Abstract
Zn2Cys6 transcription factors are unique to fungi and are involved in different regulatory functions. In this study, we have identified the Penicillium digitatum PdMut3 gene, which encodes a putative Zn (II) 2Cys6 DNA-binding protein. Elimination of PdMut3 in Pd1 strain caused increased virulence during citrus infection. The transcription of the PdMut3 gene showed a higher expression rate during fungal growth and less transcription during fruit infection. Furthermore, the deletion of the gene in the wild-type isolate of P. digitatum did not produce any modification of the sensitivity to different fungicides, indicating that the gene is not associated with resistance to fungicides. In contrast, PdMut3 null mutants showed a reduction in growth in minimal media, which was associated with severe alterations in conidiophore development and morphological alterations of the hyphae. Mutants showed greater sensitivity to compounds that interfere with the cell wall and an invasive growth block. Thus, PdMut3 might have an indirect role in fungi virulence through metabolism and peroxisomes development.
1. Introduction
During the plant–pathogen interaction, numerous reactions are triggered, allowing the progression of the infection by the pathogen. In citrus fruits, Penicillium digitatum, the main pathogen during the postharvest period, is responsible for green mold that can lead to up to 90% losses [1]. To afford new control actions in postharvest pathogens, it is necessary to understand the regulatory mechanisms that govern processes involved in fungal–plant interaction. These processes comprise germination, mycelial progression, pathogenesis/virulence, host-specificity, or fungicide resistance in order to perform an effective infection.
Usually, pathogens displayed various mechanisms to rise their virulence [2]. During pathogen–host interaction, the start point of primary infection is wounds on the surface of the fruit, where different compounds promote the germination of spores, followed by diffusion and colonization of the fruit tissue [3]. There are more and more studies involved in processes related to virulence in postharvest pathogenic fungi [4]. Progresses in fungal pathogenicity and fruit response have recently been reported in P. digitatum [5]. Many of these studies reveal the genes and molecular mechanisms underlying the infection and the increase in pathogen virulence [5,6,7,8,9,10,11,12].
In a broad sense, virulence in plant pathogenic fungi is regulated by a system of cellular pathways that react to signals faced during host infection. Despite the variety of fungi and forms of infection, the signaling components that control pathogenic progress are basically preserved [13].
Transcription factors (TFs) are proteins that control gene expression by binding to a specific DNA sequence in the promoter region. Zinc finger TFs are one of the largest groups of transcriptional regulators, many of which have been characterized [14]. Zinc cluster proteins constitute one of the broadest families of transcription regulators in eukaryotes, can act as multifunctional regulators in many biological processes, and contribute to numerous functions in both transcription and translation procedures [15]. Previous studies showed that Zn2C6 transcription factors participate in primary metabolism, including sugar, amino acid, vitamin, and uracil metabolism [16], and in secondary metabolism, such as ergosterol biosynthesis and melanin biosynthesis [17]. Additionally, Zn2C6 transcription factors can contribute to fungal development and in the response to stresses, such as heat shock, oxidative stress and high osmotic stress [18,19], and pleiotropic drug resistance [20].
Zinc finger family TFs are classified into three main classes based on the number and order of cysteine residues: Cys2His2 proteins, Cys4 zinc finger proteins, and Zn2Cys6 proteins [21]. The third type is a large transcription factor family unique to fungi. The members of this family possess a Cys6 signature sequence and coordinate two zinc atoms per monomer [22], giving rise to the so-called zinc-binding nucleus Zn (II) 2Cys6 (abbreviated Zn2C6). This third class includes many of the most relevant transcription factors and includes the Gal4p transcription factor, which is possibly the best known and most studied zinc cluster protein in Saccharomyces cerevisiae [21].
This group includes the Zn2Cys6 transcription factors that are unique in the fungal regulatory network and some of them, such as GPF1 and CNF2 TFs, that have been functionally analyzed by genetic transformation in Magnaporthe oryzae are required for virulence [14]. Another example reported is Fow2, a Zn(II)2Cys6-type transcription regulator, which controls the expression of genes involved in the pathogenicity of Fusarium oxysporum [23]. However, attributes such as growth; asexual development; and processes related to infection, pathogenicity, and tolerance to abiotic stress have been evaluated and, on occasion, no significant differences were observed between deletion transformants and isolates of type wild [14]. For instance, the MoIRR-targeted knockout transformants did not show significant differences in mycelial growth, conidial production, conidial germination, or pathogenicity compared with M. oryzae parental isolates. MoIRR is a Zn2Cys6 transcription factor with conserved domains similar to GAL4 and Fungal_TF_MHR present in some of the well-studied Zn2Cys6 transcription factors. The genetic transformation verified that the variation in the MoIRR gene was associated with resistance to the IPT fungicide [24]. In P. digitatum, different Zn2Cys6 transcription factors were identified. PdPacC mediates pH modulation and is also involved in the pathogenicity of P. digitatum [25], a calcineurin-sensitive transcription factor PdCrz1 that is related to the pathogenicity of P. digitatum through the positive regulation of the cell wall synthase genes that maintain the cell wall integrity of P. digitatum [26], and PdSte12 controls invasive growth and asexual reproduction, thus affecting virulence [8,12].
In this work, we have identified PdMut3, a Zn2Cys6 transcription with conserved GAL4-like and Fungal_TF_MHR domains, and, through evaluation of deletant mutants, we have tried to clarify the role it exerts during the fruit–pathogen interaction and what its contribution could be to the virulence or sensitivity to certain compounds.
2. Materials and Methods
2.1. Fungal Strains’ Growth and Transformation
Two P. digitatum isolates, Pd1 (CECT20795) and Pd149 (CECT2954), were used in this work, as already described [7].
Depending on the further use, all fungi were grown in either potato dextrose broth (PDB; Liofilchem Laboratories, TE, Italy) or potato dextrose agar (PDA; Liofilchem Laboratories, TE, Italy). Fungal cultures were incubated at 25 °C with continuous light for 1 to 3 days (liquid cultures) or 1 week in the dark (solid media). Spores were obtained from 1-week-old PDA plates and conidia were counted with a hemacytometer and prepared to a desired final concentration.
P. digitatum transformation was achieved with Agrobacterium tumefaciens C58C1. A. tumefaciens containing plasmid construct was grown in LB plates or LB liquid medium with 50 µg/mL Rifampycin and 100 µg/mL Kanamycin at 28 °C, as previously reported [8].
2.2. Cloning and Targeted Gene Disruption
Genomic DNA was isolated from P. digitatum mycelium, as reported by Marcet-Houben et al. [27]. All PCR amplicons described in this work were purified using Ultra Clean TM PCR Clean-up (MoBio, Solan Beach, CA, USA). Gene identity and gene cloning verification was done by DNA sequencing [28]. DNA sequences were compared with those from the EMBL database with the Washington University Basic Local Alignment Search Tool (WU-BLAST) algorithm [29].
A binary plasmid pRFHU2 [30] was used for gene disruption following a similar procedure as described previously [9].
2.3. Protein Characterization
The protein domains were analyzed using SMART (http://smart.embl-heidelberg.de, 27 of July 2021) and fungal domains were predicted using InterPro Scan search (http://www.ebi.ac.uk/interpro/search/sequence/, 27 July 2021), which showed domains and the prediction of cellular localization.
Sequence protein alignments of different P. digitatum transcription factors, including the new gene reported, were performed using the Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/, 27 September 2021), and the phylogenetic tree was constructed using the Mega 7.0 program with the maximum likelihood method.
2.4. Molecular Characterization of ΔPdMut3
Gene removal was confirmed with three independent PCR reactions: (1) amplification from the 5’ flank of each gene to hph gene, (2) amplification from the 3’ flank of each gene to hph gene, and (3) amplification of the coding region of the gene.
The evaluation of gene copy number was done using real-time quantitative PCR (qRT-PCR), with beta-tubulin as the reference gene [9].
2.5. Inhibition of Mycelial Growth In Vitro
Four fungicides commonly used during citrus postharvest handling were used for sensitivity evaluation: imazalil (IMZ) (Textar I; Tecnidex, Valencia, Spain), prochloraz (PCL) (Ascurit; Tecnidex), and philabuster (a mixture of imazalil and pyrimethanil) (PHI) (Decco Ibérica), at increasing concentrations of 0, 1, 2, 4, and 10 µg/mL, and thiabendazol (TBZ) (Textar 60 T; Tecnidex) at 0, 10, 20, 40, and 100 µg/mL. Analysis was done in triplicates in two independent experiments. The assessment of mycelial growth at different chemicals’ concentrations was performed in 96-well microtiter plates and the respective untransformed wild-type strains were assayed at the same time. Sensitivity to chemical compounds was computed as relative mycelial growth, expressed as a percentage, calculated by comparing growth in absence and presence of the chemical following the protocol previously defined [31].
Sensitivity tests were assayed transferring 5 µL of 104 conidia/mL to PDA containing a test compound and incubation at 25 °C in the dark. Tested compounds included methanol (2.5%), FeCl3 (0.2 mM), EDTA (0.05 mM), SDS (0.02%), Tween 20 (T-20, 0.5%), 1 mM H2O2, calcofluor white (CFW, 200 µM), and Congo red (CR, 50 µM). Fungal radial growth was measured at 4–7 days. Each treatment was performed in three replicates and experiments were repeated three times. Relative growth was calculated by comparing the difference in the growth of the Pd1 and the mutants expressed in percentage.
2.6. Pathogenicity Evaluation of ΔPdMut3 Mutants
‘Navel’ mature oranges without chemical treatments were used for in vivo assays and fruit inoculation was performed as reported previously [10]. Pathogenesis experiments were performed using freshly harvested oranges (Citrus sinensis) that were injured at four places around the equatorial axis and infected with 10 µL of a conidia suspension adjusted to 105 conidia/mL. They were kept at 20 °C and 90% RH. Three replicates of five fruits each were performed and the infection experiments were performed twice. As control, mocked-inoculated fruits were used. Infection progression was computed using two parameters: percentage of infected fruits (disease intensity) and diameter of macerated tissue (disease severity).
2.7. RNA Extraction and Relative Expression by RT-qPCR
The trizol method (Ambion Inc., Austin, TX, USA) was used for RNA extraction from P. digitatum frozen mycelium. The extraction of total RNA from infected samples was processed as reported previously [6].
PrimeScript™ RT reagent Kit (Takara Bio Inc., San Jose, CA, USA) was used for synthesis of the first strand of cDNA in a 20 µL reaction, following the instructions of the manufacturer. Quantitative PCR was performed as stated before [8].
Experimental values obtained were an average of two repetitions of three biological replicates. Oligos qMut3F and qMut3R were used for PdMut3 gene, and genes coding for fungal µ-tubulin (qTubF-qTubR), ribosomal protein 28S (q28SF-q28SR), and histone H3 (qH3F-qH3R) were independently used as reference genes (Table S1). LightCycler 480 Software, version 1.5 (Roche Diagnostics) was used for cycle quantification. Primer melting temperature allowed the selection of each primer set for specific amplification. Relative gene expression was carried out as previously described [7]. PrimeScript™ RT reagent Kit (Takara Bio Inc.) was used for synthesis of the first strand of cDNA in a 20 µL reaction, following the indications of the manufacturer. Quantitative PCR was performed as reported before [8]. The software LightCycler 480 SW 1.5 (Roche Diagnostics) was used for cycle point quantification. Primer melting temperature allowed the selection of each primer set for specific amplification. The relative gene expression (‘RGE’) was carried out as stated before [7].
2.8. Microscopic Visualization
Each fungal sample was stained with 50 µg/mL Calcofluor White (CFW) for 5 min in the dark. The fluorescence was examined and photographed by a Nikon E90i fluorescence microscope (Nikon Corporation, Tokyo, Japan) with DAPI filter sets. Fluorescence images were obtained by the NIS-Elements BR v2.3 software (Nikon) and processed using FIJI software [32].
2.9. Statistical Analysis
Significant differences were evaluated using analysis of variance (ANOVA) with SAS software (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as p < 0.05; when the analysis was statistically significant, Tukey’s test for separation of means was performed.
3. Results
3.1. Identification of P. digitatum PdMut3
Specific oligos (Table S1) were used based on partial sequence of a putative transcription factor gene available in our group. Primers Mut-1 and Mut2 were used to screen Pd1 genomic library. The genomic library search was carried out as reported before [7].
After complete sequence analysis, PdMut3 (PDIP_75320) was identified. PdMut3 presented an open reading frame of 3279 bp that was interrupted by four introns of 107, 54, 58, and 51 bp, placed at positions 1.. 197, 305.. 1072, 1127.. 1738, 1797.. 2181, and 2233.. > 3279 of the coding region. The deduced amino acid sequence encoded a protein of 1002 amino acids. The protein domains analyzed using SMART showed GAL4-like and Fungal_TF_MHR domains also predicted by InterPro Scan that illustrated domains and the prediction of cellular localization.
Sequence protein alignments of different P. digitatum transcription factors, including the new gene reported, were performed using the Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/, 27 September 2021), and the phylogenetic tree was constructed using the Mega 7.0 program with the maximum likelihood method (Figure 1a). Phylogenetic study shows the existence of numerous TFs that are distributed randomly in the tree and where there is no single cluster with attributed functions in a specific way. In fact, PdMut3, highlighted in green, does not cluster with another P. digitatum TF (PDIP_33600), highlighted in red, which was identified in a cDNA library constructed based on increased virulence. PDIP_33600 gene showed a high rate of transcription (fourfold compared with in vitro growth) during citrus infection, especially at early stages [33].
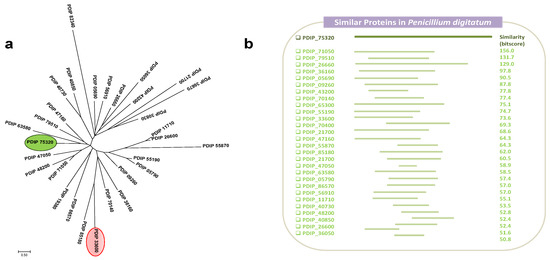
Figure 1.
(a) Unrooted maximum likelihood phylogenetic tree of selected P. digitatum TF proteins. PdMut3, the new TF described in this study, is highlighted in green, and in red, a TF found in an SSH cDNA library related to P. digitatum virulence. Each branch topology was found 100% of the time during bootstrap analysis. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. NCBI accession number is indicated for each TF protein. (b) Set of proteins within P. digitatum Pd1 that encode the same type of transcription factor corresponding to type III. The regions of greatest similarity are indicated.
Protein searching analysis showed that at least 26 TFs are present in P. digitatum that share the same typical structure with GAL4-like and Fungal_TF_MHR domains. The evaluation of the similarity between all these proteins reflected large differences between all the proteins when compared with PdMut3 (Figure 1b). Although these proteins are putative TFs and possess the GAL4-like and Fungal_TF_MHR domains, the function exerted by each of them has not been determined, nor in the signaling pathways in which they are involved.
3.2. Construction and Characterization of Deletion Mutants
A. tumefaciens-harboring plasmid pΔMut3 (Figure 2a) was used for P. digitatum Pd1 transformation. The pΔPdMut3 contained a 1.6 kb fragment from the upstream gene region amplified using the Mut-3/Mut-4 primers and a 1.8 kb fragment from the downstream gene region amplified using the Mut-5/Mut-6 primers (Table S1) adjacent to the hygromycin resistant cassette in the T-DNA region of the plasmid (Figure 2a).
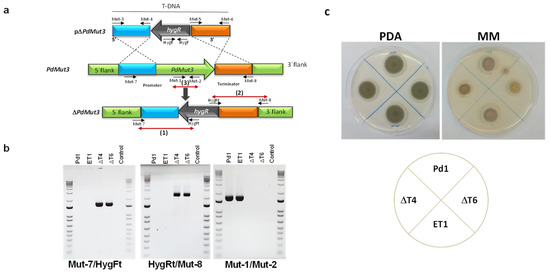
Figure 2.
(a) Diagram of wild-type locus and the PdMut3 replacement with the HygR selectable marker from pΔPdMut3 by homologous recombination to generate the ΔMut3 mutants. Red arrows define PCR fragments used for mutants’ confirmation (b) Polymerase chain reaction (PCR) evaluation of the wild-type Pd1 strain, two ΔMut3 null mutants (ΔT4, ΔT6), and the respective ectopic mutant (ET1) with analytic primers. (c) Mycelial growth of parental strain Pd1 and mutants in potato dextrose agar (PDA) and minimal medium (MM) during 4 days at 25 °C.
Deletion of the targeted gene was analysed from both flanks: (1) 5′ flank with primers Mut-7/HygFt and (2) 3′ flank with primers HygRt/Mut-8 (Table S1), confirming the presence of either 5′ flank plus promoter or 3′ flank plus terminator next to the hygromycin gene (Figure 2b). Bands were only present in deletion mutants. (3) The removal of PdMut3 gene was verified by PCR with primers Mut-1 and Mut-2. Bands were only present in the Pd1 wild-type and in ectopic transformants and were absent in deletant transformants and negative controls (Figure 2b).
To select those null mutants without additional T-DNA integrations, the copy number of integrated DNA was determined by real-time quantitative PCR (RT-qPCR). Two knockout mutants (ΔT4 and ΔT6) harboring only a single T-DNA integration and one ectopic mutant (ET1) were selected for further analysis.
Phenotypic traits of the different strains WT, deletant transformants (ΔT4 and ΔT6), and ectopic transformant (ET1) did not show differences when grown on PDA regarding growth or the rate of sporulation, but differences were observed in minimal media where deletion mutants had 35% reduced mycelial growth (Figure 2c).
3.3. Involvement of PdMut3 in Fungal Infection
Infection of different fungal strains was conducted in mature orange fruits to define if PdMut3 gene displayed a role in pathogenicity/virulence.
The analysis of the infectivity of the mutant strains against the wild-type Pd1 showed that the ectopic mutant behaved in a similar way to Pd1 strain, while surprisingly, an increase in the infectivity was observed in all deletant mutants. Although the rise was significant at early stages 3–4 dpi, no differences were observed from this point. Disease severity was also affected, but differences were observed at later time points (Figure 3a,b).
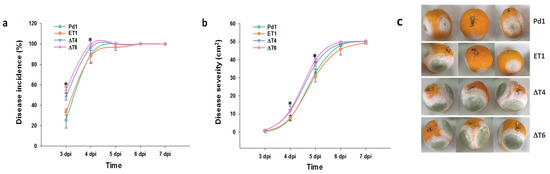
Figure 3.
Analysis of fungal infection. (a) Disease incidence (%) and (b) disease severity (cm2). Virulence assessment of Pd1, ectopic transformant ET1, and disruptant transformants (ΔT4, ΔT6). All are means of two infection experiments. Error bars represent standard deviation. * Significant differences between treatments using Tukey’s test (p < 0.05) at each dpi. (c) Representative images of infected oranges at 5 dpi.
3.4. PdMut3 Has No Contribution to Fungicide Resistance
Determination of the effect of several fungicides was carried out in the different deleting transformants. Elimination of PdMut3 gene did not affect the sensitivity of any particular chemical regardless of the type of fungicide. The results showed that all mutant strains exhibited an identical profile, which was similar to Pd1 (Figure 4).
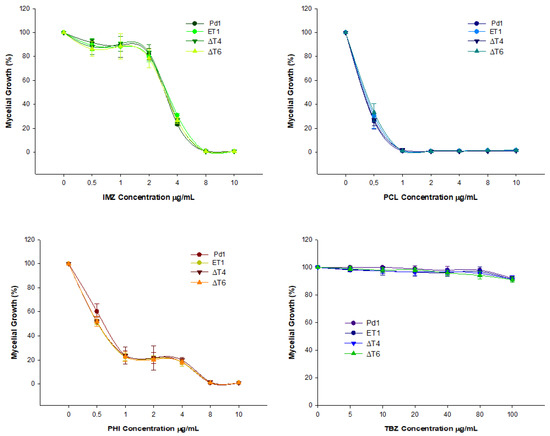
Figure 4.
Estimation of fungicide sensitivity in disruptant mutants compared with wild type Pd1. IMZ = imazalil, PCL = prochloraz, PHI = Imazalil + pyrimethanil, and TBZ = thiabendazol. The fungicides’ concentration is expressed in Δg/mL. Percentage of relative growth was calculated with respect to each strain grown without fungicide. Error bars represent standard deviation among three replicas. * Significant differences between treatments using Tukey’s test (p < 0.05).
3.5. PdMut3 Is Involved in the Maintenance of Cell-Wall Integrity
Evaluation of mycelial growth on different chemical compounds showed that Tween 20 (a polysorbitan containing lauric acid) affected all fungal strains in a similar manner by causing a 40% growth reduction. Conversely, ΔPdMut3 transformants displayed reduced radial growth by nearly 15% on PDA containing EDTA (0.05 mM), and 20% on methanol (2.5%), FeCl3 (0.2 mM), calcofluor white (CFW), and Congo red (CR). The addition of SDS (0.02%) affected ΔPdMut3 mutants drastically by inhibiting their growth, although Pd1 and ET1 also presented growth reduction (Figure 5a,b). Treatment with H2O2 confirmed that deletant mutants were able to grow better than the wild-type, being more resistant to ROS.
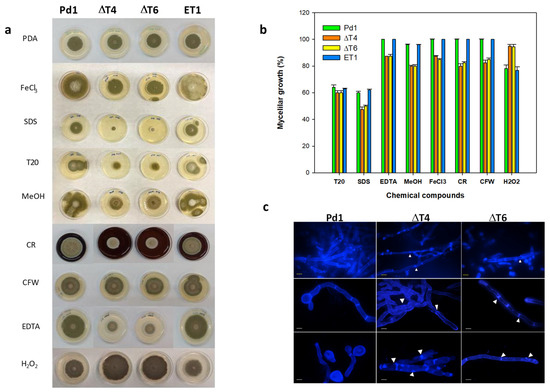
Figure 5.
(a) Fungal growth of Pd1, ET1, and ΔPdMut3 mutant (ΔT4, ΔT6) on PDA amended with different chemical compounds during 5 days: FeCl3, SDS, Tween 20 (T20), methanol (MeOH), Congo red (CR), calcofluor white (CFW), EDTA, and H2O2. (b) Percentage of growth reduction of ΔPdMut3 in relation to that grown on PDA amended with different concentrations of chemical compounds after 7 days. (c) The effects on the cell wall of spores and mycelia of P. digitatum strains observed under a fluorescence microscope after staining with calcofluor white (CFW). White arrows point out strangulation in the hyphae and variation in the septum. Yellow bars correspond to 20 µm and white bars to 40 µm.
Changes in mycelial morphology of deletion mutants were analyzed by fluorescence microscopy under CFW staining that binds chitin. The mycelium of both deletant mutants ΔT4 and ΔT6 exhibited strong microscopic alterations (Figure 5c). The areas of the septum were thinner and more diffuse and, in some cases, showed strangulation in the hyphae. Mutants exhibited intense CFW staining and occasionally broke down and released the intracellular content (Figure 5c).
3.6. Transcriptional Profiling of PdMut3
Examination of P. digitatum transcription factor gene expression was conducted using RT-qPCR. As shown in Figure 6a, gene transcription in axenic growth increased over time. No discrepancies were observed between parental strain Pd1 and the ectopic strain, while both deletant mutants assayed exhibited no expression at all.
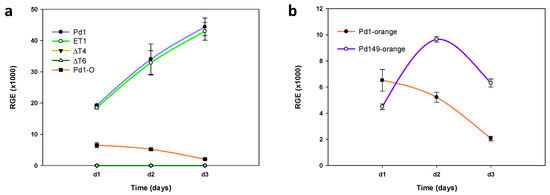
Figure 6.
Analysis of PdMut3 relative gene expression (RGE). (a) Time course evaluation of gene expression of Pd1, ectopic mutant ET1, and deletant mutants (ΔT4 and ΔT6) grown in PDB liquid culture at 25 °C and time course comparison of gene expression of Pd1 during orange infection. (b) Time course evaluation of gene expression of Pd1-virulent P. digitatum strain and Pd149-low virulent strain during orange infection. In all cases, d1, d2, and d3 correspond to 1 dpi, 2 dpi, and 3 dpi, respectively. The expression levels are relative to three reference genes: ribosomal 28S RNA, β-tubulin, and histone H3. Error bars indicate standard deviations of three biological replicates.
Comparison of PdMut3 gene expression in Pd1 strain in vitro and in vivo revealed that the expression dropped drastically during infection. Furthermore, the transcription tendency was just the opposite to that observed in vitro and the expression during the infection progressively decreased over time (Figure 6a).
Given the results observed during infection, a comparison was made of two isolates of P. digitatum that vary in their degree of virulence. The results obtained during infection (Pd149) showed transcription around two times higher at 48 h after infection compared with Pd1, and the transcription decreased with time, but maintained the difference with respect to the virulent strain (Figure 6b).
4. Discussion
P. digitatum is one of the most relevant pathogens responsible for postharvest losses in citrus [1]. The study of fruit–pathogen interactions has shown greater interest and advances have been made in the knowledge of the pathogenicity of fungi. In the citrus–P. digitatum interaction, the availability of the entire genome has greatly facilitated a better understanding of the pathogenicity of P. digitatum at molecular levels [33].
In this work, we addressed the identification of PdMut3 that encodes a Zn2Cys6 transcription factor with conserved GAL4-like and Fungal_TF_MHR domains. Remarkably, it should be noted that the most relevant pathogenicity elements described to date, which are also responsible for the virulence of P. digitatum in citrus, include mainly transcription factors, enzymes related to the cell wall, protein kinases, and fungal transporters [5]. The phylogenetic analyses carried out made it possible to determine the presence of a large number of transcription factors with similar topology that could not be grouped around PdMut3. Furthermore, another transcription factor identified in a virulence gene-enriched cDNA library that showed high gene transcription during citrus infection [33] is phylogenetically distant from PdMut3. Therefore, it is difficult to establish a clear function for the different transcription factors.
The functional activity of PdMut3 has been achieved by gene elimination. The deletion mutants were confirmed molecularly and, from the point of view of their axenic growth, differences in growth on solid media were observed. The mutants presented decreased growth on minimal media, but not in PDA, suggesting that metabolism is likely affected. In addition, the growth of the mutants in PDA medium supplemented with different compounds showed that the elimination of PdMut3 affects the cell wall integrity. A decrease in mycelial growth in the presence of CFW and CR was observed and growth was also affected in the presence of methanol, FeCl3, and chelators such as EDTA, indicating that metabolism is altered.
The increase in CFW sensitivity is indicative of alterations in the cell wall and chitin content. Damage to the cell wall structure was confirmed by microscopic studies performed with CFW staining, where the mutants showed cell disorganization, strangulation in hyphae, and chitin deposits. These effects have already been described in other cases where the cell wall was clearly damaged, for instance, in PdCrz1 mutants [26]. This result was also similar to the one observed with the addition of antifungal proteins on cell wall chitin [34]. The cell membrane plays a relevant role in maintaining cell viability because it represents a barrier by separating the cell from its environment, and is also a means of exchange of substances and energy between the cell and the surrounding environment. Generally, its integrity is highly related to many metabolic processes [35].
In this study, we evaluated the possible implication of PdMut3 in the sensitivity to certain fungicides owing to the detected alteration of the cell wall and because many TFs are involved in stress responses and pleiotropic drug resistance [20]. Previous studies performed with MoIRR, a Zn2Cys6 transcription factor of M. oryzae, which has the conserved GAL4-like and Fungal_TF_MHR domains, revealed that the knockout of MoIRR gene did not exhibit relevant differences attributable to variations in pathogenicity/virulence pathogenicity compared with M. oryzae parental isolates, but MoIRR gene was associated with resistance to IPT fungicide [24]. However, the tests carried out in PdMut3 mutants showed that this gene does not have a relevant role in the resistance to fungicides and knock-out mutants showed exactly the same resistance pattern for the four chemical compounds tested. This effect is in agreement with a study carried out with the C6 transcription factor PdSte12, which showed that it does not intervene in fungicide activity through triggering of a fungal signaling pathway [8].
In the search for the role performed by PdMut3, infective capacity studies were carried out in oranges with knockout mutants. The results were very surprising and, instead of showing a decrease in virulence, as might be expected for a relevant role in pathogenesis/virulence, just the opposite was detected. The ΔPdMut3 mutants showed a higher infective capacity during the initial stages, as demonstrated by both the incidence and the severity of the disease, particularly in the early stages. It is well known that the progression of P. digitatum during infection includes spore germination, germ tube, growth, differentiation of conidiophores stems, and formation of phialids and new conidia [36]. Therefore, initial states of infection are crucial. Previous studies have shown that several genes control the different steps of growth and development of fungi, and the elimination of any of these genes leads to growth defects and loss of pathogenicity of pathogens [2]. Nevertheless, in the RNA-seq studies performed in order to identify genes involved in virulence in P. digitatum, PdbrlA (PDIP_05330), a C2H2 zinc-finger transcription factor, was down-regulated, while the genes PdVEA1 (PDIP_21430) and PdVelB (PDIP_64730) were up-regulated, being related not only to fungal development, but also to oxidative stress sensitivity [37].
The elucidation of the possible function of the PdMut3 gene led us to study its expression pattern. In in vitro studies, the expression rate showed an increasing level over time. According to what was seen in the infection assays, it was observed that the wild-type strain Pd1 had a much lower level of expression during infection, which confirms that it does not play a role in the infectious capacity in a direct way. This is contrary to what was previously described for other TFs such as PdPacC [25], PdCrz1 [26], PdsreA, PdsreB [38,39], or PdSte12 [8,12], which all contribute in some way to the virulence of P. digitatum.
Previous works showed that Mut3p protein has been associated with peroxisomes and methanol metabolism [40]. Multiple metabolic processes occur in peroxisomes, thus they play a crucial role in the development and pathogenesis of fungi and virulence [41,42,43,44,45]. For instance, peroxisomal metabolic function is required for appressorium-mediated plant infection by Colletotrichum lagenarium [42], peroxisomal fatty acid-oxidation occurred during plant infection in M. grisea [44], and peroxisome functions are of vital importance to pathogenesis of the tangerine pathotype of Alternaria alternata [45]. Taking all this together, it could be possible that PdMut3 plays an indirect role in fungal infectivity through control of metabolism, as deletion of this gene leads to increased infectivity. We suggest that PdMut3 could play a role in the control of peroxisome development, probably by means of a negative control that promotes its degradation.
Peroxisomes are small conserved organelles that carry out a variety of critical functions. In fact, peroxisomes participate in the uptake of reactive oxygen species (ROS), through catalases and peroxidases, which are abundant in peroxisomes [46]. In Hansenula polymorpha, peroxisome degradation resulted in increased ROS formation [47]. In the early stages of infection, the oxidative stress sensitivity should occur in P. digitatum, when the citrus fruit has a series of oxidative bursts in response to infection [48]. Our hypothesis would be that, if PdMut3 regulates the degradation of peroxisomes, the knockout mutants would allow the increase of catalases, helping the degradation of the H2O2 produced as a defense response of the host [49] and improving the infectivity of P. digitatum, as has been observed in deletant mutants. This would be in agreement with the results obtained in the presence of H2O2, where the deletant mutants were able to grow better than the wild-type Pd1. This effect was also supported by the higher transcription rate shown in the less-virulent strain (Pd149) during infection.
Moreover, in fungi, peroxisomes have been shown to be required for sexual and asexual reproduction [50]. Different TFs appear to be involved in the regulation of peroxisomal proteins [40]. A correlation between PdMut3 and PdSte12, clearly involved in initial stages of P. digitatum–citrus infection and impaired in asexual reproduction during orange fruit infection [8], was established. In previous studies, we showed that PdSte12-disruptant mutants displayed increased PdMut3 gene expression of 3–4-fold [12], so PdSte12 could negatively regulate PdMut3.
The involvement of peroxisomes in cell wall integrity has also been reported in other fungal pathogens such as Fusarium graminearum, M. oryzae, and A. alternata [45,51,52,53]. As described above, in this study, deletant mutants showed a clear effect on cell wall integrity, which indicates that peroxisomes metabolism could be altered, affecting infection capacity.
Nevertheless, it would be necessary to go deeper into the process that regulates the expression of the numerous genes involved in the proliferation and degradation of peroxisomes to determine the relative contribution of PdMut3 to each of them. More research will be needed to understand all the processes in which peroxisomes are involved that likely will shed some light on how P. digitatum responds to stress and nutrient availability during penetration and colonization of citrus fruits.
5. Conclusions
To improve the postharvest disease control technology of citrus fruits, it is important to explore fungal infection mechanism. The infection mechanisms between P. digitatum and citrus depend on several factors that mediate and affect this interaction.
This study provides the identification and characterization of PdMut3, a new Zn2Cys6 transcription factor with conserved GAL4-like and Fungal_TF_MHR domains of P. digitatum. Functional analysis carried out by gene elimination confirms that PdMut3 in not involved in fungicide sensitivity, but appears to be indirectly implicated in fungal virulence. Mutants demonstrate higher infectivity in citrus fruit, although this was not correlated with a higher gene transcription rate. Our hypothesis is that PdMut3 could be related to metabolism through peroxisomes development, regulating their degradation, and could also be negatively controlled by PdSte12 transcription factor involved in the Fus3 MAPK metabolic pathway.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof7100828/s1, Table S1: Oligo sequence used in this study.
Author Contributions
Conceptualization, P.S.-T.; methodology, P.S.-T. and M.d.R.-C.; formal analysis, M.d.R.-C. and P.S.-T.; investigation, M.d.R.-C. and P.S.-T.; resources, P.S.-T.; data curation, P.S.-T.; writing—original draft preparation P.S.-T.; writing—review and editing, P.S.-T.; project administration, P.S.-T.; funding acquisition, P.S.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds of AGL2008-04828-C03 and AGL2011-30519-C03-02 projects from the Ministry of Science and Innovation (Spain). M. de Ramón-Carbonell was the recipient of a fellowship from Instituto Nacional de Investigaciones Agrarias (INIA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palou, L. Penicillium digitatum and Pencillium italicum (Green Mold, Blue Mold). In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 45–102. [Google Scholar]
- Pérez-Nadales, E.; Nogueira, M.F.A.; Baldin, C.; Castanheira, S.; Ghalid, M.; Grund, E.; Lengeler Marchegiani, E.; Mehrotra, P.V.; Moretti, M.; Naik, V.; et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Gen. Biol. 2014, 70, 42–67. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Margosan, D.A.; Mlikota Gabler, F.; Goodwine, W.R. Influence of pH and NaHCO3 on effectiveness of imazalil to inhibit germination of Penicillium digitatum and to control postharvest green mold on citrus fruit. Plant Dis. 2005, 89, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Chen, T.; Li, B.Q.; Qin, G.Z.; Tian, S.T. Molecular basis of pathogenesis of postharvest pathogenic Fungi and control strategy in fruits: Progress and prospect. Mol. Hortic. 2021, 1, 2. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Y.; Cao, H.; Li, Z. Citrus Postharvest Green Mold: Recent Advances in Fungal Pathogenicity and Fruit Resistance. Microorganisms 2020, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, M.; Ballester, A.R.; González-Candelas, L. Identification and functional analysis of Penicillium digitatum genes putatively involved in virulence towards citrus fruit. Mol. Plant Pathol. 2015, 16, 262–275. [Google Scholar] [CrossRef] [PubMed]
- de Ramón-Carbonell, M.; López-Pérez, M.; González-Candelas, L.; Sánchez-Torres, P. PdMFS1 transporter contributes to Penicilliun digitatum fungicide resistance and fungal virulence during citrus fruit infection. J. Fungi 2019, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- de Ramón-Carbonell, M.; Sánchez-Torres, P. The transcription factor PdSte12 contributes to Penicillium digitatum virulence during citrus fruit infection. Postharvest Biol. Technol. 2017, 125, 129–139. [Google Scholar] [CrossRef]
- de Ramón-Carbonell, M.; Sánchez-Torres, P. Involvement of Penicillium digitatum PdSUT1 in fungicide sensitivity and virulence during citrus fruit infection. Microbiol. Res. 2017, 203, 57–67. [Google Scholar] [CrossRef] [PubMed]
- de Ramón-Carbonell, M.; Sánchez-Torres, P. PdSlt2 Penicillium digitatum mitogen-activated-protein kinase controls sporulation and virulence during citrus fruit infection. Fungal Biol. 2017, 121, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- de Ramón-Carbonell, M.; Sánchez-Torres, P. Significance of 195 bp-enhancer of PdCYP51B in the acquisition of Penicillium digitatum DMI resistance and increase of fungal virulence. Pest. Biochem. Physiol. 2020, 165, 104522. [Google Scholar] [CrossRef]
- Vilanova, L.; Teixido, N.; Torres, R.; Usall, J.; Vinas, I.; Sánchez-Torres, P. Relevance of the transcription factor PdSte12 in Penicillium digitatum conidiation and virulence during citrus fruit infection. Int. J. Food Microbiol. 2016, 235, 93–102. [Google Scholar] [CrossRef]
- Rispail, N.; Di Pietro, A. The two-component histidine-kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 395–407. [Google Scholar] [CrossRef]
- Lu, J.; Cao, H.; Zhang, L.; Huang, P.; Lin, F. Systematic analysis of Zn2Cys6 transcription factors required for development and pathogenicity by high-throughput gene knockout in the rice blast fungus. PLoS Pathog. 2014, 10, e1004432. [Google Scholar] [CrossRef]
- Kim, J.H.; Polish, J.; Johnston, M. Specificity and regulation of DNA binding by the yeast glucose transporter gene repressor Rgt1. Mol. Cell Biol. 2003, 23, 5208–5216. [Google Scholar] [CrossRef]
- Iraqui, I.; Vissers, S.; André, B.; Urrestarazu, A. Transcriptional induction by aromatic amino acids in Saccharomyces cerevisiae. Mol. Cell Biol. 1999, 19, 3360–3371. [Google Scholar] [CrossRef]
- Tsuji, G.; Kenmochi, Y.; Takano, Y.; Sweigard, J.; Farrall, L.; Furusawa, I.; Horino, O.; Kubo, Y. Novel fugal transcriptional activa-tors, Cmr1p of Colletotrichum lagenarium and Pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear clus-ter DNA-binding motifs and regulate transcription of melanin biosyn-thesis genes in a developmentally specific manner. Mol. Microbiol. 2000, 38, 940–954. [Google Scholar] [PubMed]
- Ohm, R.A.; de Jong, J.F.; de Bekker, C.; Wösten, H.A.B.; Lugones, L.G. Transcription factor genes of Schizophyllum commune involved in regulation of mushroom formation. Mol. Microbiol. 2011, 15, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; de Larrinoa, I.F.; Tudzynski, B. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 2008, 7, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Hellauer, K.; Akache, B.; MacPherson, S.; Sirard, E.; Turcotte, B. Zinc cluster protein Rdr1p is a transcriptional repressor of the PDR5 gene encoding a multidrug transporter. J. Biol. Chem. 2002, 277, 17671–17676. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, S.; Larochelle, M.; Turcotte, B. A fungal family of transcriptional regulators: The zinc clusterproteins. Microbiol. Mol. Biol. Rev. 2006, 70, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, M.; Drouin, S.; Robert, F.; Turcotte, B. Oxidative stress-activated zinc cluster protein Stb5 has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Mol. Cell Biol. 2006, 26, 6690–6701. [Google Scholar] [CrossRef]
- Imazaki, I.; Kurahashi, M.; Iida, Y.; Tsuge, T. Fow2, a Zn (II) 2Cys6-type transcription regulator, controls plant infection of the vascular wilt fungus Fusarium oxysporum. Mol. Microbiol. 2007, 63, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Meng, F.-Z.; Zhang, M.-M.; Yin, L.-F.; Yin, W.-X.; Lin, Y.; Hsiang, T.; Peng, Y.-L.; Wang, Z.-H.; Luo, C.-X. A Putative Zn2Cys6 Transcription Factor Is Associated With Isoprothiolane Resistance in Magnaporthe oryzae. Front. Microbiol. 2018, 9, 2608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, X.; Xu, Q.; González-Candelas, L.; Li, H. The pH signaling transcription factor PacC is required for full virulence in Penicillium digitatum. Appl. Microbiol. Biotechnol. 2013, 97, 9087–9098. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Q.; Sun, X.; Li, H. The calcineurin-responsive transcription factor Crz1 is required for conidation, full virulence and DMI resistance in Penicillium digitatum. Microbiol. Res. 2013, 168, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Marcet-Houben, M.; Ballester, A.R.; de la Fuente, B.; Harries, E.; Marcos, J.F.; González-Candelas, L.; Gabaldón, T. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genom. 2012, 13, 646. [Google Scholar] [CrossRef]
- Prober, J.M.; Trainor, G.L.; Dam, R.J.; Hobbs, F.W.; Robertson, C.W.; Zagursky, R.J.; Cocuzza, A.J.; Jensen, M.A.; Baumeister, K. A system for rapid DNA sequencing with fluorescent chain terminating dideoxynucleotides. Science 1987, 238, 336–341. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Frandsen, R.J.N.; Andersson, J.A.; Kristensen, M.B.; Giese, H. Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol. Biol. 2008, 9, 70. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Tuset, J.J. Molecular insights into fungicide resistance in sensitive and resistant Penicillium digitatum strains infecting citrus. Postharvest Biol. Technol. 2011, 59, 159–165. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Ballester, A.R.; González-Candelas, L. Identification and transcriptional profiling of Penicillium digitatum virulence genes during citrus fruit interaction. In preparation.
- Gandía, M.; Garrigues, S.; Bolós, B.; Manzanares, P.; Marcos, J.F. The myosin motor domain-containing chitin synthases are involved in cell wall integrity and sensitivity to antifungal proteins in Penicillium digitatum. Front. Microbiol. 2019, 10, 2400. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Qian, X.; Dhanasekaran, S.; Boateng, N.; Yan, X.; Zhu, H.; He, F.; Zhang, H. Study on the Infection Mechanism of Penicillium digitatum on Postharvest Citrus (Citrus reticulata Blanco) Based on Transcriptomics. Microorganisms 2019, 7, 672. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, Y.; Wu, Z.; Li, N.; Chen, Y.; Qin, T.; Geng, H.; Xiong, L.; Liu, D. A novel sterol regulatory element-binding protein gene (sreA) identified in Penicillium digitatum is required for prochloraz resistance, full virulence and erg11 (cyp51) regulation. PLoS ONE 2015, 10, e0117115. [Google Scholar] [CrossRef] [PubMed]
- Ruan, R.; Wang, M.; Liu, X.; Sun, X.; Chung, K.R.; Li, H. Functional analysis of two sterol regulatory element binding proteins in Penicillium digitatum. PLoS ONE 2017, 12, e0176485. [Google Scholar] [CrossRef]
- Leao-Helder, A.N.; Krikken, A.M.; van der Klei, I.J.; Kiel, J.A.; Veenhuis, M. Transcriptional down-regulation of peroxisome numbers affects selective peroxisome degradation in Hansenula polymorpha. J. Biol. Chem. 2003, 278, 40749–40756. [Google Scholar] [CrossRef]
- Fujihara, N.; Sakaguchi, A.; Tanaka, S.; Fujii, S.; Tsuji, G.; Shiraishi, T.; O’Connell, R.; Kubo, Y. Peroxisome biogenesis factor PEX13 is required for appressorium-mediated plant infection by the anthracnose fungus Colletotrichum orbiculare. Mol. Plant-Microbe Interact. 2010, 23, 436–445. [Google Scholar] [CrossRef]
- Kimura, A.; Takano, Y.; Furusawa, I.; Okuno, T. Peroxisomal metabolic function is required for appressorium-mediated plant infection by Colletotrichum lagenarium. Plant Cell 2001, 13, 1945–1957. [Google Scholar] [CrossRef]
- Min, K.; Son, H.; Lee, J.; Choi, G.J.; Kim, J.C.; Lee, Y.W. Peroxisome function is required for virulence and survival of Fusarium graminearum. Mol. Plant-Microbe Interact. 2012, 25, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Soanes, D.M.; Kershaw, M.J.; Talbot, N.J. Functional analysis of lipid metabolism in Magnaporthe grisea reveals a requirement for peroxisomal fatty acid beta-oxidation during appressorium mediated plant infection. Mol. Plant-Microbe Interact. 2007, 20, 475–491. [Google Scholar] [CrossRef]
- Wu, P.-C.; Chen, Y.-K.; Yago, J.I.; Chung, K.-R. Peroxisomes Implicated in the Biosynthesis of Siderophores and Biotin, Cell Wall Integrity, Autophagy, and Response to Hydrogen Peroxide in the Citrus Pathogenic Fungus Alternaria alternata. Front. Microbiol. 2021, 12, 645792. [Google Scholar] [CrossRef]
- Antonenkov, V.D.; Grunau, S.; Ohlmeier, S.; Hiltunen, J.K. Peroxisomes are oxidative organelles. Antioxid. Redox Signal. 2010, 13, 525–537. [Google Scholar] [CrossRef] [PubMed]
- van Zutphen, T.; Veenhuis, M.; van der Klei, I.J. Damaged peroxisomes are subject to rapid autophagic degradation in the yeast Hansenula polymorpha. Autophagy 2011, 7, 863–872. [Google Scholar] [CrossRef][Green Version]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Macarisin, D.; Cohen, L.; Eick, A.; Rafael, G.; Belausov, E.; Wisniewski, M.; Droby, S. Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathology 2007, 97, 1491–1500. [Google Scholar] [CrossRef]
- Peraza-Reyes, L.; Berteaux-Lecellier, V. Peroxisomes and sexual development in fungi. Front. Physiol. 2013, 6, 244. [Google Scholar] [CrossRef]
- Goh, J.; Jeon, J.; Kim, K.S.; Park, J.; Park, S.-Y.; Lee, Y.-H. The PEX7-mediated peroxisomal import systemis required for fungal development and pathogenicity in Magnaporthe oryzae. PLoS ONE 2011, 6, e28220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Wang, J.; Zhang, Z.; Wang, Y.; Liu, M.; Jiang, H.; Chai, R.; Mao, X.; Qiu, H.; Liu, F.; et al. Mopex19, which is essential for maintenance of peroxisomal structure and Woronin bodies, is required for metabolism and development in the rice blast fungus. PLoS ONE 2014, 9, e85252. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Liang, Y.; Yu, J. FgPEX4 is involved in development, pathogenicity, and cell wall integrity in Fusarium graminearum. Curr. Genet. 2019, 65, 747–758. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).