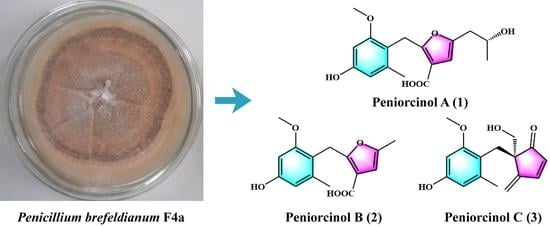
Novel Antioxidants and α-Glycosidase and Protein Tyrosine Phosphatase 1B Inhibitors from an Endophytic Fungus Penicillium brefeldianum F4a
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Material
2.3. Fermentation and Extraction
2.4. Bioactivity-Guided Isolation
2.5. Spectral Data
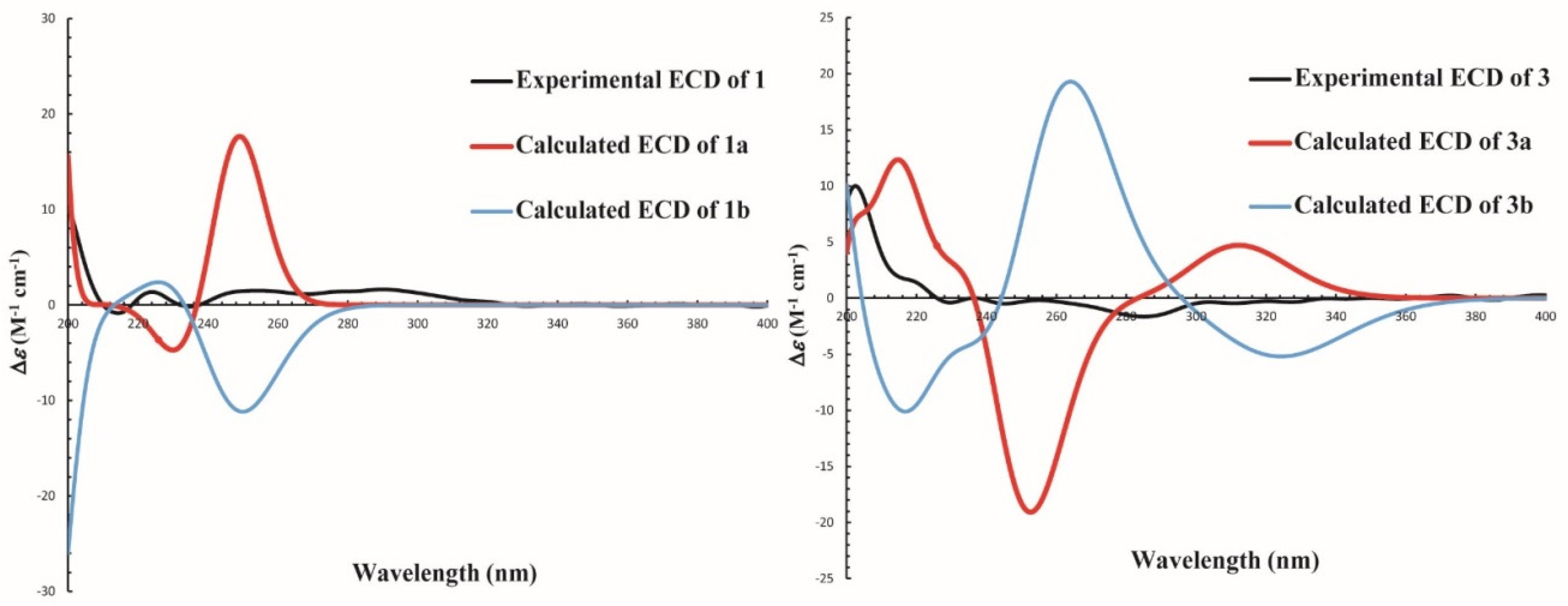
2.6. ECD Calculations
2.7. Antioxidant Assay
2.7.1. DPPH Radical Scavenging Assay
2.7.2. ABTS Radical Scavenging Assay
2.8. Antidiabetic Assay In Vitro
2.8.1. α-Glycosidase Inhibitory Assay
2.8.2. PTP1B Inhibitory Assay
3. Results and Discussion
3.1. Bioactivity-Guided Compound Isolation
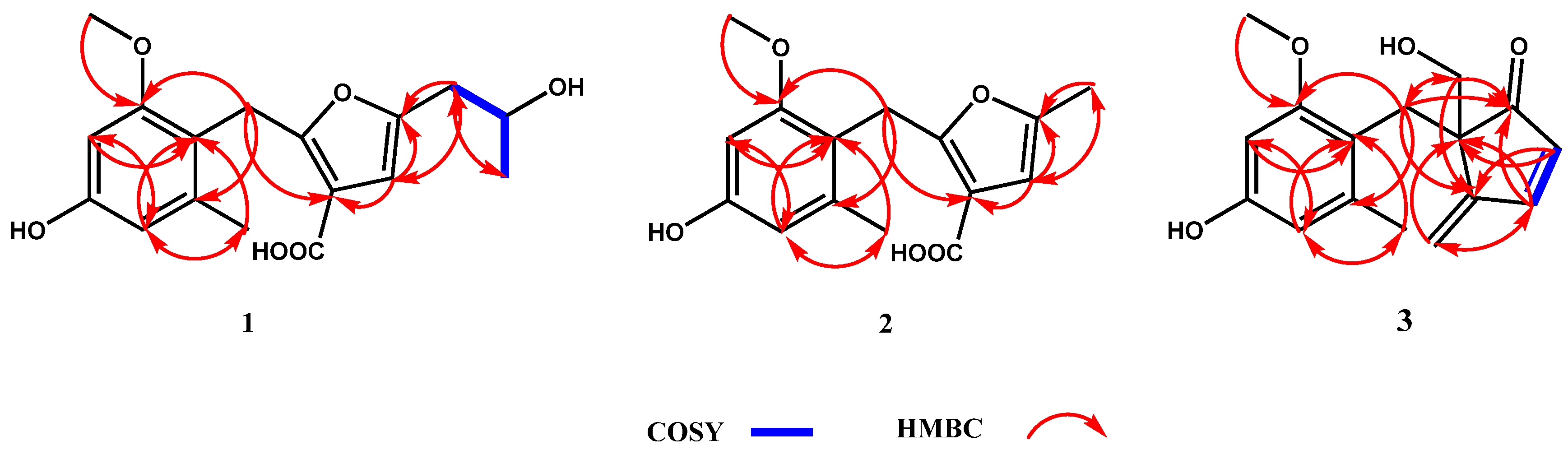
3.2. Structure Elucidation
3.3. Antioxidant Activities
3.4. α-Glycosidase and PTP1B Inhibition Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stadtman, E.R. Role of Oxidant Species in Aging. Curr. Med. Chem. 2004, 11, 1105–1112. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Erbağcı, M.O.; Tuna, G.; Köse, S.; Dal-Bekar, N.E.; Akış, M.; Kant, M.; Altunyurt, S.; Işlekel, G.H. Association between early oxidative DNA damage and iron status in women with gestational diabetes mellitus. Reprod. Toxicol. 2021, 103, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, S.H.; Pouramir, M.; Zabihi, E.; Golpour, M.; Aghajanpour-Mir, M. The Effect of Arbutin on The Expression of Tumor Suppressor P53, BAX/BCL-2 Ratio and Oxidative Stress Induced by Tert-Butyl Hydroperoxide in Fibroblast and LNcap Cell Lines. Cell J. 2020, 22, 532–541. [Google Scholar]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-C.; Shen, X.-F.; Meng, X.-L. A traditional Chinese medicine JiuHuangLian (Rhizoma coptidis steamed with rice wine) reduces oxidative stress injury in type 2 diabetic rats. Food Chem. Toxicol. 2013, 59, 222–229. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Faghihi, N.; Mohammadi, M.T.; Sahebkar, A. Effects of atorvastatin on myocardial oxidative and nitrosative stress in diabetic rats. Comp. Haematol. Int. 2018, 27, 691–697. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Mohammadi, M.T.; Sahebkar, A. Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed. Pharmacother. 2018, 98, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 1–30. [Google Scholar] [CrossRef]
- Lin, X.-P.; Ai, W.; Li, M.; Zhou, X.-F.; Liao, S.-R.; Wang, J.-F.; Liu, J.; Yang, B.; Liu, Y.-H. Collacyclumines A–D from the endophytic fungus Colletotrichum salsolae SCSIO 41021 isolated from the mangrove Kandelia candel. Phytochemistry 2019, 171, 112237. [Google Scholar] [CrossRef]
- Torres-Mendoza, D.; Ortega, H.E.; Cubilla-Rios, L. Patents on endophytic fungi related to secondary metabolites and biotrans-formation applications. J. Fungi 2020, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.E.; Torres-Mendoza, D.; Caballero E., Z.; Cubilla-Rios, L. Structurally Uncommon Secondary Metabolites Derived from Endophytic Fungi. J. Fungi 2021, 7, 570. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Moawad, A.S.; Bahr, H.S.; Abdelmohsen, U.R.; Mohammed, R. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Adv. 2020, 10, 22058–22079. [Google Scholar] [CrossRef]
- Koul, M.; Singh, S. Penicillium spp.: Prolific producer for harnessing cytotoxic secondary metabolites. Anticancer. Drugs 2017, 28, 11–30. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.C.; Ogawa, C.Y.; Rodrigues, L.D.C.; de Medeiros, L.S.; Veiga, T.A.M. Penicillium genus as a source for anti-leukemia compounds: An overview from 1984 to 2020. Leuk. Lymphoma 2021, 62, 2079–2093. [Google Scholar] [CrossRef]
- Mei, R.-Q.; Wang, B.; Zeng, W.-N.; Huang, G.-L.; Chen, G.-Y.; Zheng, C.-J. Bioactive isocoumarins isolated from a mangrove-derived fungus Penicillium sp. MGP11. Nat. Prod. Res. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Malika, A.; Ardalania, H.; Anama, S.; McNaira, L.M.; Kromphardtd, K.J.K.; Frandsend, R.J.N.; Franzyka, H.; Staerka, D.; Kongstad, K.T. Antidiabetic xanthones with α-glucosidase inhibitory activities from an endophytic Penicillium canescens. Fitoterapia 2020, 142, 104522. [Google Scholar] [CrossRef]
- Pan, H.Q.; Hu, J.C.; Wang, S.J. A Plant Endophytic Fungus Eupenicillium brefeldianum F4a and Its Application. China Patent No. ZL201410072690.1, 19 December 2017. [Google Scholar]
- Autschbach, J. Time-Dependent Density Functional Theory for Calculating Origin-Independent Optical Rotation and Rotatory Strength Tensors. ChemPhysChem 2011, 12, 3224–3235. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1, Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Bai, Y.; Chang, J.-W.; Xu, Y.; Cheng, D.; Liu, H.-X.; Zhao, Y.-L.; Yu, Z.-G. Antioxidant and Myocardial Preservation Activities of Natural Phytochemicals from Mung Bean (Vigna radiata L.) Seeds. J. Agric. Food Chem. 2016, 64, 4648–4655. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Chang, J.-W.; Wang, X.-X.; Zhao, Y.-L.; Yu, Z.-G. Bioactives from stems and leaves of mung beans (Vigna radiata L.). J. Funct. Foods 2016, 25, 314–322. [Google Scholar] [CrossRef]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glycosidase and α-amylase inhibition. J. Food Sci. 2010, 75, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-H.; Niu, X.-L.; Xiao, T.; Lu, J.-J.; Li, W.; Zhao, Y.-Q. Chemical profile and inhibition of α-glycosidase and protein tyrosine phosphatase 1B (PTP1B) activities by flavonoids from licorice (Glycyrrhiza uralensis Fisch). J. Funct. Foods 2015, 14, 324–336. [Google Scholar] [CrossRef]
- Jouda, J.B.; Kusari, S.; Lamshöft, M.; Talontsi, F.M.; Meli, C.D.; Wandji, J.; Spiteller, M. Penialidins A-C with strong antibacterial activities from Penicillium sp., an endophytic fungus harboring leaves of Garcinia nobilis. Fitoterapia 2014, 98, 209–214. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Yu, L.-Y.; Wang, Q.-Q.; Ding, W.-J.; Chen, Z.; Ma, Z.-J. New brefeldins and penialidins from marine fungus Penicillium janthinellum DT-F29. Nat. Prod. Res. 2018, 32, 282–286. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, H.Y.; Wu, C.S.; Jiao, Y.; Li, M.; Wang, Y.Y.; Wang, S.Q.; Zhao, Z.T.; Lou, H.X. Austdiol, fulvic acid and citromy-cetin derivatives from an endolichenic fungus, Myxotrichum sp. Phytochem. Lett. 2013, 6, 662–666. [Google Scholar] [CrossRef]
- Liu, K.-W.; Li, Z.-L.; Pu, S.-B.; Xu, D.-R.; Zhou, H.-H.; Shen, W.-B. Chemical Constituents of the Rhizome of Typhonium giganteum. Chem. Nat. Compd. 2014, 50, 155. [Google Scholar] [CrossRef]
- Elsayed, Y.; Refaat, J.; Abdelmohsen, U.R.; Ahmed, S.; Fouad, M.A. Rhodozepinone, a new antitrypanosomal azepino-diindole alkaloid from the marine sponge-derived bacterium Rhodococcus sp. UA13. Med. Chem. Res. 2017, 26, 2751–2760. [Google Scholar] [CrossRef]
- Tokes, A.L.; Bognar, R. Synthesis of spiro chroman-4-ones. Flavonoids Bioflavonoids. Proc. Hung. Bioflavonoid Symp. 1977, 5, 151–158. [Google Scholar]
- Fu, J.; Hu, L.W.; Shi, Z.; Sun, W.; Yue, D.; Wang, Y.; Ma, X.P.; Ren, Z.H.; Zuo, Z.C.; Peng, G.N.; et al. Two metabolites isolated from endophytic fungus Coniochaeta sp. F-8 in Ageratina adenophora exhibit antioxidative activity and cytotoxicity. Nat. Prod. Res. 2019, 35, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Heacock, P.M.; Hertzler, S.R.; Williams, J.A.; Wolf, W.B. Effects of a medical food containing an herbal α-glycosidase inhibitor on postprandial glycemia and insulinemia in healthy adults. J. Amer. Dietetic Assoc. 2005, 105, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.; Nesi, I.; Caselli, A.; Paoli, P. Natural α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors: A Source of Scaffold Molecules for Synthesis of New Multitarget Antidiabetic Drugs. Molecules 2021, 26, 4818. [Google Scholar] [CrossRef] [PubMed]

| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 209.0, C | |||||
| 2 | 164.6, C | 164.7, C | 6.17, d, (5.6) | 132.5, CH | ||
| 3 | 100.9, C | 100.5, C | 7.76, d, (5.6) | 158.6, CH | ||
| 4 | 5.92, s | 100.8, CH | 5.89, s | 100.0, CH | 150.8, C | |
| 5 | 160.4, C | 159.3, C | 55.7, C | |||
| 1′ | 116.9, C | 116.9, C | 114.6, C | |||
| 2′ | 158.6, C | 158.6, C | 158.8, C | |||
| 3′ | 6.14, d, (2.1) | 96.8, CH | 6.14, d, (1.8) | 96.7, CH | 6.09, d, (2.2) | 96.3, CH |
| 4′ | 155.7, C | 155.7, C | 156.2, C | |||
| 5′ | 6.09, d, (2.1) | 108.9, CH | 6.09, d, (1.8) | 108.9, CH | 6.08, d, (2.2) | 108.9, CH |
| 6′ | 138.1, C | 138.1, C | 138.4, C | |||
| 7′ | 3.45, s | 19.6, CH2 | 3.45, s | 19.5, CH2 | 2.65, d, (14.0) 2.55, d, (14.0) | 28.8, CH2 |
| 8′ | 2.16, s | 19.9, CH3 | 2.16, s | 19.9, CH3 | 2.01, s | 20.4, CH3 |
| 9′ | 3.60, s | 55.3, OCH3 | 3.60, s | 55.3, OCH3 | 3.52, s | 54.4, OCH3 |
| 10′ | 10.76, s | 164.3, C | 10.74, s | 164.2, C | 5.23, s 4.96, s | 111.9, CH2 |
| 11′ | 2.39, m | 42.7, CH2 | 2.09, s | 19.2, CH3 | 3.63, d, (10.3) 3.55, d, (10.3) | 64.7, CH2 |
| 12′ | 3.86, m | 64.0, CH | ||||
| 13′ | 1.06, d, (6.2) | 23.4, CH3 | ||||
| Compd. | α-Glycosidase Inhibitory Assay | PTP1B Inhibitory Assay | DPPH• Assay | ABTS•+ Assay |
|---|---|---|---|---|
| EC50 (μM) a | EC50 (μM) a | EC50 (μM) a | EC50 (μM) a | |
| 1 | >50 | >50 | >100 | 21.93 ± 1.06 |
| 2 | >50 | >50 | >100 | 28.20 ± 2.87 |
| 3 | 38.93 ± 4.90 | >50 | >100 | 32.67 ± 3.86 |
| 4 | >50 | >50 | >100 | 14.54 ± 0.46 |
| 5 | >50 | >50 | 28.42 ± 3.16 | 7.61 ± 0.46 |
| 6 | >50 | >50 | 30.07 ± 2.83 | 14.96 ± 2.57 |
| 7 | >50 | 8.87 ± 0.91 | >100 | >100 |
| 8 | >50 | 11.68 ± 1.03 | >100 | 21.48 ± 0.88 |
| 9 | >50 | >50 | >100 | 30.02 ± 4.17 |
| Acarbose | 2.62 ± 0.06 | ND | ND | ND |
| Na3VO4 | ND | 2.38 ± 0.07 | ND | ND |
| L-Ascorbic acid | ND | ND | 6.12 ± 0.17 | 18.81 ± 3.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Yi, P.; Zhang, S.; Hu, J.; Pan, H. Novel Antioxidants and α-Glycosidase and Protein Tyrosine Phosphatase 1B Inhibitors from an Endophytic Fungus Penicillium brefeldianum F4a. J. Fungi 2021, 7, 913. https://doi.org/10.3390/jof7110913
Bai Y, Yi P, Zhang S, Hu J, Pan H. Novel Antioxidants and α-Glycosidase and Protein Tyrosine Phosphatase 1B Inhibitors from an Endophytic Fungus Penicillium brefeldianum F4a. Journal of Fungi. 2021; 7(11):913. https://doi.org/10.3390/jof7110913
Chicago/Turabian StyleBai, Yan, Ping Yi, Songya Zhang, Jiangchun Hu, and Huaqi Pan. 2021. "Novel Antioxidants and α-Glycosidase and Protein Tyrosine Phosphatase 1B Inhibitors from an Endophytic Fungus Penicillium brefeldianum F4a" Journal of Fungi 7, no. 11: 913. https://doi.org/10.3390/jof7110913
APA StyleBai, Y., Yi, P., Zhang, S., Hu, J., & Pan, H. (2021). Novel Antioxidants and α-Glycosidase and Protein Tyrosine Phosphatase 1B Inhibitors from an Endophytic Fungus Penicillium brefeldianum F4a. Journal of Fungi, 7(11), 913. https://doi.org/10.3390/jof7110913