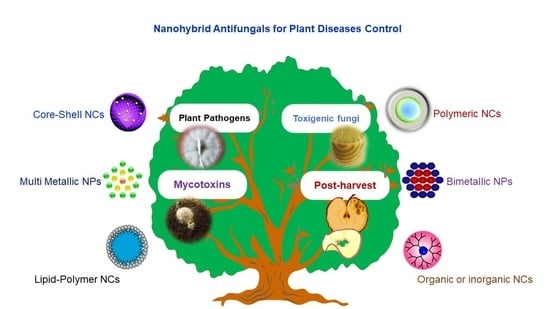
Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives
Abstract
1. Introduction
2. Nanotechnology Advances for Improved Pathogen Diagnosis
3. Nanocomposites and Their Mode of Action on the Fungal Phytopathogens
3.1. Antifungal Mechanism of Nanoparticles/Nanocomposites
3.2. Nano-Hybrid Antifungals for Control of Toxigenic Fungi and Mycotoxins Degradations
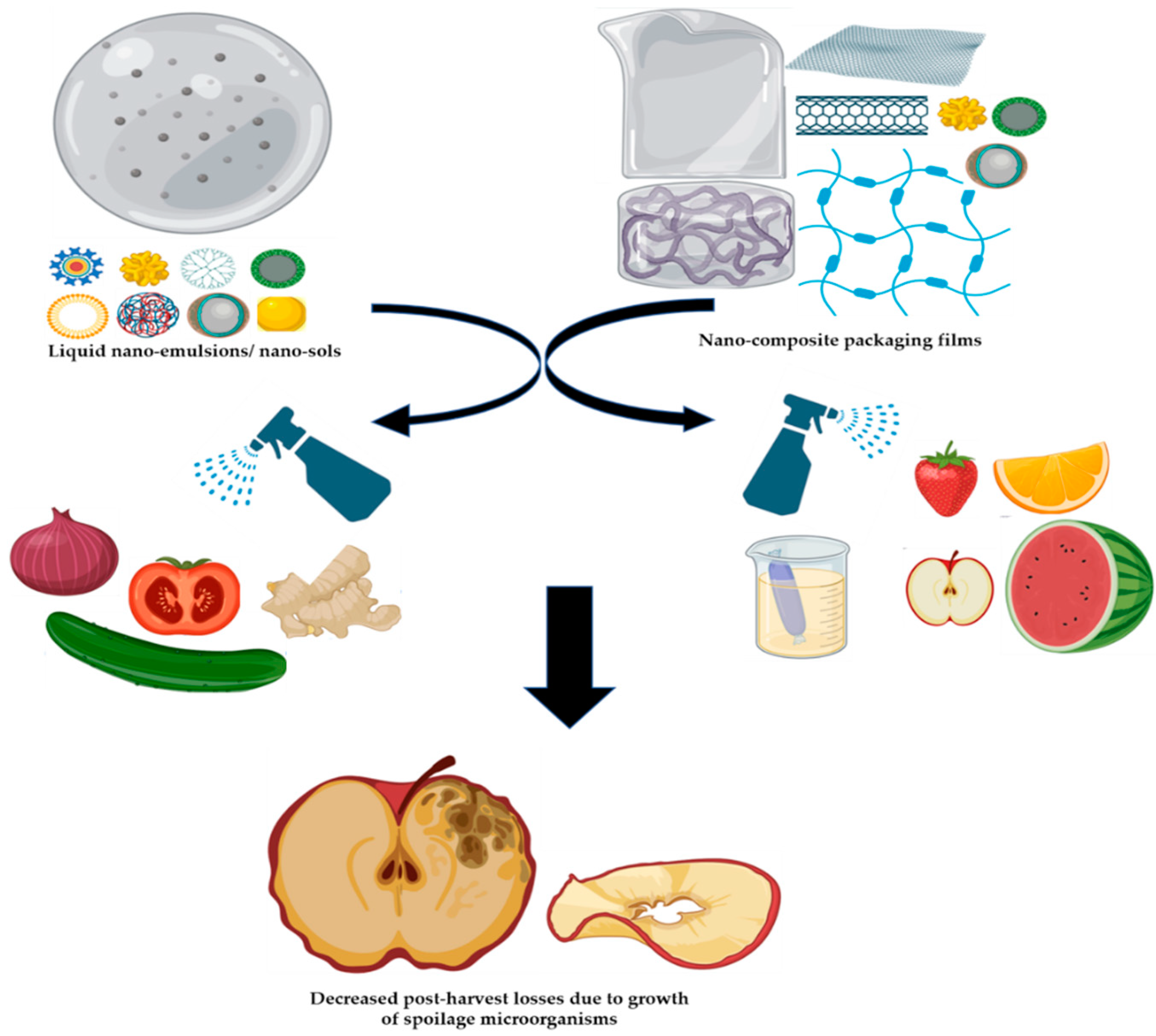
3.3. Postharvest Management of Nanocomposite Against Pathogenic Fungi
4. Challenges
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Kalia, A.; Gosal, S.K. Effect of pesticide application on soil microorganisms. Arch. Agron. Soil Sci. 2011, 57, 569–596. [Google Scholar] [CrossRef]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale Self-Assembly for Therapeutic Delivery. Front. Bioeng. Biotechnol. 2020, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kamrul Hasan, M.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Use of Nanoparticles and Nanoformulations in Agriculture. J. Nanosci. Nanotechnol. 2017, 17, 8699–8730. [Google Scholar] [CrossRef]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; Do Espirito Santo Pereira, A.; De Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 1–19. [Google Scholar] [CrossRef]
- Rangaraj, S.; Gopalu, K.; Muthusamy, P.; Rathinam, Y.; Venkatachalam, R.; Narayanasamy, K. Augmented biocontrol action of silica nanoparticles and Pseudomonas fluorescens bioformulant in maize (Zea mays L.). RSC Adv. 2014, 4, 8461–8465. [Google Scholar] [CrossRef]
- Paek, S.M.; Oh, J.M.; Choy, J.H. A lattice-engineering route to heterostructured functional nanohybrids. Chem. Asian J. 2011, 6, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Spadola, G.; Sanna, V.; Bartoli, J.; Carcelli, M.; Pelosi, G.; Biscegliel, F.; Restivo, F.M.; Degola, F.; Rogolino, D. Thiosemicarbazone nano-formulation for the control of Aspergillus flavus. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K. Nanodiagnostics for plant pathogens. Environ. Chem. Lett. 2017, 15, 7–13. [Google Scholar] [CrossRef]
- Rad, F.; Mohsenifar, A.; Tabatabaei, M.; Safarnejad, M.R.; Shahryari, F.; Safarpour, H.; Foroutan, A.; Mardi, M.; Davoudi, D.; Fotokian, M. Detection of Candidatus Phytoplasma aurantifolia with a quantum dots fret-based biosensor. J. Plant Pathol. 2012, 94, 525–534. [Google Scholar] [CrossRef]
- Yao, K.S.; Li, S.J.; Tzeng, K.C.; Cheng, T.C.; Chang, C.Y.; Chiu, C.Y.; Liao, C.Y.; Hsu, J.J.; Lin, Z.P. Fluorescence Silica Nanoprobe as a Biomarker for Rapid Detection of Plant Pathogens. Adv. Mater. Res. 2009, 79–82, 513–516. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Agrawal, V.V.; Kumar, A. An attempt to develop surface plasmon resonance based immunosensor for Karnal bunt (Tilletia indica) diagnosis based on the experience of nano-gold based lateral flow immuno-dipstick test. Thin Solid Films 2010, 519, 1156–1159. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, F.; Liu, S.Y.; Xu, Q.; Huang, J.Y.; Dong, X.Y.; Yu, J.H.; Yang, Q.; Di Zhao, Y.; Chen, H. Electrocatalytic oxidation of phytohormone salicylic acid at copper nanoparticles-modified gold electrode and its detection in oilseed rape infected with fungal pathogen Sclerotinia sclerotiorum. Talanta 2010, 80, 1277–1281. [Google Scholar] [CrossRef]
- Schwenkbier, L.; Pollok, S.; König, S.; Urban, M.; Werres, S.; Cialla-May, D.; Weber, K.; Popp, J. Towards on-site testing of Phytophthora species. Anal. Methods 2015, 7, 211–217. [Google Scholar] [CrossRef]
- Ariffin, S.A.B.; Adam, T.; Hashim, U.; Faridah, S.; Zamri, I.; Uda, M.N.A. Plant diseases detection using nanowire as biosensor transducer. Adv. Mater. Res. 2014, 832, 113–117. [Google Scholar] [CrossRef]
- Ashfaq, M.; Talreja, N.; Chuahan, D.; Srituravanich, W. Polymeric Nanocomposite-Based Agriculture Delivery System: Emerging Technology for Agriculture. Genet. Eng. Glimpse Tech. Appl. 2020, 1–16. [Google Scholar] [CrossRef]
- Winter, J.; Nicolas, J.; Ruan, G. Hybrid nanoparticle composites. J. Mater. Chem. B 2020, 8, 4713–4714. [Google Scholar] [CrossRef]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.A. In situ synthesis of hybrid inorganic-polymer nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Salah Eldin, R.E.; Abu Serea, E.S.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Idumah, C.I.; Zurina, M.; Ogbu, J.; Ndem, J.U.; Igba, E.C. A review on innovations in polymeric nanocomposite packaging materials and electrical sensors for food and agriculture. Compos. Interfaces 2020, 27, 1–72. [Google Scholar] [CrossRef]
- Sadeghi, R.; Rodriguez, R.J.; Yao, Y.; Kokini, J.L. Advances in Nanotechnology as They Pertain to Food and Agriculture: Benefits and Risks. Annu. Rev. Food Sci. Technol. 2017, 8, 467–492. [Google Scholar] [CrossRef]
- Padmanaban, V.C.; Giri Nandagopal, M.S.; Madhangi Priyadharshini, G.; Maheswari, N.; Janani Sree, G.; Selvaraju, N. Advanced approach for degradation of recalcitrant by nanophotocatalysis using nanocomposites and their future perspectives. Int. J. Environ. Sci. Technol. 2016, 13, 1591–1606. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile Ball-Milling Synthesis of CuO/Biochar Nanocomposites for Efficient Removal of Reactive Red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef]
- Kalia, A.; Sharma, S.P.; Kaur, H.; Kaur, H. Novel nanocomposite-based controlled-release fertilizer and pesticide formulations: Prospects and challenges. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystem; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–134. [Google Scholar]
- Guha, T.; Gopal, G.; Kundu, R.; Mukherjee, A. Nanocomposites for Delivering Agrochemicals: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Min, J.S.; Kim, K.S.; Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, S.B.; Jung, M.; Lee, Y.S. Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant Pathol. J. 2009, 25, 376–380. [Google Scholar] [CrossRef]
- Sidhu, A.; Bala, A.; Singh, H.; Ahuja, R.; Kumar, A. Development of MgO-sepoilite Nanocomposites against Phytopathogenic Fungi of Rice (Oryzae sativa): A Green Approach. ACS Omega 2020, 5, 13557–13565. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Chen, J.; Sun, L.; Cheng, Y.; Lu, Z.; Shao, K.; Li, T.; Hu, C.; Han, H. Graphene Oxide-Silver Nanocomposite: Novel Agricultural Antifungal Agent against Fusarium graminearum for Crop Disease Prevention. ACS Appl. Mater. Interfaces 2016, 8, 24057–24070. [Google Scholar] [CrossRef]
- Wang, X.; Cai, A.; Wen, X.; Jing, D.; Qi, H.; Yuan, H. Graphene oxide-Fe3O4 nanocomposites as high-performance antifungal agents against Plasmopara viticola. Sci. China Mater. 2017, 60, 258–268. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Domínguez-Espíndola, R.B.; Casas-Flores, S.; Patrón-Soberano, O.A.; Camposeco-Solis, R.; Lee, S.W. Antifungal nanocomposites inspired by titanate nanotubes for complete inactivation of Botrytis cinerea isolated from tomato infection. ACS Appl. Mater. Interfaces 2016, 8, 31625–31637. [Google Scholar] [CrossRef]
- Hoseinzadeh, A.; Habibi-Yangjeh, A.; Davari, M. Antifungal activity of magnetically separable Fe3O4/ZnO/AgBr nanocomposites prepared by a facile microwave-assisted method. Prog. Nat. Sci. Mater. Int. 2016, 26, 334–340. [Google Scholar] [CrossRef]
- Bhavyasree, P.G.; Xavier, T.S. Green synthesis of Copper Oxide/Carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon 2020, 6, e03323. [Google Scholar] [CrossRef] [PubMed]
- Olad, A. Polymer–Clay nanocomposites. In Advanced Polymeric Materials: Structure Property Relationships; CRC Press: Boca Raton, FL, USA, 2011; pp. 349–396. ISBN 9780203492901. [Google Scholar]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef]
- Sampathkumar, K.; Tan, K.X.; Loo, S.C.J. Developing Nano-Delivery Systems for Agriculture and Food Applications with Nature-Derived Polymers. iScience 2020, 23, 101055. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Youssef, K.; Hashim, A.F. Inhibitory effect of clay/chitosan nanocomposite against Penicillium digitatum on citrus and its possible mode of action. Jordan J. Biol. Sci. 2020, 13, 349–355. [Google Scholar]
- Al-Dhabaan, F.A.; Shoala, T.; Ali, A.A.M.; Alaa, M.; Abd-Elsalam, K. Chemically-Produced Copper, Zinc Nanoparticles and Chitosan-Bimetallic Nanocomposites and Their Antifungal Activity against Three Phytopathogenic Fungi. Int. J. Agric. Technol. 2017, 13, 753–769. [Google Scholar]
- Abd-Elsalam, K.A.; Vasil’kov, A.Y.; Said-Galiev, E.E.; Rubina, M.S.; Khokhlov, A.R.; Naumkin, A.V.; Shtykova, E.V.; Alghuthaymi, M.A. Bimetallic blends and chitosan nanocomposites: Novel antifungal agents against cotton seedling damping-off. Eur. J. Plant Pathol. 2018, 151, 57–72. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Choudhary, A. An In Vitro Study of The Antifungal Activity of Silver/Chitosan Nanoformulations Against Important Seed Borne Pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef]
- Mathew, T.V.; Kuriakose, S. Photochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groups. Mater. Sci. Eng. C 2013, 33, 4409–4415. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in chitosan-cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Kalia, A.; Abd-Elsalam, K.A.; Kuca, K. Zinc-based nanomaterials for diagnosis and management of plant diseases: Ecological safety and future prospects. J. Fungi 2020, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef]
- Patra, P.; Mitra, S.; Debnath, N.; Goswami, A. Biochemical-, biophysical-, and microarray-based antifungal evaluation of the buffer-mediated synthesized nano zinc oxide: An in vivo and in vitro toxicity study. Langmuir 2012, 28, 16966–16978. [Google Scholar] [CrossRef]
- Shoeb, M.; Singh, B.R.; Khan, J.A.; Khan, W.; Singh, B.N.; Singh, H.B.; Naqvi, A.H. ROS-dependent anticandidal activity of zinc oxide nanoparticles synthesized by using egg albumen as a biotemplate. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Thipe, V.C.; Thatyana, M.; Ajayi, F.R.; Njobeh, P.B.; Katti, K.V. Hybrid nanomaterials for detection, detoxification, and management mycotoxins. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 271–285. ISBN 9780128213544. [Google Scholar]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.H.; Marty, J.L. Nano-aptasensing in mycotoxin analysis: Recent updates and progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef]
- Santos, A.O.; Vaz, A.; Rodrigues, P.; Veloso, A.C.A.; Venâncio, A.; Peres, A.M. Thin films sensor devices for mycotoxins detection in foods: Applications and challenges. Chemosensors 2019, 7, 3. [Google Scholar] [CrossRef]
- Ananikov, V.P. Organic-inorganic hybrid nanomaterials. Nanomaterials 2019, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Pandey, M.K.; Sumana, R.; Sumana, G. Electrochemical Aflatoxin B1 immunosensor based on the use of graphene quantum dots and gold nanoparticles. Microchim. Acta 2019, 186, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hamza, Z.; El-Hashash, M.; Aly, S.; Hathout, A.; Soto, E.; Sabry, B.; Ostroff, G. Preparation and characterization of yeast cell wall beta-glucan encapsulated humic acid nanoparticles as an enhanced aflatoxin B1 binder. Carbohydr. Polym. 2019, 203, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Kráĺová, K. Nanocomposites: Synergistic nanotools for management of mycotoxigenic fungi. In Nanomycotoxicology; Academic Press: Cambridge, MA, USA, 2020; pp. 349–383. [Google Scholar]
- Pirouz, A.A.; Selamat, J.; Iqbal, S.Z.; Mirhosseini, H.; Karjiban, A.R.; Bakar, F.A. The use of innovative and efficient nanocomposite (magnetic graphene oxide) for the reduction on of Fusarium mycotoxins in palm kernel cake. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xie, W. Detoxification of Aflatoxin B 1 by magnetic graphene composite adsorbents from contaminated oils. J. Hazard. Mater. 2020, 381, 120915. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; Shami, A.; Said-Galive, E.; Shtykova, E.V.; Naumkin, A.V. Silver/Chitosan Nanocomposites: Preparation and Characterization and Their Fungicidal Activity against Dairy Cattle Toxicosis Penicillium expansum. J. Fungi 2020, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Shami, A.; Rubina, M.S.; Abramchuk, S.S.; Shtykova, E.V.; Vasil’kov, A. Copper-Chitosan Nanocomposite Hydrogels Against Aflatoxigenic Aspergillus flavus from Dairy Cattle Feed. J. Fungi 2020, 6, 112. [Google Scholar] [CrossRef]
- Tarus, B.K.; Mwasiagi, J.I.; Fadel, N.; Al-Oufy, A.; Elmessiry, M. Electrospun cellulose acetate and poly(vinyl chloride) nanofiber mats containing silver nanoparticles for antifungi packaging. SN Appl. Sci. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- González-Estrada, R.; Blancas-Benítez, F.; Velázquez-Estrada, R.M.; Montaño-Leyva, B.; Ramos-Guerrero, A.; Aguirre-Güitrón, L.; Moreno-Hernández, C.; Coronado-Partida, L.; Herrera-González, J.A.; Rodríguez-Guzmán, C.A.; et al. Alternative Eco-Friendly Methods in the Control of Post-Harvest Decay of Tropical and Subtropical Fruits. Mod. Fruit Ind. 2020. [Google Scholar] [CrossRef]
- Roberto, S.R.; Youssef, K.; Hashim, A.F.; Ippolito, A. Nanomaterials as alternative control means against postharvest diseases in fruit crops. Nanomaterials 2019, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, R.R. Postharvest Diseases of Fruits and Vegetables and Their Management. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–52. ISBN 9780128126981. [Google Scholar]
- Pétriacq, P.; López, A.; Luna, E. Fruit decay to diseases: Can induced resistance and priming help? Plants 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of banana postharvest quality using a novel soybean protein isolate/cinnamaldehyde/zinc oxide bionanocomposite coating strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Jagana, D.; Hegde, Y.R.; Lella, R. Green Nanoparticles—A Novel Approach for the Management of Banana Anthracnose Caused by Colletotrichum musae. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1749–1756. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA film incorporated with ZnO nanoparticle on the quality attributes of fresh-cut apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef]
- Chowdappa, P.; Shivakumar, G.; Chettana, C.S.; Madhura, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. African J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar] [CrossRef]
- Dubey, P.K.; Shukla, R.N.; Srivastava, G.; Mishra, A.A.; Pandey, A. Study on Quality Parameters and Storage Stability of Mango Coated with Developed Nanocomposite Edible Film. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2899–2935. [Google Scholar] [CrossRef]
- Zahid, N.; Ali, A.; Manickam, S.; Siddiqui, Y.; Maqbool, M. Potential of chitosan-loaded nanoemulsions to control different Colletotrichum spp. and maintain quality of tropical fruits during cold storage. J. Appl. Microbiol. 2012, 113, 925–939. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, D.H.; Pham, D.D.; Nguyen, Q.H.; Hoang, D.Q. New oligochitosan-nanosilica hybrid materials: Preparation and application on chili plants for resistance to anthracnose disease and growth enhancement. Polym. J. 2017, 49, 861–869. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Huynh, T.N.; Hoang, D.; Nguyen, D.H.; Nguyen, Q.H.; Tran, T.H. Functional Nanostructured Oligochitosan–Silica/Carboxymethyl Cellulose Hybrid Materials: Synthesis and Investigation of Their Antifungal Abilities. Polymers 2019, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Noman, M.; Luo, J.; Muhammad, S.; Shahid, M.; Ali, M.A.; Zhang, M.; Li, B. Bioengineered chitosan-magnesium nanocomposite: A novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int. J. Biol. Macromol. 2020, 24, S0141–S8130. [Google Scholar] [CrossRef]
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.A.; Mohamed, M.A. Reduced Graphene Oxide Nanosheet-Decorated Copper Oxide Nanoparticles: A Potent Antifungal Nanocomposite against Fusarium Root Rot and Wilt Diseases of Tomato and Pepper Plants. Nanomaterials 2020, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, A.; Casillas-Santana, M.Á.; Martínez-Castañón, G.A.; Slavin, Y.N.; Zhao, W.; Asnis, J.; Häfeli, U.O.; Bach, H. Effective Control of Molds Using a Combination of Nanoparticles. PLoS ONE 2017, 12, e0169940. [Google Scholar] [CrossRef]
- De La Rosa-García, S.C.; Martínez-Torres, P.; Gómez-Cornelio, S.; Corral-Aguado, M.A.; Quintana, P.; Gómez-Ortíz, N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, L.; Le, X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ. Pollut. 2017, 230, 302–310. [Google Scholar] [CrossRef]
- Shen, C.X.; Zhang, Q.F.; Li, J.; Bi, F.C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef]
- Rienzie, R.; Adassooriya, N.M. Nanomaterials: Ecotoxicity, Safety, and Public Perception. In Nanomaterials: Ecotoxicity, Safety, and Public Perception; Springer International Publishing: Berlin, Germany, 2018; pp. 207–234. ISBN 9783030051440. [Google Scholar]
- Zheng, L.P.; Zhang, Z.; Zhang, B.; Wang, J.W. Antifungal properties of Ag-SiO2 core-shell nanoparticles against phytopathogenic fungi. Adv. Mater. Res. 2012, 476, 814–818. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Carbone, S.; Gatti, A.; Vianello, G.; Nannipieri, P. Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
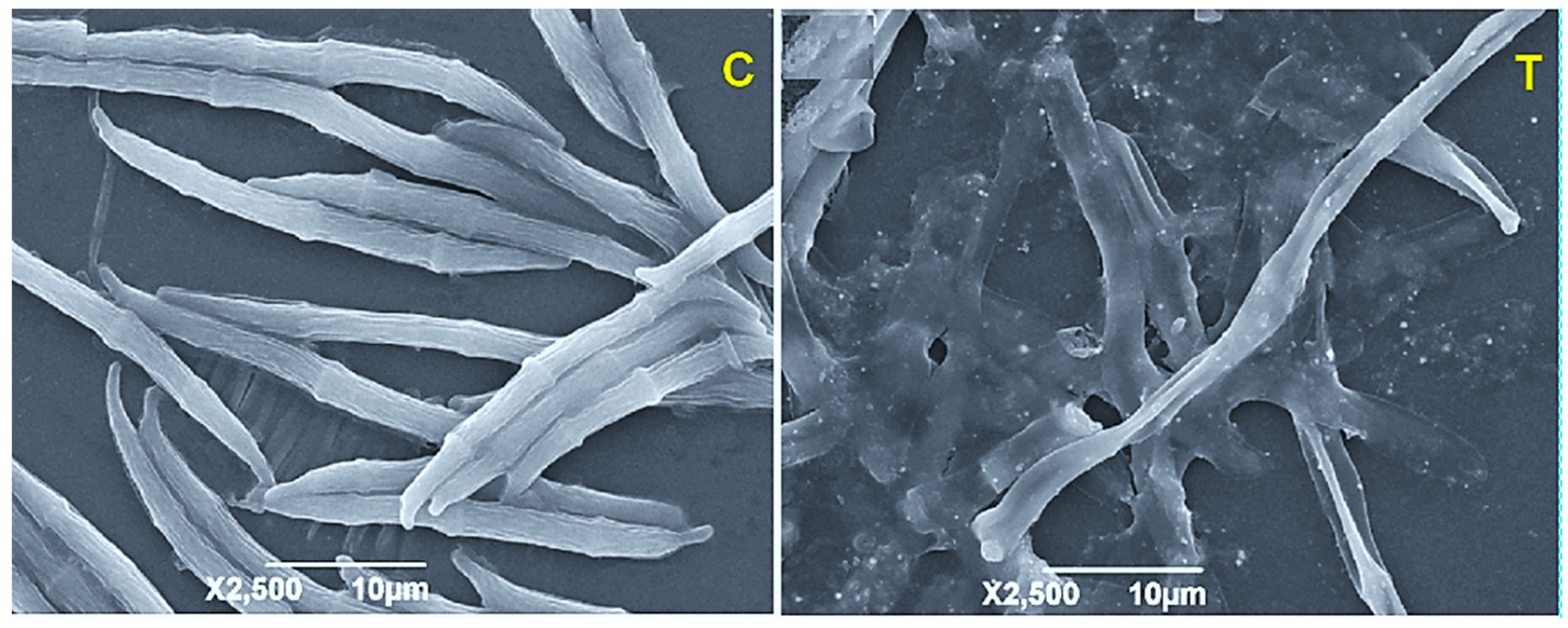
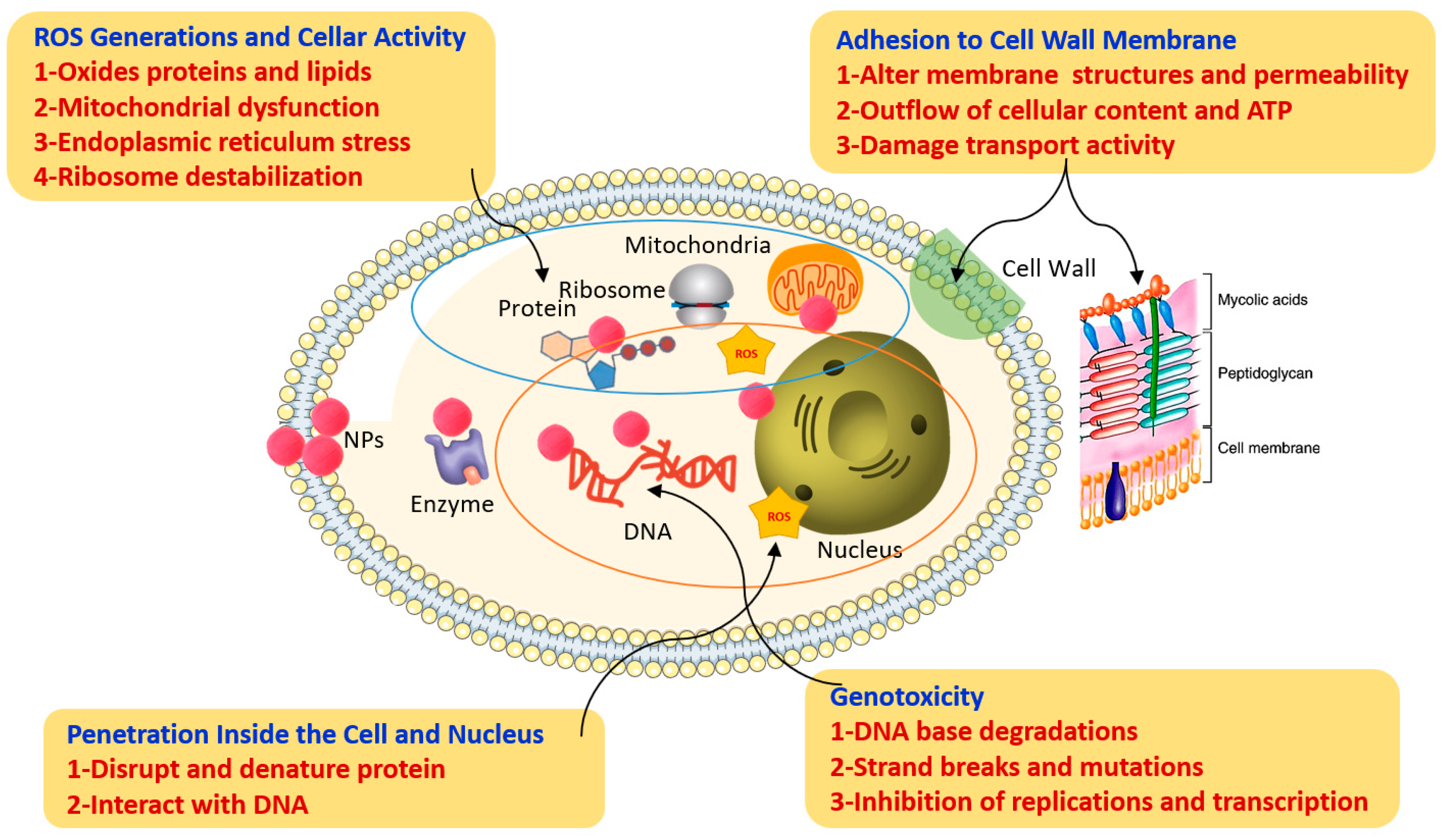


| Type of Nanocomposite Applied | Method of Synthesis | Effective Working Concentration | Application Method | Pathogen Studied | Remarks | References |
|---|---|---|---|---|---|---|
| Inorganic-inorganic composites | ||||||
| AgNPs-titanate nanotubes | Photo-assisted functionalization of AgNPs on hydrothermal micro-wave-assisted synthesis of titanate nanotubes | 30 mg/30 mL | In vitro study performed in PDB supplemented with 30 mg photo-activated AgNP-titanate nanotube composite | Botrytis cinerea | Fungal conidial death due to ROS damage | [40] |
| Fe3O4/ZnO/AgBr | Microwave-assisted synthesis | 1:8 weight ratio nanocomposite | In vitro spore broth incubation study performed in a cylindrical Pyrex reactor | Fusarium graminearum, Fusarium oxysporum | Complete inactivation of test fungi within one hour of incubation with the nanocomposite | [41] |
| Bimetallic (Au/Ag) NPs with metal oxide NPs(ZnO NPs) | Physical mixture technique | 50:10 μg/mL | In vitro poison food study involving the addition of NP suspension in SDB | Aspergillus flavus/A. fumigatus | -Augmented inhibition of fungal growth by bimetallic and metal oxide NPs | [90] |
| ZnO:Mg(OH)2 composite | Hydrothermal/co-precipitation technique | 5 to 0.002 mg mL−1 | In vitro study involving DMSO dissolved NPs supplemented in PDB | Colletotrichum gloeosporioides | -Addition of MgO diminished the antifungal potential of ZnO NPs | [91] |
| Inorganic-carbon composites | ||||||
| CuO NPs functionalized graphene-like carbon composite | Green synthesis using Adhatoda vasica leaf extract and 0.01 M CuSO4 | 5:4 ratio proportion of leaf extract: CuSO4 | In vitro agar well diffusion assay on PDA media | Aspergillus niger, Candida albicans | growth inhibition due to the disruption of the cell membranes | [42] |
| GO-AgNPs | Interfacial electrostatic self-assembly synthesis | 9.37 µg/mL-MIC value | In vitro assay using growth media and detached wheat leaf bioassay | Fusarium graminearum | Improved anti-fungal efficiency [>3-fold for AgNPs and >7-fold over pure GO] through two mechanisms (physical injury and ROS mediated chemical injury) | [38] |
| Inorganic-organic composites | ||||||
| ZnO NPs/CS-Zn-CuNPs | Wet chemical method | 0 to 90 µg mL−1 | In vitro study involved supplementation of nanocomposite in PDA media | Alternaria alternata, B. cinerea, R. solani | -Highest mycelial inhibition by chitosan mixed Zn-Cu nanocomposite | [48] |
| Cu-/Zn-chitosan and bimetallic nanocomposites | Wet chemical synthesis | 30, 60, and 100 μg mL−1 | -In vitro study using agar based media -In vivo seed priming assay for damping-off disease in cotton cultivar Giza 92 seedlings | Rhizoctonia solani | -highest hyphal inhibition at 100 μg mL−1 -Augmented effect of bimetallic NC along with biocontrol fungus (Trichoderma)to suppress disease in vivo | [49] |
| Clay-chitosan nanocomposite | Anion-exchange technique | 5 to 60 μg mL−1 | -In vitro study using PDA -In vivo assay in mature fruits of Citrus sinensis (L. Osbeck) cv. Valencia | Penicillium digitatum | -Complete inhibition of fungal hyphae in different weight ratios of clay/chitosan nanocomposite (1:0.5, 1:1, 1:2) (conc.20 μg mL−1) | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuthaymi, M.A.; C., R.; P., R.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. J. Fungi 2021, 7, 48. https://doi.org/10.3390/jof7010048
Alghuthaymi MA, C. R, P. R, Kalia A, Bhardwaj K, Bhardwaj P, Abd-Elsalam KA, Valis M, Kuca K. Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. Journal of Fungi. 2021; 7(1):48. https://doi.org/10.3390/jof7010048
Chicago/Turabian StyleAlghuthaymi, Mousa A., Rajkuberan C., Rajiv P., Anu Kalia, Kanchan Bhardwaj, Prerna Bhardwaj, Kamel A. Abd-Elsalam, Martin Valis, and Kamil Kuca. 2021. "Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives" Journal of Fungi 7, no. 1: 48. https://doi.org/10.3390/jof7010048
APA StyleAlghuthaymi, M. A., C., R., P., R., Kalia, A., Bhardwaj, K., Bhardwaj, P., Abd-Elsalam, K. A., Valis, M., & Kuca, K. (2021). Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. Journal of Fungi, 7(1), 48. https://doi.org/10.3390/jof7010048