Spent Pleurotus ostreatus Substrate Has Potential for Managing Fusarium Wilt of Banana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Culture Conditions, and Plant Material
2.2. In Vitro Interactions of Pleurotus ostreatus and Fusarium oxysporum f. sp. cubense
2.3. Effect of Extracts from Spent P. ostreatus Substrate (SpoS) on F. oxysporum f. sp. cubense (Foc) Mycelia Growth
2.4. Greenhouse Evaluation of Spent P. ostreatus Substrate (SpoS) against Fusarium oxysporum f. sp. cubense
2.5. Data Analysis
3. Results
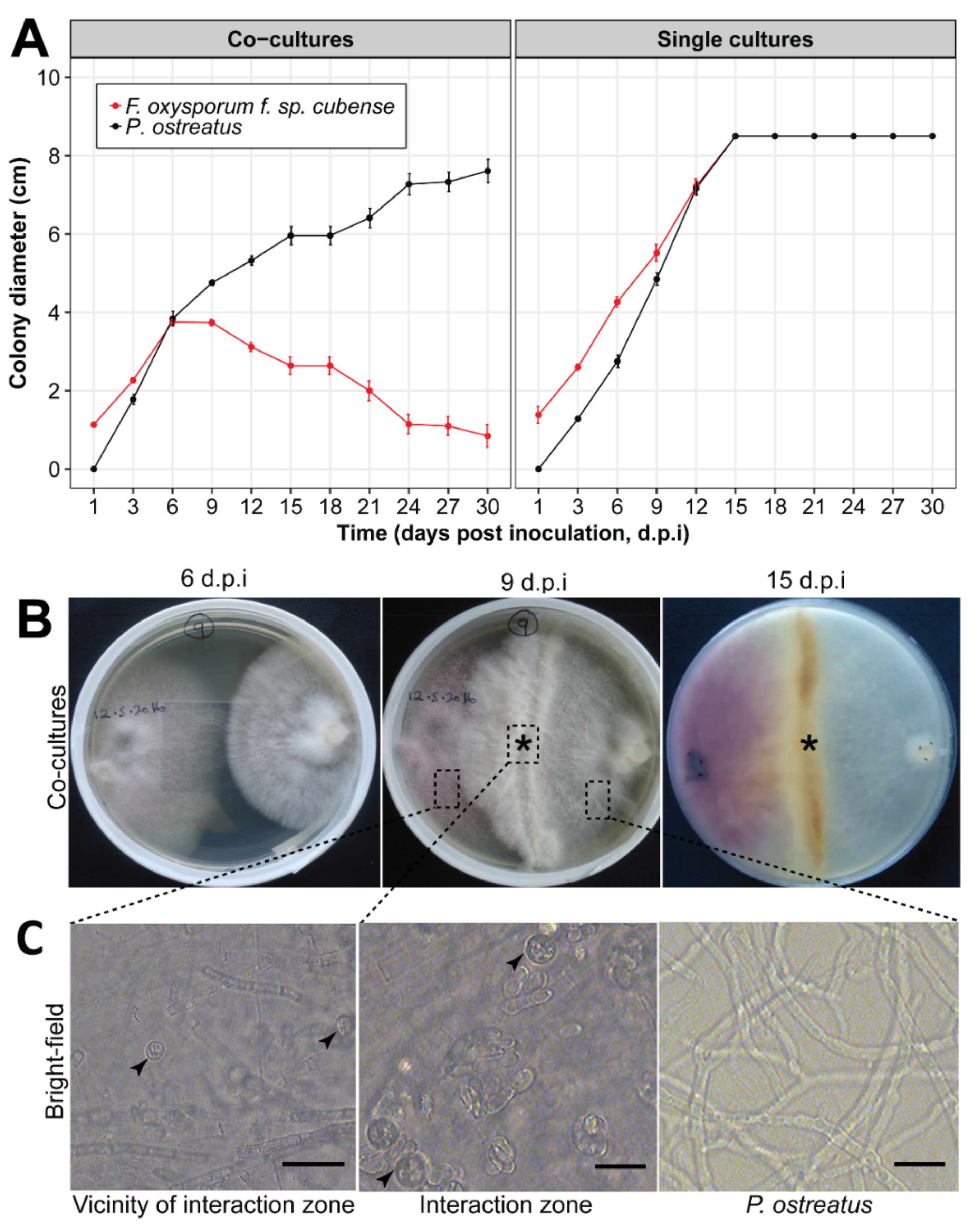
3.1. In Vitro Interaction between Pleurotus ostreatus and Fusarium oxysporum f. sp. cubense
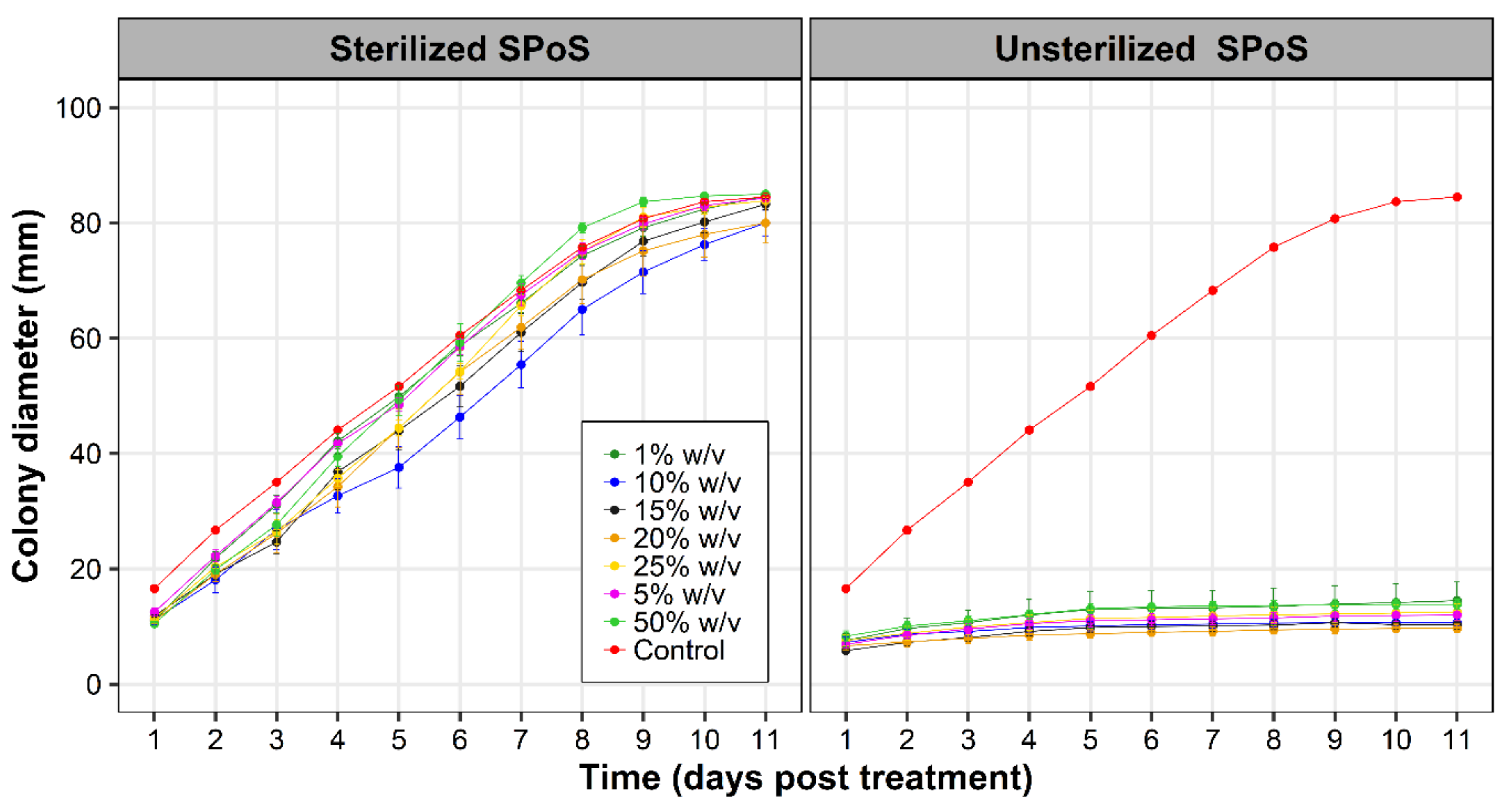
3.2. Effect of SPoS Extracts on Fusarium oxysporum f. sp. cubense Growth
3.3. Greenhouse Evaluation of Spent P. ostreatus Substrate (SpoS) against Fusarium oxysporum f. sp. cubense
3.4. Suppression of Foc in ‘Ndizi’ Plants by SPoS in Naturally Foc-Infested Soils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010, 85, 1321–1337. [Google Scholar] [CrossRef]
- de Carvalho, C.S.M.; Sales-Campos, C.; de Andrade, M.C.N. Mushrooms of the Pleurotus genus: A review of cultivation techniques. Interciencia 2010, 35, 177–182. [Google Scholar]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; López-Lucendo, M.F.; Pérez-Boada, M.; Rencoret, J.; Gutiérrez, A.; Martínez, A.T. A secretomic view of woody and nonwoody lignocellulose degradation by Pleurotus ostreatus. Biotechnol. Biofuels 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.C.; McMullan, J.T.; McCahey, S. An initial assessment of spent mushroom compost as a potential energy feedstock. Bioresour. Techhnol. 2001, 79, 227–230. [Google Scholar] [CrossRef]
- Prabu, M.; Jeyanthi, C.; Kumuthakalavalli, R. Spent mushroom substrate: An enriched organic manure for improving the yield of Vigna unguiculata (L.) Walp (Cowpea) leguminous crop. Scrut. Int. Res. J. Agric. Plant Biotechnol. Bioprod. 2014, 1, 7–14. [Google Scholar]
- Jasińska, A. Spent mushroom compost (SMC)–retrieved added value product closing loop in agricultural production. Acta Agrar. Debr. 2018, 150, 185–202. [Google Scholar] [CrossRef]
- Paszczynski, A.; Crawford, R. Recent advances in the use of fungi in environmental remediation and biotechnology. Soil. Biochem. 2000, 10, 379–422. [Google Scholar]
- Pointing, S. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar]
- Velázquez-Cedeño, M.; Farnet, A.M.; Billette, C.; Mata, G.; Savoie, J.M. Interspecifc interactions with Trichoderma longibrachiatum induce Pleurotus ostreatus defence reactions based on the production of laccase isozymes. Biotechnol. Lett. 2007, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Increase of laccase activity during interspecific interactions of white-rot fungi. FEMS Microbiol. Ecol. 2004, 50, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Ferreira, I.C.; Dias, J.F.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, A.D.M.; Griffith, G.S.; Wildman, H.G. Induction of metabolic and morphogenetic changes during mycelia interactions among species of higher fungi. Biochem. Soc. Trans. 1994, 22, 389–394. [Google Scholar] [CrossRef]
- Phan, C.W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Dianez, F.; Santos, M.; Carretero, F.; Gea, F.J.; Castaneda, C.; Navarro, M.J.; Yau, J.A. Control of Phytophthora capsici and Phytophthora parasitica on pepper (Capsicum annuum L.) with compost teas from different sources, and their effects on plant growth promotion. Phytopathol. Mediterr. 2014, 53, 216–228. [Google Scholar]
- Adedeji, K.O.; Aduramigba, M.A.O. In vitro evaluation of spent mushroom compost on growth of Fusarium oxysporium f. sp. lycopersici. Adv. Plants Agric. Res. 2016, 4, 332–339. [Google Scholar]
- Viljoen, A. The status of Fusarium wilt (Panama disease) of banana in South Africa. S. Afr. J. Sci. 2002, 98, 341–344. [Google Scholar]
- Ploetz, R.C. Fusarum wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Dita, M.; Teixeira, L.A.J.; O’Neill, W.; Pattison, A.B.; Weinert, M.P.; Li, C.Y.; Zheng, S.J.; Staver, C.; Thangavelu, R.; Viljoen, A. Current state of Fusarium wilt of banana in the subtropics. Acta Hort. 1272, 2020, 45–56. [Google Scholar] [CrossRef]
- State of Queensland. Guide to Alternative Hosts of Panama Disease in Australia; Hort Innovation Queensland: Queensland, Australia, 2020; p. 47. [Google Scholar]
- Zhang, N.; Xin, H.E.; Zhang, J.; Raza, W.; Yang, X.-M.; Ruan, Y.-Z.; Shen, Q.-R.; Huang, Q.-W. Suppression of Fusarium wilt of banana with application of bio-organic fertilizers. Pedosphere 2014, 24, 613–624. [Google Scholar] [CrossRef]
- Akyuz, M.; Kirbag, S. Antimicrobial activity of Pleurotus eryngii var. ferulae grown on various agro-wastes. Eur. Asian, J. BioSci. 2009, 3, 58–63. [Google Scholar] [CrossRef]
- Mishra, P.K.; Fox, R.T.; Culham, A. Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusaria. FEMS Microbiol. Lett. 2003, 218, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Pegg, K.G.; Moore, N.Y.; Sorensen, S. Fusarium wilt in the Asian Pacific region. In Proceedings of the International Symposium on Recent Development in Banana Cultivation Technology, Pingtung, Taiwan, 14–18 December 1992; Valmayor, R.V., Hwang, S.C., Ploetz, R.C., Lee, S.W., Roa, V.N., Eds.; Taiwan Banana Research Institute: Pingtung, Taiwan, 1993; pp. 255–314. [Google Scholar]
- Molina, A.B.; Sinohin, V.O.; Fabregar, E.G.; Ramillete, E.B.; Yi, G.; Sheng, O.; Viljoen, A. Resistance to Fusarium oxysporum f. sp. cubense tropical race 4 in African bananas. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): IX 1114, Brisbane, Australia, 17–22 August 2014; pp. 107–110. [Google Scholar]
- Szczech, M.M. Suppressiveness of vermicompost against Fusarium wilt of tomato. J. Phytopathol. 1999, 147, 155–161. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Intrigliolo, F.; Pascual, J.A. Citrus compost and its water extract for cultivation of melon plants in greenhouse nurseries. Evaluation of nutri-active and biocontrol effects. Bioresour. Technol. 2008, 99, 8722–8728. [Google Scholar] [CrossRef]
- Viljoen, A.; Mahuku, G.; Massawe, C.; Ssali, R.T.; Kimunye, J.; Mostert, G.; Ndayihanzamaso, P.; Coyne, D.L. Banana Pests and Diseases: Field Guide for Disease Diagnostics and Data Collection; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 19 July 2021).
- Wickham, H. Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009; p. 35. [Google Scholar]
- Spraker, J.E.; Sanchez, L.M.; Lowe, T.M.; Dorrestein, P.C.; Keller, N.P. Ralstonia solanacearum lipopeptide induces chlamydospore development in fungi and facilitates bacterial entry into fungal tissues. Int. Soc. Microbial. Ecol. J. 2016, 10, 2317–2330. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Estrella, F.; Bustamante, M.A.; Moral, R.; Vargas-García, M.C.; López, M.J.; Moreno, J. In vitro control of Fusarium wilt using Agro-industrial sub-product-based composts. J. Plant Pathol. 2012, 94, 59–70. [Google Scholar]
- Yusidah, I.; Istifadah, N. The abilities of spent mushroom substrate to suppress basal rot disease (Fusarium oxysporum f. sp. cepae) in shallot. Int. J. Biosci. 2018, 13, 440–448. [Google Scholar]
- Owaid, M.N.; AL Saeedi, S.S.S.; Abed, I.A.; Shahbazi, P.; Sabaratnam, V. Antifungal activities of some Pleurotus species (Higher Basidiomycetes). Walailak J. Sci. Technol. 2017, 14, 215–224. [Google Scholar]
- Chu, K.T.; Xia, L.; Ng, T.B. Pleurostrin, an antifungal peptide from the oyster mushroom. Peptides 2005, 26, 2098–2103. [Google Scholar] [CrossRef]
- Rosa, L.H.; Machado, K.M.G.; Jacob, C.C.; Capelari, M.; Rosa, C.A.; Zani, C.L. Screening of Brazilian Basidiomycetes for antimicrobial activity. Mem. Inst. Oswaldo Cruz, Rio de Janeiro 2003, 98, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Gold, C.S.; Kiggundu, A.; Abera, R.; Ssendege, A.M.K.; Karamura, E.B.; Bagamba, F.; Wejuli, M.; Karamura, D.; Kalyebara, R. Geographic shifts in highland banana production in Uganda. Acta Hort. 2000, 540, 55–62. [Google Scholar] [CrossRef]
- Schwartz, H.F.; Root Rots of Dry Beans. Fact Sheet No. 2.938. Crop Series: Diseases. Colorado State University Cooperative Extension Service. 2021. Available online: https://extension.colostate.edu/docs/pubs/crops/02938.pdf (accessed on 8 November 2021).

| Treatments | Corm Damage Score (±sd) | Plant Girth (cm ± sd) | Plant Height (cm ± sd) | Dry Root Weight (g ± sd) |
|---|---|---|---|---|
| Foc-infested soil | 2.75 ± 0.50 | -0.40 ± 0.34 | −0.90 ± 0.52 | 5.20 ± 1.41 |
| Foc-infested soil + SPoS | 0.88 ± 0.83 | 0.43 ± 0.69 | 0.74 ± 0.48 | 3.79 ± 1.30 |
| Sterile soil + SPoS | 0.00 ± 0.00 | 1.08 ± 1.42 | 2.38 ± 1.55 | 3.02 ± 1.81 |
| Control (sterile soil) | 0.00 ± 0.00 | 1.60 ± 1.80 | 2.28 ± 2.14 | 4.53 ± 1.55 |
| p value | 1.53e-05 | 0.1718 | 0.0040 | 0.2714 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocimati, W.; Were, E.; Tazuba, A.F.; Dita, M.; Zheng, S.-J.; Blomme, G. Spent Pleurotus ostreatus Substrate Has Potential for Managing Fusarium Wilt of Banana. J. Fungi 2021, 7, 946. https://doi.org/10.3390/jof7110946
Ocimati W, Were E, Tazuba AF, Dita M, Zheng S-J, Blomme G. Spent Pleurotus ostreatus Substrate Has Potential for Managing Fusarium Wilt of Banana. Journal of Fungi. 2021; 7(11):946. https://doi.org/10.3390/jof7110946
Chicago/Turabian StyleOcimati, Walter, Evans Were, Anthony Fredrick Tazuba, Miguel Dita, Si-Jun Zheng, and Guy Blomme. 2021. "Spent Pleurotus ostreatus Substrate Has Potential for Managing Fusarium Wilt of Banana" Journal of Fungi 7, no. 11: 946. https://doi.org/10.3390/jof7110946
APA StyleOcimati, W., Were, E., Tazuba, A. F., Dita, M., Zheng, S.-J., & Blomme, G. (2021). Spent Pleurotus ostreatus Substrate Has Potential for Managing Fusarium Wilt of Banana. Journal of Fungi, 7(11), 946. https://doi.org/10.3390/jof7110946







