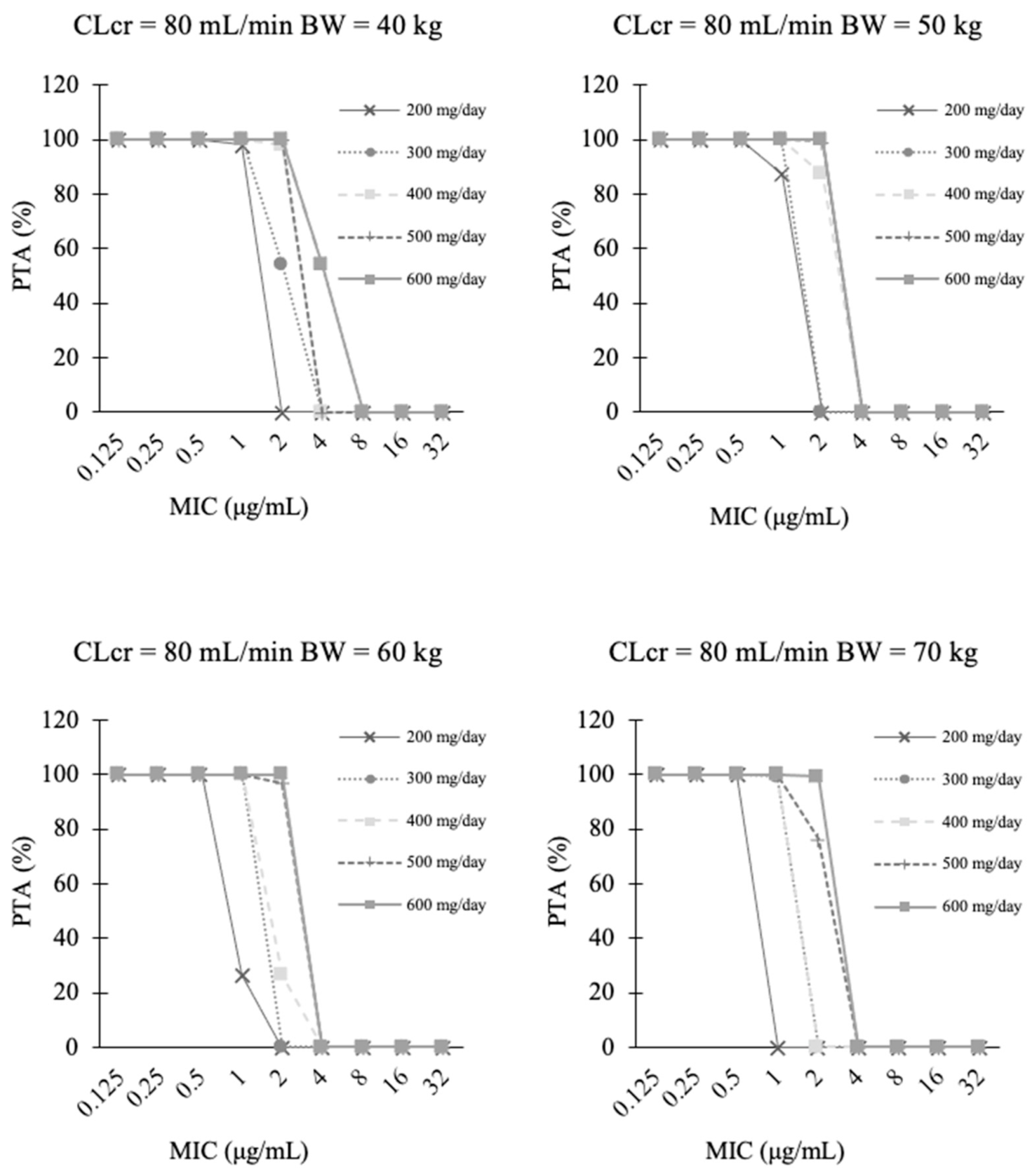
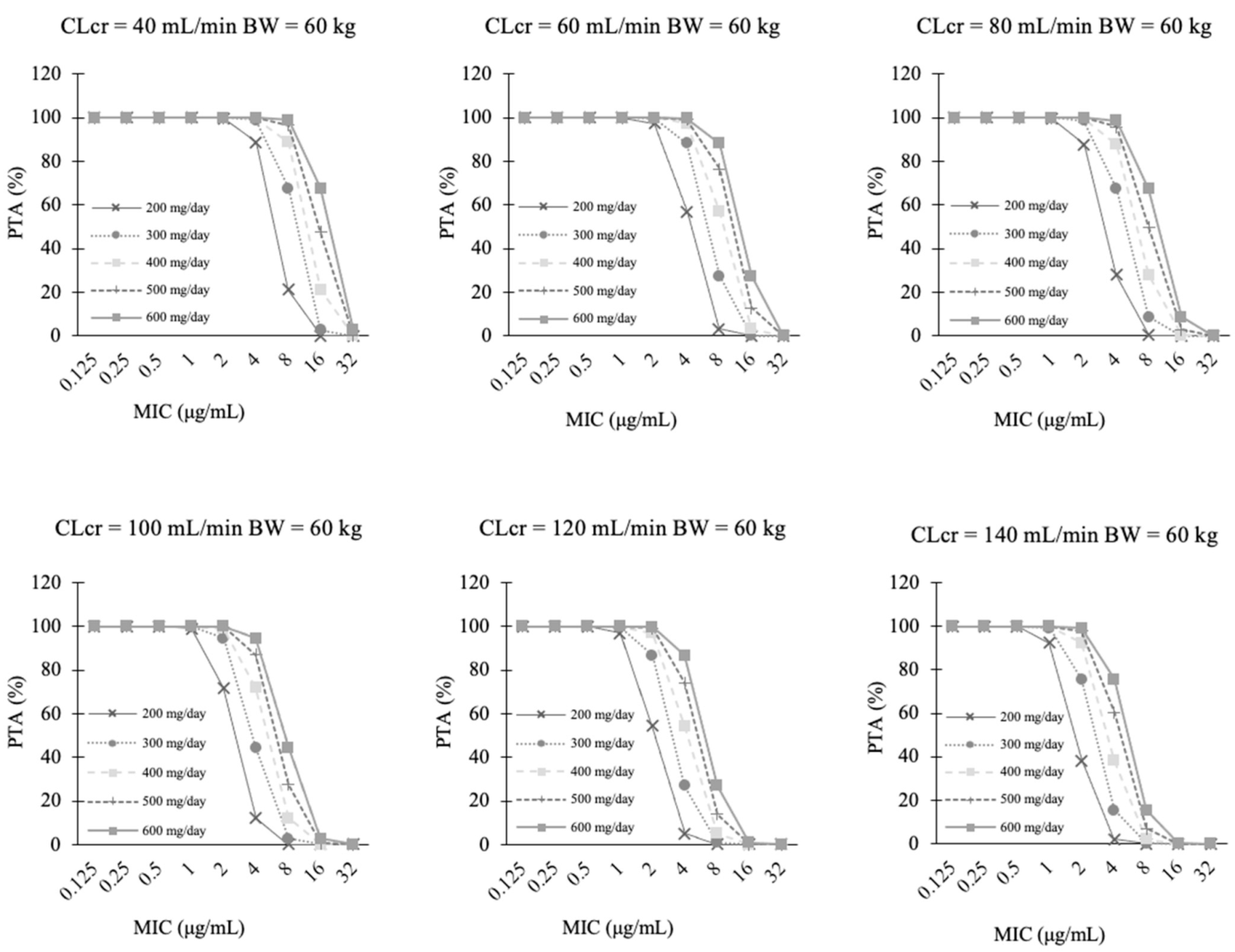
3.4. PTA Analysis
3.5. Verification of the Optimal Dose of Fluconazole
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Fleming, S.; Yannakou, C.K.; Haeusler, G.M.; Clark, J.; Grigg, A.; Heath, C.H.; Bajel, A.; van Hal, S.J.; Chen, S.C.; Milliken, S.T.; et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern. Med. J. 2014, 44, 1283–1297. [Google Scholar] [CrossRef]
- Mellinghoff, S.C.; Panse, J.; Alakel, N.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Kiehl, M.; Koldehoff, M.; Krause, S.W.; et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann. Hematol. 2018, 97, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Teng, J.C.; Slavin, M.A.; Teh, B.W.; Lingaratnam, S.M.; Ananda-Rajah, M.R.; Worth, L.J.; Seymour, J.F.; Thursky, K.A. Epidemiology of invasive fungal disease in lymphoproliferative disorders. Haematologica 2015, 100, e462–e466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togano, T.; Suzuki, Y.; Nakamura, F.; Tse, W.; Kume, H. Epidemiology of visceral mycoses in patients with acute leukemia and myelodysplastic syndrome: Analyzing the national autopsy database in Japan. Med. Mycol. 2021, 59, 50–57. [Google Scholar] [CrossRef]
- Kontoyiennis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the transplant- associated infection surveillance network (TRANSNET) database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Tamura, K.; Niki, Y.; Izumikawa, K.; Oka, S.; Ogawa, K.; Kadota, J.; Kamei, K.; Kanda, Y.; Kiuchi, T.; et al. Executive summary of japanese domestic guidelines for management of deep-seated mycosis 2014. Med. Mycol. J. 2016, 57, 117–163. [Google Scholar] [CrossRef] [PubMed]
- Zonios, D.I.; Bennett, J.E. Update on azole antifungals. Semin. Respir. Crit. Care Med. 2008, 29, 198–210. [Google Scholar] [CrossRef]
- Tashiro, S.; Osa, S.; Igarashi, Y.; Watabe, Y.; Liu, X.; Enoki, Y.; Taguchi, K.; Mayumi, T.; Miyazaki, Y.; Takesue, Y.; et al. Echinocandins versus non-echinocandins for the treatment of invasive candidiasis: A meta-analysis of randomized controlled trials. J. Infect. Chemother. 2020, 26, 1164–1176. [Google Scholar] [CrossRef]
- Rodríguez-Tudela, J.L.; Almirante, B.; Rodríguez-Pardo, D.; Laguna, F.; Donnelly, J.P.; Mouton, J.W.; Pahissa, A.; Cuenca-Estrella, M. Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob. Agents Chemother. 2007, 51, 3599–3604. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Goodman, J.L.; Winston, D.J.; Greenfield, R.A.; Chandrasekar, P.H.; Fox, B.; Kaizer, H.; Shadduck, R.K.; Shea, T.C.; Stiff, P.; Friedman, D.J. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N. Engl. J. Med. 1992, 326, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Japan Society for Hematopoietic Cell Transplantation (JSHCT). Guideline for Prophylaxis and Treatment of Fungal Infection. JSHCT Monograph Vol 46. September 2017. Available online: https://www.jshct.com/uploads/files/guideline/01_04_shinkin.pdf (accessed on 13 September 2021).
- Imataki, O.; Kami, M.; Kim, S.W.; Gotoh, M.; Komaba, S.; Kasai, M.; Hashino, S.; Naito, K.; Masuda, M.; Anan, K.; et al. A nationwide survey of deep fungal infections and fungal prophylaxis after hematopoietic stem cell transplantation in Japan. Bone Marrow Transplant. 2004, 33, 1173–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornely, O.A.; Ullmann, A.J.; Karthaus, M. Evidence-based assessment of primary antifungal prophylaxis in patients with hematologic malignancies. Blood 2003, 101, 3365–3372. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Lugano, M.; Righi, E.; Della Rocca, G.; Bassetti, M.; Hope, W.; Pea, F. Population pharmacokinetics of fluconazole in liver transplantation: Implications for target attainment for infections with Candida albicans and non-albicans spp. Eur. J. Clin. Pharmacol. 2018, 74, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Hirata, K.; Hirata, R.; Yamazaki, H.; Yamamoto, Y.; Hayashi, H.; Matsumoto, Y. Population pharmacokinetics of fluconazole after administration of fosfluconazole and fluconazole in critically ill patients. J. Clin. Pharm. Ther. 2012, 37, 356–363. [Google Scholar] [CrossRef]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.O.; Roberts, M.S.; Sinnollareddy, M.G.; Roger, C.; et al. Effect of obesity on the population pharmacokinetics of fluconazole in critically Ill patients. Antimicrob. Agents Chemother. 2016, 60, 6550–6557. [Google Scholar] [CrossRef] [Green Version]
- Mclachlan, A.J.; Tett, S.E. Pharmacokinetics of fluconazole in people with HIV infection: A population analysis. Br. J. Clin. Pharmacol. 1996, 41, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Kim, J.; Yim, H.; Hur, J.; Song, W.; Lee, J.; Jeon, S.; Hong, T.; Woo, H.; Yim, D.S. Population pharmacokinetic analysis of fluconazole to predict therapeutic outcome in burn patients with candida infection. Antimicrob. Agents Chemother. 2013, 57, 1006–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Joynt, G.M.; Choi, G.Y.S.; Gomersall, C.D.; Lipman, J. How to optimise antimicrobial prescriptions in the Intensive Care Unit: Principles of individualised dosing using pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 2012, 39, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically Ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312. [Google Scholar] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Turpin, R.S.; Garey, K.W. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 2007, 51, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. M60: Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Pfaller, M.A.; Andes, D.; Diekema, D.J.; Espinel-Ingroff, A.; Sheehan, D. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: Time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 2010, 13, 180–195. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kawabe, K.; Suzuki, T.; Sano, K.; Ide, K.; Nishigaki, T.; Enoki, Y.; Taguchi, K.; Koike, H.; Kato, H.; et al. Species distribution of candidemia and their susceptibility in a single japanese university hospital: Prior micafungin use affects the appearance of candida parapsilosis and elevation of micafungin mics in non-parapsilosis candida species. J. Fungi 2021, 7, 596. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.W.; Farrow, P.R.; Faulkner, J.K. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev. Infect. Dis. 1990, 12 (Suppl. S3), S318–S326. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, M.J.; Jevons, S.; Tarbit, M.H. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob. Agents Chemother. 1985, 28, 648–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiba, K.; Saito, A.; Miyahara, T. Pharmacokinetic evaluation of fluconazole in healthy volunteers. Jpn. J. Antibiot. 1989, 42, 17–30. [Google Scholar]
- Izumisawa, T.; Kaneko, T.; Soma, M.; Imai, M.; Wakui, N.; Hasegawa, H.; Horino, T.; Takahashi, N. Augmented renal clearance of vancomycin in hematologic malignancy patients. Biol. Pharm. Bull. 2019, 42, 2089–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, S.; Fdez De Gatta, M.; Calvo, M.V.; Caballero, D.; Dominguez-Gil, A.; Lanao, J.M. Population pharmacokinetics of amikacin in patients with haematological malignancies. J. Antimicrob. Chemother. 1999, 44, 235–242. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatr. Am. J. Health Pharm. 2020, 77, 835–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; Macdougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the british society for medical mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louie, A.; Drusano, G.L.; Banerjee, P.; Liu, Q.F.; Liu, W.; Kaw, P.; Shayegani, M.; Taber, H.; Miller, M.H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 1998, 42, 1105–1109. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 2011, 86, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ueno, K.; Yoshimura, H.; Morii, M.; Takada, M.; Sawai, T.; Mitsutake, K.; Shibakawa, M. Fluconazole-induced convulsions at serum trough concentrations of approximately 80 mg/mL. Ther. Drug Monit. 2000, 22, 635–636. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Median or Number | Range |
|---|---|---|
| Number of patients (male/female) | 31/23 | – |
| Body weight (kg) | 57.6 | 39.8–99.1 |
| Height (cm) | 162.6 | 143.6–187.7 |
| Age (years) | 53 | 20–77 |
| BMI (kg/m2) | 21.7 | 15.8–29.1 |
| BSA (m2) | 1.62 | 1.31–2.23 |
| CG-CLcr (mL/min) | 87.1 | 31.1–193.6 |
| eGFRcre (mL/min) | 71.2 | 25.9–148.5 |
| eGFRcre (mL/min/1.73 m2) | 69.2 | 31.6–157.5 |
| Disease | ||
| Acute myeloid leukemia (AML)/Myelodysplastic syndromes (MDS) | 10 | – |
| Acute lymphoid leukemia (ALL) | 6 | – |
| non-Hodgkin lymphoma (NHL) | 28 | – |
| Hodgkin lymphoma (HL) | 2 | – |
| Multiple myeloma (MM) | 3 | – |
| Others | 5 | – |
| Treatment | ||
| Chemotherapy | 38 | – |
| Autologous peripheral blood stem cell transplantation | 9 | – |
| Bone marrow transplantation | 2 | – |
| Cord blood transplantation | 2 | – |
| Allogeneic peripheral blood stem cell transplantation | 1 | – |
| Others | 2 | – |
| Parameter | Final Model | Bootstrap Method n = 1000 | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | CV (%) | 95% CI | Median | 95% CI | |
| CL/F (L/h) = θ₁ × (CLcr/5.2)θ₂ × e0.16 | ||||||
| θ₁ (L/h) | 1.03 | 0.08 | 7.38 | 0.88–1.18 | 1.03 | 0.86–1.19 |
| θ₂ (L/h) | 1.05 | 0.14 | 13.7 | 0.77–1.33 | 1.07 | 0.81–1.37 |
| Vd/F (L) = θ₃ × (BW/57.6)θ₄ | ||||||
| θ₃ (L) | 62.3 | 8.87 | 14.2 | 44.7–79.9 | 64.3 | 47.5–87.9 |
| θ₄ (L) | 1.06 | 0.36 | 34.4 | 0.34–1.78 | 1.08 | 0.08–2.24 |
| ka (/h) | 0.34 | 0.12 | 35.1 | 0.10–0.58 | 0.41 | 0.15–0.77 |
| PTA by MIC (μg/mL) and f AUC/MIC of 50 | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 (0–24 h) | Day 8 (168–192 h) | Day 15 (336–360 h) | ||||||||||||||||||||||||
| Dose (mg/day) | BW (kg) | CLcr (mL/min) | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 |
| 200 | 40 | |||||||||||||||||||||||||
| 40 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 60 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 80 | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 100 | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 120 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 140 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 50 | ||||||||||||||||||||||||||
| 40 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 60 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 80 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 100 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 120 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 140 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 60 | ||||||||||||||||||||||||||
| 40 | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 60 | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 80 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 100 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 120 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 140 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 70 | ||||||||||||||||||||||||||
| 40 | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 60 | + | + | + | - | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 80 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 100 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 120 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 140 | + | + | + | - | - | - | - | - | + | + | + | + | - | - | - | - | + | + | + | + | - | - | - | - | ||
| 400 | 40 | |||||||||||||||||||||||||
| 40 | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 60 | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 80 | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 100 | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 120 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 140 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 50 | ||||||||||||||||||||||||||
| 40 | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 60 | + | + | + | + | + | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 80 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 100 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 120 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 140 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 60 | ||||||||||||||||||||||||||
| 40 | + | + | + | + | - | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 60 | + | + | + | + | - | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 80 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 100 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 120 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 140 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 70 | ||||||||||||||||||||||||||
| 40 | + | + | + | + | - | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 60 | + | + | + | + | - | - | - | - | + | + | + | + | + | + | - | - | + | + | + | + | + | + | - | - | ||
| 80 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 100 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 120 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| 140 | + | + | + | + | - | - | - | - | + | + | + | + | + | - | - | - | + | + | + | + | + | - | - | - | ||
| CLcr (Cockcroft-Gault Equation) | ||||||
|---|---|---|---|---|---|---|
| 40–60 mL/min | 60–80 mL/min | 80–100 mL/min | 100–120 mL/min | 120–140 mL/min | ||
| Loading dose (Required in the situation neutropenia is predicted within a week) | ||||||
| Body | 40–50 kg | 400 mg/day | 500 mg/day | 500 mg/day | 500 mg/day | 600 mg/day |
| Weight | 50–60 kg | 500 mg/day | 500 mg/day | 500 mg/day | 600 mg/day | 600 mg/day |
| 60–70 kg | 500 mg/day | 600 mg/day | 600 mg/day | 600 mg/day | 700 mg/day | |
| Maintenance dose | ||||||
| Body | 40–50 kg | 200 mg/day | 300 mg/day | 300 mg/day | 400 mg/day | 400 mg/day |
| Weight | 50–60 kg | 200 mg/day | 300 mg/day | 300 mg/day | 400 mg/day | 400 mg/day |
| 60–70 kg | 200 mg/day | 300 mg/day | 300 mg/day | 400 mg/day | 400 mg/day | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, Y.; Isono, H.; Enoki, Y.; Taguchi, K.; Miyazaki, T.; Kunimoto, H.; Koike, H.; Hagihara, M.; Matsumoto, K.; Nakajima, H.; et al. Population Pharmacokinetic Analysis and Dosing Optimization of Prophylactic Fluconazole in Japanese Patients with Hematological Malignancy. J. Fungi 2021, 7, 975. https://doi.org/10.3390/jof7110975
Sakamoto Y, Isono H, Enoki Y, Taguchi K, Miyazaki T, Kunimoto H, Koike H, Hagihara M, Matsumoto K, Nakajima H, et al. Population Pharmacokinetic Analysis and Dosing Optimization of Prophylactic Fluconazole in Japanese Patients with Hematological Malignancy. Journal of Fungi. 2021; 7(11):975. https://doi.org/10.3390/jof7110975
Chicago/Turabian StyleSakamoto, Yasutaka, Hikaru Isono, Yuki Enoki, Kazuaki Taguchi, Takuya Miyazaki, Hiroyoshi Kunimoto, Hirofumi Koike, Maki Hagihara, Kenji Matsumoto, Hideaki Nakajima, and et al. 2021. "Population Pharmacokinetic Analysis and Dosing Optimization of Prophylactic Fluconazole in Japanese Patients with Hematological Malignancy" Journal of Fungi 7, no. 11: 975. https://doi.org/10.3390/jof7110975
APA StyleSakamoto, Y., Isono, H., Enoki, Y., Taguchi, K., Miyazaki, T., Kunimoto, H., Koike, H., Hagihara, M., Matsumoto, K., Nakajima, H., Sahashi, Y., & Matsumoto, K. (2021). Population Pharmacokinetic Analysis and Dosing Optimization of Prophylactic Fluconazole in Japanese Patients with Hematological Malignancy. Journal of Fungi, 7(11), 975. https://doi.org/10.3390/jof7110975






