Physiological Basis of Smut Infectivity in the Early Stages of Sugar Cane Colonization
Abstract
1. Introduction
2. What Is the Life Cycle of the Ustilaginales-Like Sporisorium scitamineum?
3. A Quorum Signal (QS) Triggers the Infection
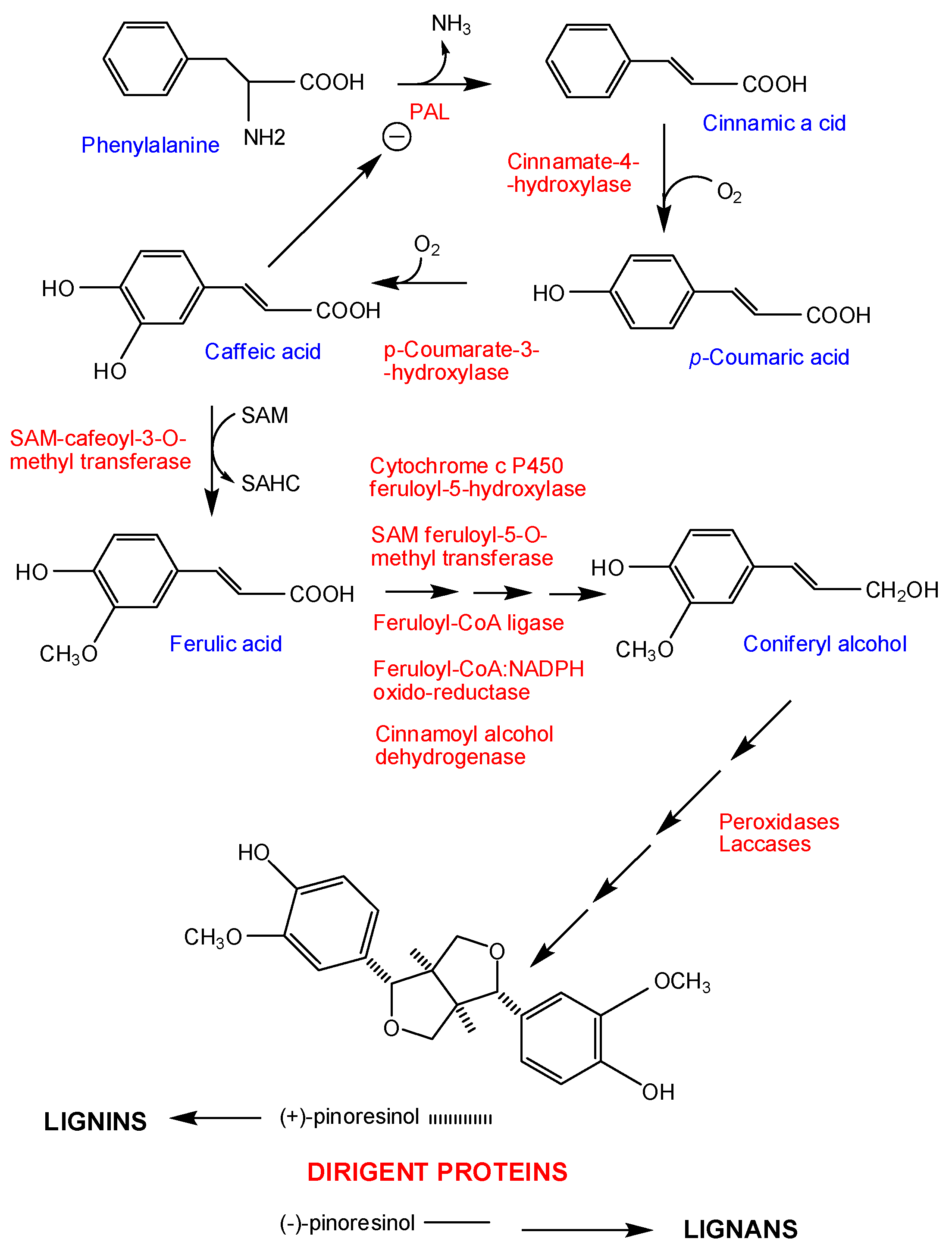
4. Pathogenicity Factors
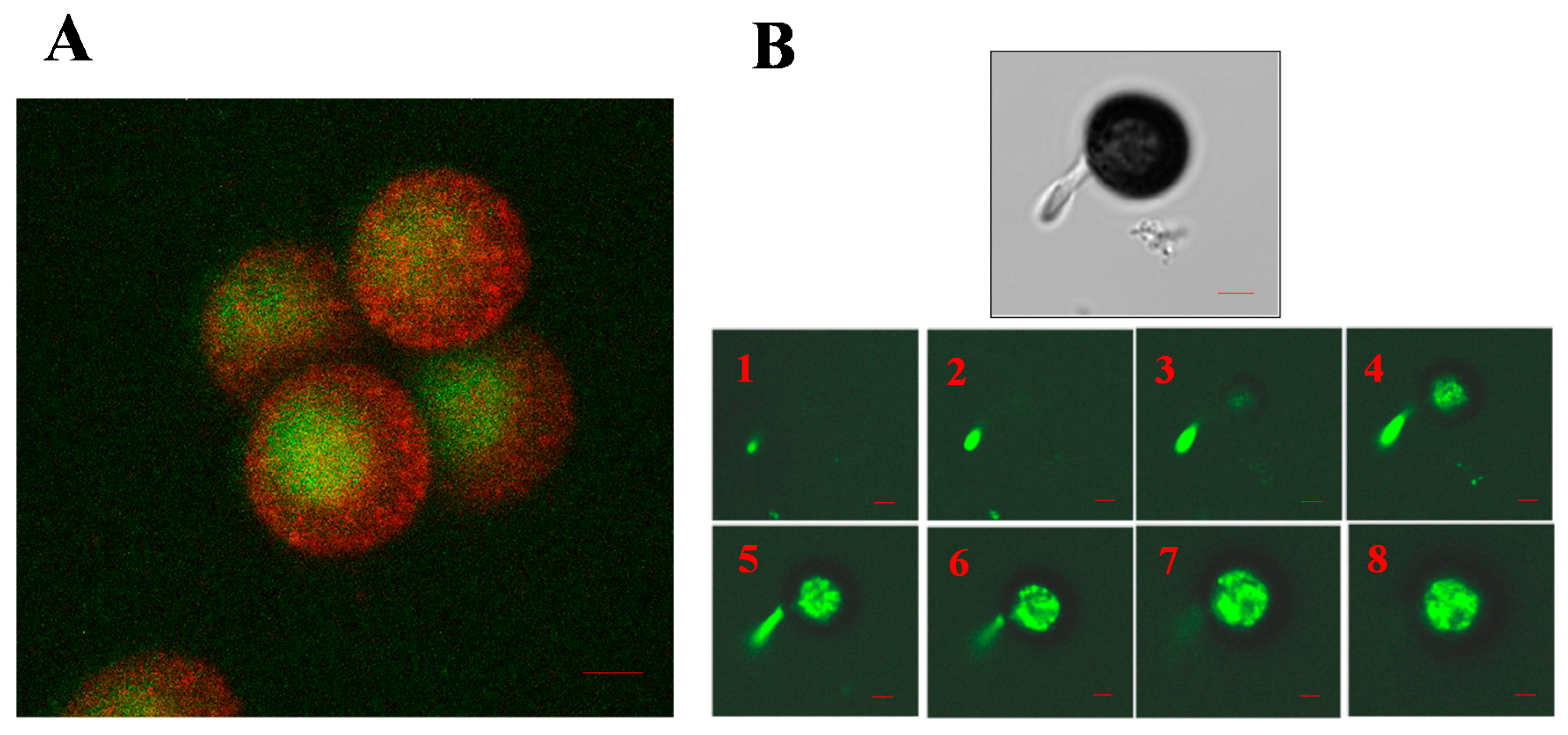
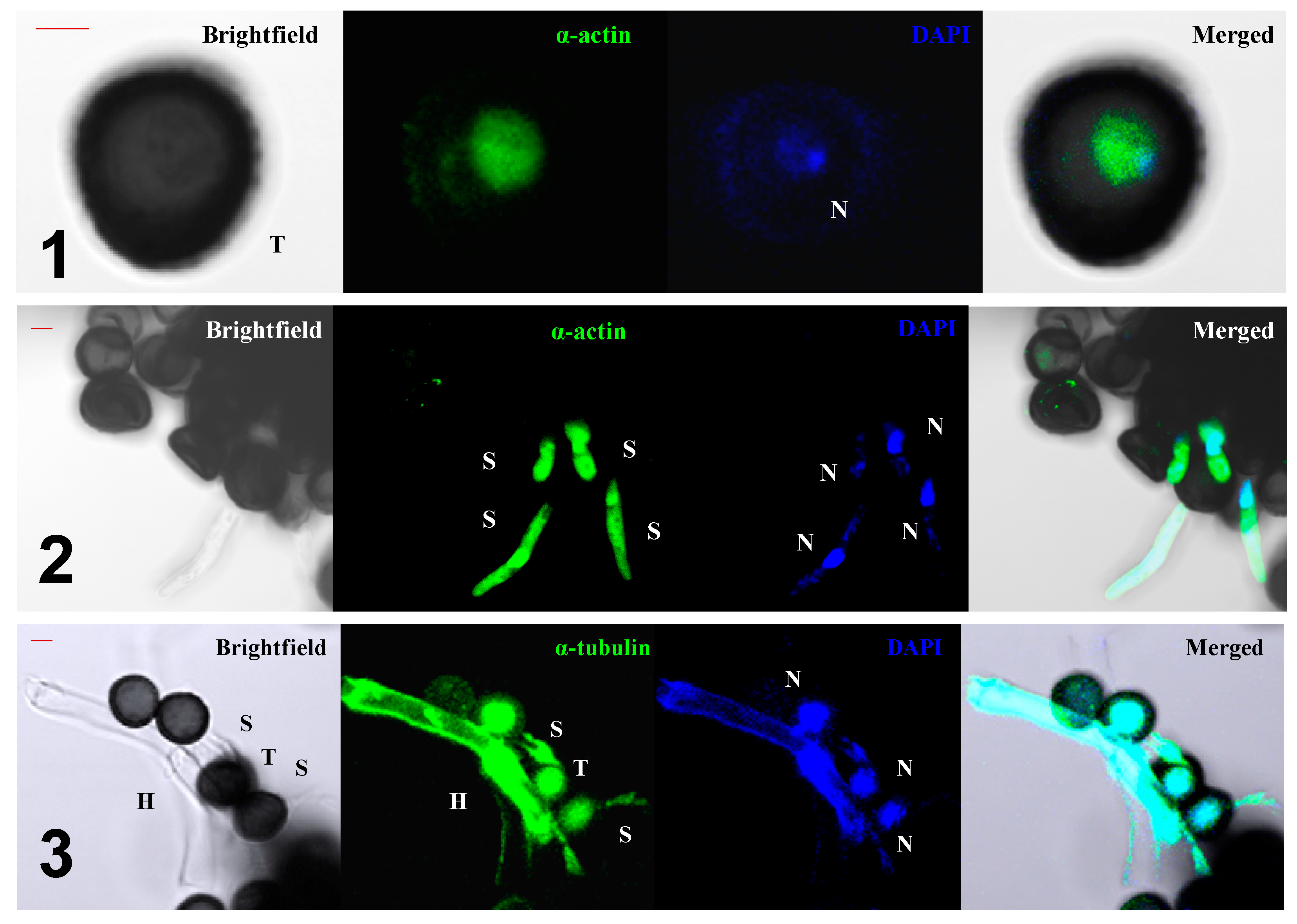
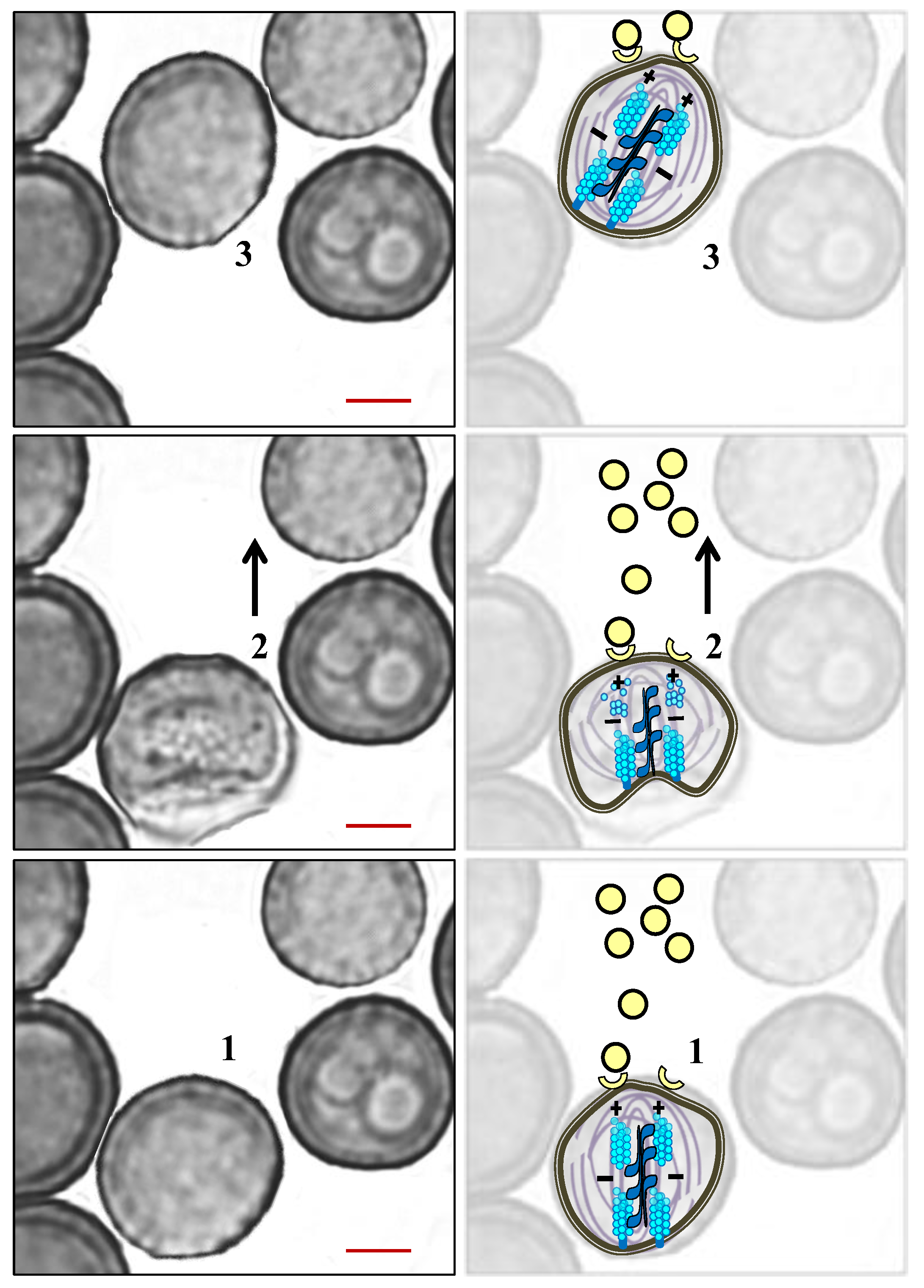
5. The Role of the Cytoskeleton in Teliospore Germination
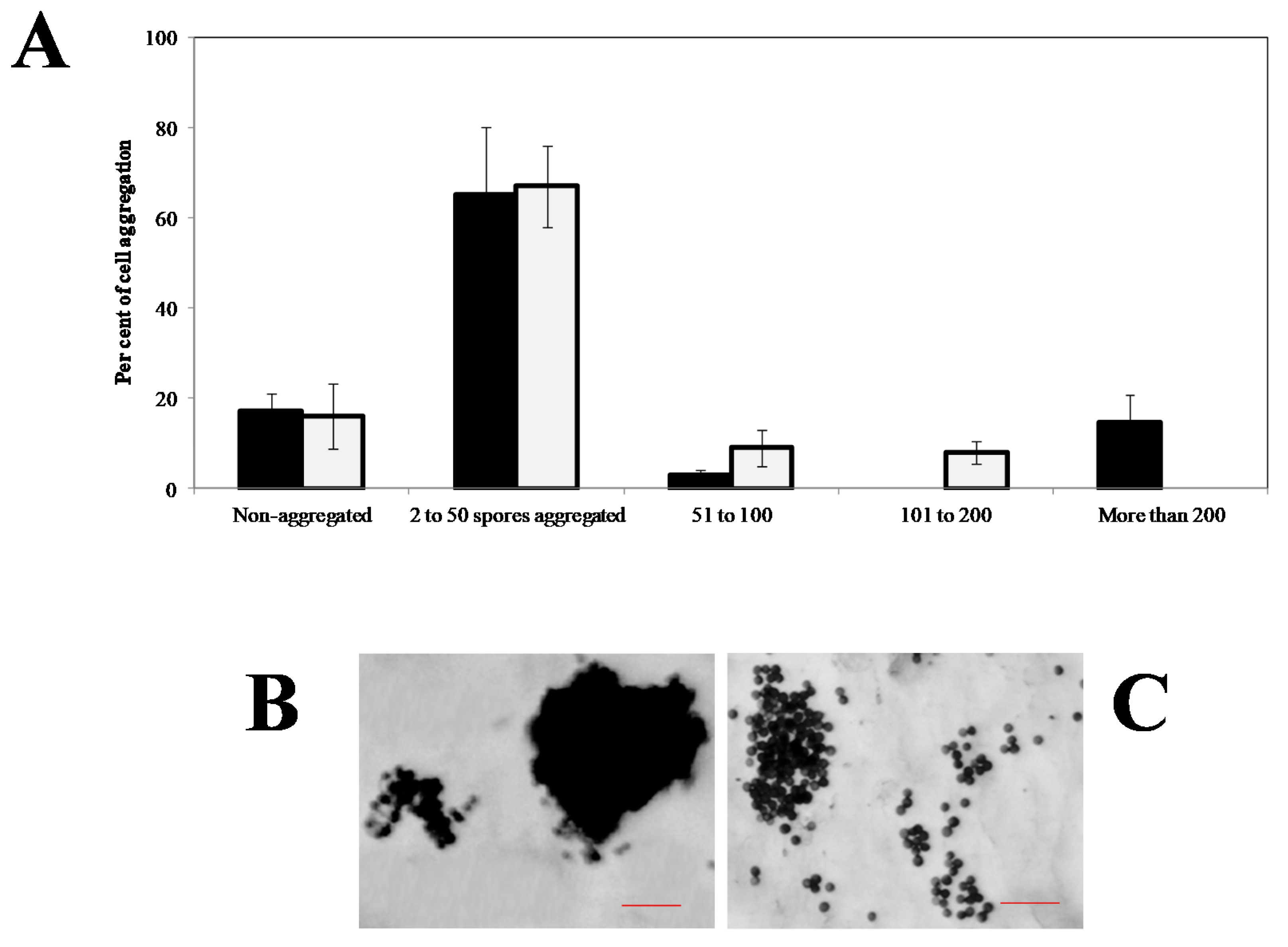
6. The Role of the Cytoskeleton in Teliospore Motility
7. The GTPases as Mediators of the Signal of Organization of the Cytoskeleton
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- La O-Hechavarría, M.; Zardón-Navarro, M.A.; Arencibia-Rodríguez, A.; Rodríguez-Lema, E.; Acevedo-Rojas, R.; Mesa-López, J.M. Técnicas para el estudio de la interacción caña de azúcar-Sporisorium scitamineum. Agron. Mesoamer. 2011, 22, 157–165. [Google Scholar] [CrossRef][Green Version]
- Magarey, R.; Croft, B.; Braithwaite, K.; James, A. A smut incursion in the major Eastern-Australian sugarcane production area. In Technologies to Improve Sugar Productivity in Developing Countries; Li, Y.R., Solomon, S., Eds.; Agriculture Press: Beijing, China, 2006; pp. 333–336. [Google Scholar]
- Boller, T.; He, S.Y. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, B.J.; Innes, R.W. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat. Immunol. 2006, 7, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.; Zhang, Z.; Saalbach, G.; Thordal-Christensen, H. A proteomics study of barley powdery mildew haustoria. Proteomics 2009, 9, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.C.; Ramakrishnan, K. Infection of the bud, establishment in the host and production of whips in sugarcane smut (Ustilago scitaminea) of sugarcane. Proc. Intern. Soc. Sugarcane Technol. 1980, 17, 1452–1455. [Google Scholar]
- Snetselaar, K.M.; Mims, C.W. Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia 1992, 84, 193–203. [Google Scholar] [CrossRef]
- Santiago, R.; Alarcón, B.; de Armas, R.; Vicente, C.; Legaz, M.E. Changes in cinnamylalcohol dehydrogenases activities from sugarcane cultivars inoculated with Sporisorium scitamineum sporidia. Physiol. Plant. 2012, 145, 245–259. [Google Scholar] [CrossRef]
- Legaz, M.E.; Millanes, A.M.; Fontaniella, B.; Piñon, D.; de Armas, R.; Rodríguez, C.W.; Solas, M.T.; Vicente, C. Ultrastructural alterations of sugarcane leaves caused by common sugarcane pathogens. Belg. J. Bot. 2006, 139, 79–91. [Google Scholar]
- Alexopoulos, C.J.; Mims, C.W.; Blackwell, M. Characteristics of fungi. In Introductory Mycology, 4th ed.; Alexopoulos, C.J., Mims, C.W., Blackwell, M., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 1996; pp. 25–56. [Google Scholar]
- Webster, J. Ustilaginomycetes: Smut fungi and their allies. In Introduction to Fungi; Webster, J., Weber, R.W.S., Eds.; Cambridge University Press: New York, NY, USA, 1989; pp. 63–657. [Google Scholar]
- Martínez-Espinoza, A.D.; García-Pedrajas, M.D.; Gold, S.E. The Ustilaginales as plant pests and model systems. Fungal Gen. Biol. 2002, 35, 1–20. [Google Scholar] [CrossRef]
- Kurihara, L.J.; Beh, C.T.; Latterich, M.; Schekman, R.; Rose, M.D. Nuclear congression and membrane fusion: Two distinct events in the yeast karyogamy pathway. J. Cell Biol. 1994, 126, 911–923. [Google Scholar] [CrossRef]
- Banuett, F.; Herskowitz, I. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 1996, 122, 2965–2976. [Google Scholar] [PubMed]
- Waller, J.M. Sugarcane smut (Ustilago scitaminea) in Kenya. II Infection and resistance. Trans. British Mycol. Soc. 1970, 54, 405–414. [Google Scholar] [CrossRef]
- Villajuana-Bonequi, M.; Matei, A.; Ernst, C.; Hallab, A.; Usadel, B.; Doehlemann, G. Cell type specific transcriptional reprogramming of maize leaves during Ustilago maydis induced tumor formation. Sci. Rep. 2019, 9, 1–15. [Google Scholar]
- Que, Y.; Su, Y.; Guo, J.; Wu, Q.; Xu, L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. PLoS ONE 2014, 9, e106476. [Google Scholar] [CrossRef] [PubMed]
- Galloway, W.R.J.D.; James, T.; Hodgkinson, J.T.; Bowden, S.; Welch, M.; Spring, D.R. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012, 20, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Khmel, I.A. Quorum-sensing regulation of gene expression: Fundamental and applied aspects and the role in bacterial communication. Mikrobiologiia 2006, 75, 457–464. [Google Scholar] [CrossRef]
- Moleleki, L.N.; Pretorius, R.G.; Tanui, C.K.; Mosina, G.; Theron, J. A quorum sensing-defective mutant of Pectobacterium carotovorum ssp. brasiliense 1692 is attenuated in virulence and unable to occlude xylem tissue of susceptible potato plant stems. Mol. Plant Pathol. 2017, 18, 32–44. [Google Scholar] [CrossRef]
- Hogan, D.A. Talking to themselves: Autoregulation and quorum sensing in fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar] [CrossRef]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal Quorum-Sensing Molecules and Inhibitors with Potential Antifungal Activity: A Review. Molecules 2019, 24, 1950. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Sahu, P. Perspective of Quorum Sensing Mechanism in Candida albicans. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Bramhachari, P.V., Ed.; Springer: Singapore, 2018. [Google Scholar]
- Macko, V.; Staples, R.C.; Gershon, H.; Renwick, J.A. Self-inhibitor of bean rust uredospores: Methyl 3,4-dimethoxycinnamate. Science 1970, 170, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, B.T.; Lingappa, Y. Role of auto-inhibitors on mycelial growth and dimorphism of Glomerella cingulata. J. Gen. Microbiol. 1969, 56, 35–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yajima, A. Recent progress in the chemistry and chemical biology of microbial signaling molecules: Quorum-sensing pheromones and microbial hormones. Tetrahedron Lett. 2014, 55, 2773–2780. [Google Scholar] [CrossRef]
- Vitale, S.; Di Pietro, A.; Turrà, D. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat. Microbiol. 2019, 4, 1443–1449. [Google Scholar] [CrossRef]
- Sánchez-Elordi, E.; Morales de los Ríos, L.; Vicente, C.; Legaz, M.E. Sugar cane arginase competes with the same fungal enzyme as a false quorum signal against smut teliospores. Phytochem. Lett. 2015, 14, 115–122. [Google Scholar] [CrossRef]
- Millanes, A.M.; Vicente, C.; Legaz, M.E. Sugarcane glycoproteins bind to surface, specific ligands and modify cytoskeleton arrangement of Ustilago scitaminea teliospores. J. Plant Interact. 2008, 3, 95–110. [Google Scholar] [CrossRef]
- Sanchez-Elordi, E.; de los Rios, L.M.; Diaz, E.M.; Avila, A.; Legaz, M.E.; Vicente, C. Defensive glycoproteins from sugar cane plants induced chemotaxis, cytoagglutination and death of smut teliospores. J. Plant Pathol. 2016, 98, 493–501. [Google Scholar]
- Speth, E.B.; Lee, Y.N.; He, S.Y. Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr. Opin. Plant Biol. 2007, 10, 580–586. [Google Scholar] [CrossRef]
- Soyer, J.L.; El Ghalid, M.; Glaser, N.; Ollivier, B.; Linglin, J.; Grandaubert, J.; Balesdent, M.H.; Connolly, L.R.; Freitag, M.; Rouxel, T.; et al. Epigenetic control of effector gene expression in the plant pathogenic fungus Leptosphaeria maculans. PLoS Gen. 2014, 10, e1004227. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Xu, L.; Wu, Q.; Liu, Y.; Ling, H.; Liu, Y.; Zhang, Y.; Guo, J.; Su, Y.; Chen, J.; et al. Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genom. 2014, 15, 996. [Google Scholar] [CrossRef] [PubMed]
- de Armas, R.; Santiago, R.; Legaz, M.E.; Vicente, C. Levels of phenolic compounds and enzyme activity can be used to screen for resistance of sugarcane to smut (Ustilago scitaminea). Austral. Plant Pathol. 2007, 36, 32–38. [Google Scholar] [CrossRef]
- Sánchez-Elordi, E.; Sterling, R.M.; Santiago, R.; de Armas, R.; Vicente, C.; Legaz, M.E. Increase in cytotoxic lignans production after smut infection in sugar cane plants. J. Plant Physiol. 2020, 244, 153087. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Elordi, E.; Contreras, R.; de Armas, R.; Benito, M.C.; Alarcón, B.; de Oliveira, E.; Del Mazo, C.; Díaz-Peña, E.M.; Santiago, R.; Vicente, C.; et al. Differential expression of SofDIR16 and SofCAD genes in smut resistant and susceptible sugarcane cultivars in response to Sporisorium scitamineum. J. Plant Physiol. 2018, 226, 103–113. [Google Scholar] [CrossRef]
- Barnabas, L.; Ashwin, N.M.R.; Kaverinathan, K.; Trentin, A.R.; Pivato, M.; Sundar, A.R.; Malathi, P.; Viswanathan, R.; Carletti, P.; Arrigoni, G.; et al. In vitro secretomic analysis identifies putative pathogenicity-related proteins of Sporisorium scitamineum–The sugarcane smut fungus. Fungal Biol. 2017, 121, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Silva, N.S.; Schaker, P.D.C.; Rody, H.V.S.; Maia, T.; Garner, C.M.; Gassmann, W.; Monteiro-Vitorello, C.B. Leaping into the Unknown World of Sporisorium scitamineum Candidate Effectors. J. Fungi 2020, 6, 339. [Google Scholar] [CrossRef]
- Dutheil, J.Y.; Mannhaupt, G.; Schweizer, G.; Sieber, C.M.K.; Münsterkötter, M.; Güldener, U.; Schirawski, J.; Kahmann, R. A Tale of genome compartmentalization: The evolution of virulence clusters in smut fungi. Genome Biol. Evol. 2016, 8, 681–704. [Google Scholar] [CrossRef]
- Su, Y.; Xu, L.; Wang, S.; Wang, Z.; Yang, Y.; Chen, Y.; Que, Y. Identification, phylogeny, and transcript of chitinase family genes in sugarcane. Nat. Sci. Rep. 2015, 5, 10708. [Google Scholar] [CrossRef]
- Su, Y.; Guo, J.; Ling, H.; Chen, S.; Wang, S.; Xu, L.; Allan, A.C.; Que, Y. Isolation of a novel peroxisomal catalase gene from Sugarcane, which is responsive to biotic and abiotic stresses. PLoS ONE 2014, 9, e84426. [Google Scholar] [CrossRef]
- Sánchez-Elordi, E.; de Los Ríos, L.M.; Vicente, C.; Legaz, M.E. Polyamines levels increase in smut teliospores after contact with sugarcane glycoproteins as a plant defensive mechanism. J. Plant Res. 2019, 132, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Santiago, L.; Guzmán-de-Peña, D.; Ruiz-Herrera, J. Life without putrescine: Disruption of the gene-encoding polyamine oxidase in Ustilago maydis odc mutants. FEMS Yeast Res. 2010, 10, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Olvera, L.; Xoconostle-Cázares, B.; Ruiz-Herrera, J. Cloning and disruption of the ornithine decarboxylase gene of Ustilago maydis: Evidence for a role of polyamines in its dimorphic transition. Microbiology 1997, 143, 2237–2245. [Google Scholar] [CrossRef]
- Chang, C.; Cai, E.; Deng, Y.Z.; Mei, D.; Qiu, S.; Chen, B.; Zhang, L.; Jiang, Z. cAMP/PKA signalling pathway regulates redox homeostasis essential for Sporisorium scitamineum mating/filamentation and virulence. Environ. Microbiol. 2019, 21, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Atanasov, K.E.; Arafaty, N.; Murillo, E.; Tiburcio, A.F.; Zeier, J.; Alcázar, R. Putrescine elicits ROS-dependent activation of the salicylic acid pathway in Arabidopsis thaliana. Plant Cell Environ. 2020, 43, 2755–2768. [Google Scholar] [CrossRef]
- Sánchez-Elordi, E.; Baluška, F.; Echevarría, C.; Vicente, C.; Legaz, M.E. Defence sugarcane glycoproteins disorganize microtubules and prevent nuclear polarization and germination of Sporisorium scitamineum teliospores. J. Plant Physiol. 2016, 200, 111–123. [Google Scholar] [CrossRef]
- Sánchez-Elordi, E.; Baluska, F.; Vicente, C.; Legaz, M.E. Sugarcane glycoproteins control dynamics of cytoskeleton during teliospore germination of Sporisorium scitamineum. Mycol. Progr. 2019, 18, 1121–1134. [Google Scholar] [CrossRef]
- Fuchs, U.; Manns, I.; Steinber, G. Microtubules are dispensable for initial pathogenic development but required for long-distance hyphal growth in the corn smut Ustilago maydis. Mol. Biol. Cell 2005, 16, 2746–2758. [Google Scholar] [CrossRef]
- Wendland, J.; Philippsen, P. Determination of cell polarity in germinated spores and hyphal tips of the filamentous ascomycete Ashbya gossypii requires a rhoGAP homolog. J. Cell Sci. 2000, 113, 1611–1621. [Google Scholar]
- Kazami, S.; Usui, T.; Osada, H. Actin stress fiber retraction and aggresome formation is a common cellular response to actin toxins. Biosci. Biotechnol. Biochem. 2011, 75, 1853–1855. [Google Scholar] [CrossRef]
- Taxis, C.; Maeder, C.; Reber, S.; Rathfelder, N.; Miura, K.; Greger, K.; Stelzer, E.H.; Knop, M. Dynamic organization of the actin cytoskeleton during meiosis and spore formation in budding yeast. Traffic 2006, 7, 1628–1642. [Google Scholar] [CrossRef] [PubMed]
- Fontaniella, B.; Márquez, A.; Rodríguez, C.W.; Piñón, D.; Solas, M.T.; Vicente, C.; Legaz, M.E. A role for sugarcane glycoproteins in the resistance of sugarcane to Ustilago scitaminea. Plant Physiol. Biochem. 2002, 40, 881–889. [Google Scholar] [CrossRef]
- Bachewich, C.; Heath, I.B. Radial F-actin arrays precede new hypha formation in Saprolegnia: Implications for establishing polar growth and regulating tip morphogenesis. J. Cell Sci. 1998, 111, 2005–2016. [Google Scholar] [PubMed]
- Millanes, A.M.; Fontaniella, B.; Legaz, M.E.; Vicente, C. Glycoproteins from sugarcane plants regulate cell polarity of Ustilago scitaminea teliospores. J. Plant Physiol. 2005, 162, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987, 105, 1473–1478. [Google Scholar] [CrossRef]
- Kovacs, M.; Toth, J.; Hetenyi, C.; Malnasi-Csizmadia, A.; Sellers, J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004, 279, 35557–35563. [Google Scholar] [CrossRef]
- Vasquez, R.J.; Howell, B.; Yvon, A.M.C.; Wadsworth, P.; Cassimeris, L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 1997, 8, 973–985. [Google Scholar] [CrossRef]
- Snyder, M.; Gehrung, S.; Page, B.D. Studies concerning the temporal andgenetic control of cell polarity in Saccharomyces cerevisiae. J. Cell Biol. 1991, 11, 515–532. [Google Scholar] [CrossRef]
- Pillai, M.C.; Baldwin, J.D.; Cherr, G.N. Early development in an algalgametophyte: Role of the cytoskeleton in germination and nucleartranslocation. Protoplasma 1992, 170, 34–45. [Google Scholar] [CrossRef]
- Åström, H.; Sorri, O.; Raudaskoski, M. Role of microtubules in the movementof the vegetative nucleus and generative cell in tobacco pollen tubes. Sex. Plant Reprod. 1995, 8, 61–69. [Google Scholar] [CrossRef]
- Brand, A.; Gow, N.A.R. Tropic orientation responses of pathogenic fungi. In Morphogenesis and Pathogenicity in Fungi; Pérez-Martin, J., Di Pietro, A., Eds.; Springer: Berlin, Germany, 2012; pp. 21–41. [Google Scholar]
- Sánchez-Elordi, E.; Vicente-Manzanares, M.; Díaz, E.; Legaz, M.E.; Vicente, C. Plant–pathogen interactions: Sugarcane glycoproteins induce chemotaxis of smut teliospores by cyclic contraction and relaxation of the cytoskeleton. S. Afr. J. Bot. 2016, 105, 66–78. [Google Scholar] [CrossRef]
- Yao, J.; Allen, C. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J. Bacteriol. 2006, 188, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Schwarzerová, K.; Vondráková, Z.; Fischer, L.; Boríková, P.; Bellinvia, E.; Eliásová, K.; Havelková, L.; Fiserová, J.; Vágner, M.; Opatrný, Z. The role of actin isoforms in somatic embryogenesis in Norway spruce. BMC Plant Biol. 2010, 10, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Sánchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 2004, 4, 110–122. [Google Scholar] [CrossRef]
- Raftopoulou, M.; Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef]
- Caron, E. Rac signalling: A radical view. Nat. Cell Biol. 2003, 5, 185–187. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Strange, P.G. Use of the GTPγS ([35S]GTPγS and Eu-GTPγS) binding assay for analysis of ligand potency and efficacy at G protein-coupled receptors. Br. J. Pharmacol. 2010, 161, 1238–1249. [Google Scholar] [CrossRef]
- Peterson, D.A.; Peterson, D.C.; Reeve, H.L.; Archer, S.L.; Weir, E.K. GTP (γS) and GDP (βS) as electron donors: New wine in old bottles. Life Sci. 1999, 65, 1135–1140. [Google Scholar] [CrossRef]
- Delley, P.A.; Hall, M.N. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 1999, 147, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Choi, C.K.; Shin, E.Y.; Schwartz, M.A.; Kim, E.G. Myosin II directly binds and inhibits dbl family guanine nucleotide exchange factors: A possible link to rho family GTPases. J. Cell Biol. 2010, 190, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Even-Ram, S.; Doyle, A.D.; Conti, M.A.; Matsumoto, K.; Adelstein, R.S.; Yamada, K.M. Myosin IIA regulates cell motility and actomyosin microtubule crosstalk. Nat. Cell Biol. 2007, 9, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, F.N.; Van Delft, S.; Kain, H.E.; Van Der Kammen, R.A.; Collard, J.G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999, 1, 242–248. [Google Scholar] [CrossRef]
- Bosgraaf, L.; van Haastert, P.J. The regulation of myosin II in Dictyostelium. Eur. J. Cell Biol. 2006, 85, 969–979. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Koach, M.A.; Whitmore, L.; Lamers, M.L.; Horwitz, A.F. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 2008, 183, 543–554. [Google Scholar] [CrossRef]
- Choi, C.K.; Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.A.; Mogilner, A.; Horwitz, A.R. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008, 10, 1039–1050. [Google Scholar] [CrossRef]
- ToliT-Nørrelykke, I.M. Push-me-pull-you: How microtubules organize the cell interior. Eur. Biophys. J. 2008, 37, 1271–1278. [Google Scholar] [CrossRef]
- Waterman-Storer, C.M.; Salmon, E.D. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr. Opin. Cell Biol. 1999, 11, 61–67. [Google Scholar] [CrossRef]
- Rodriguez, O.C.; Schaefer, A.W.; Mandato, C.A.; Forscher, P.; Bement, W.M.; Waterman-Storer, C.M. Conserved microtubule–actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 2003, 5, 599–609. [Google Scholar] [CrossRef]
- Murata, T.; Sano, T.; Sasabe, M.; Shigenori Nonaka, S.; Higashiyama, T.; Hasezawa, S.; Machida, Y.; Hasebe, M. Mechanism of microtubule array expansion inthe cytokinetic phragmoplast. Nat. Commun. 2013, 4, 1967. [Google Scholar] [CrossRef] [PubMed]
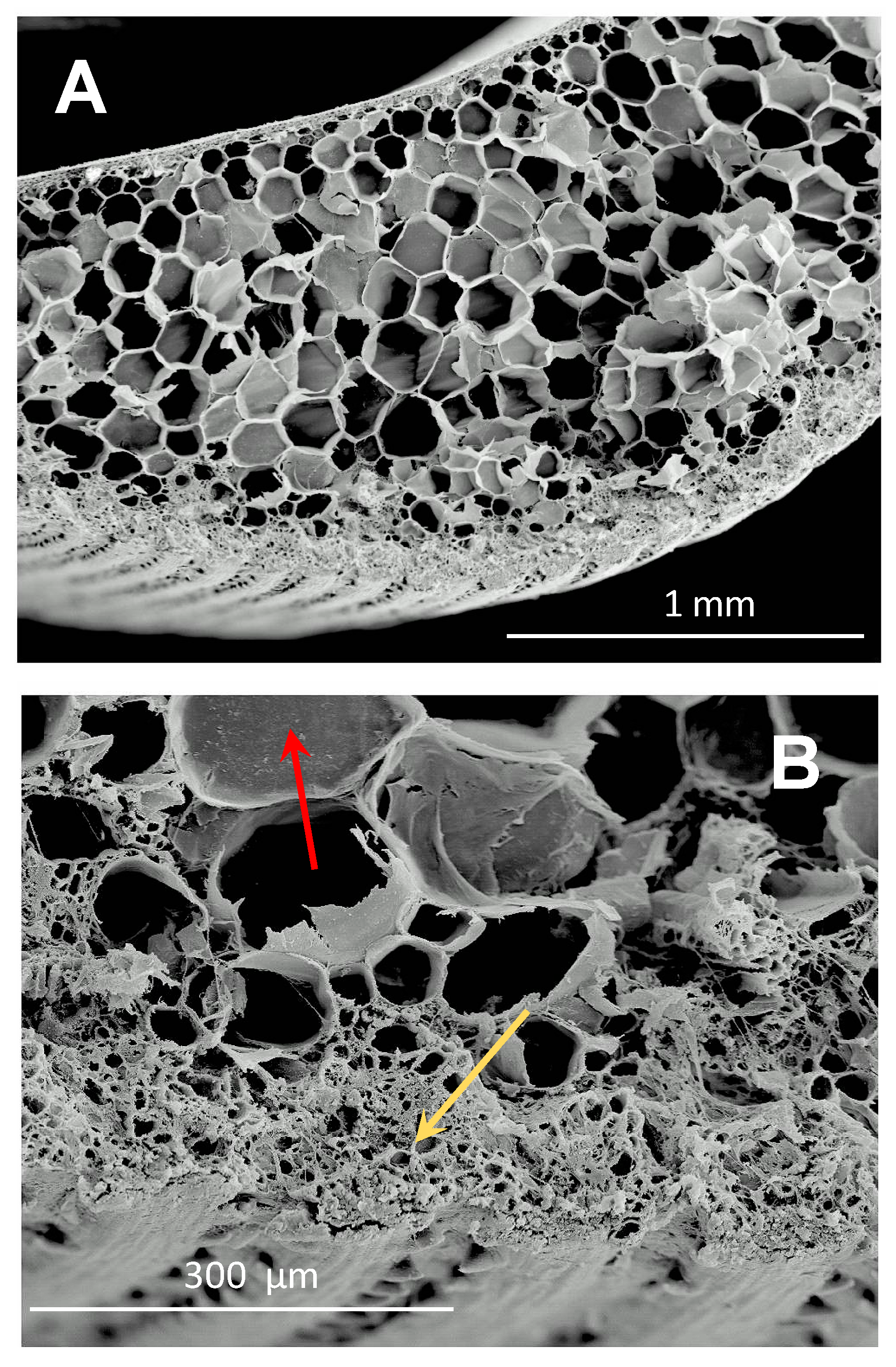

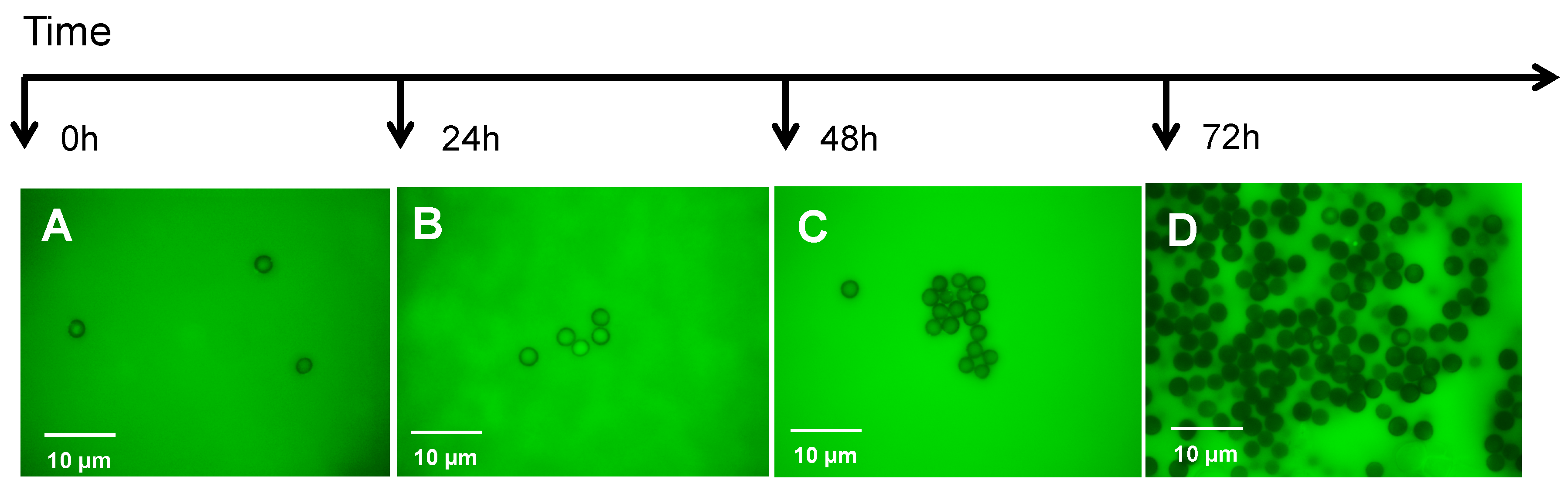
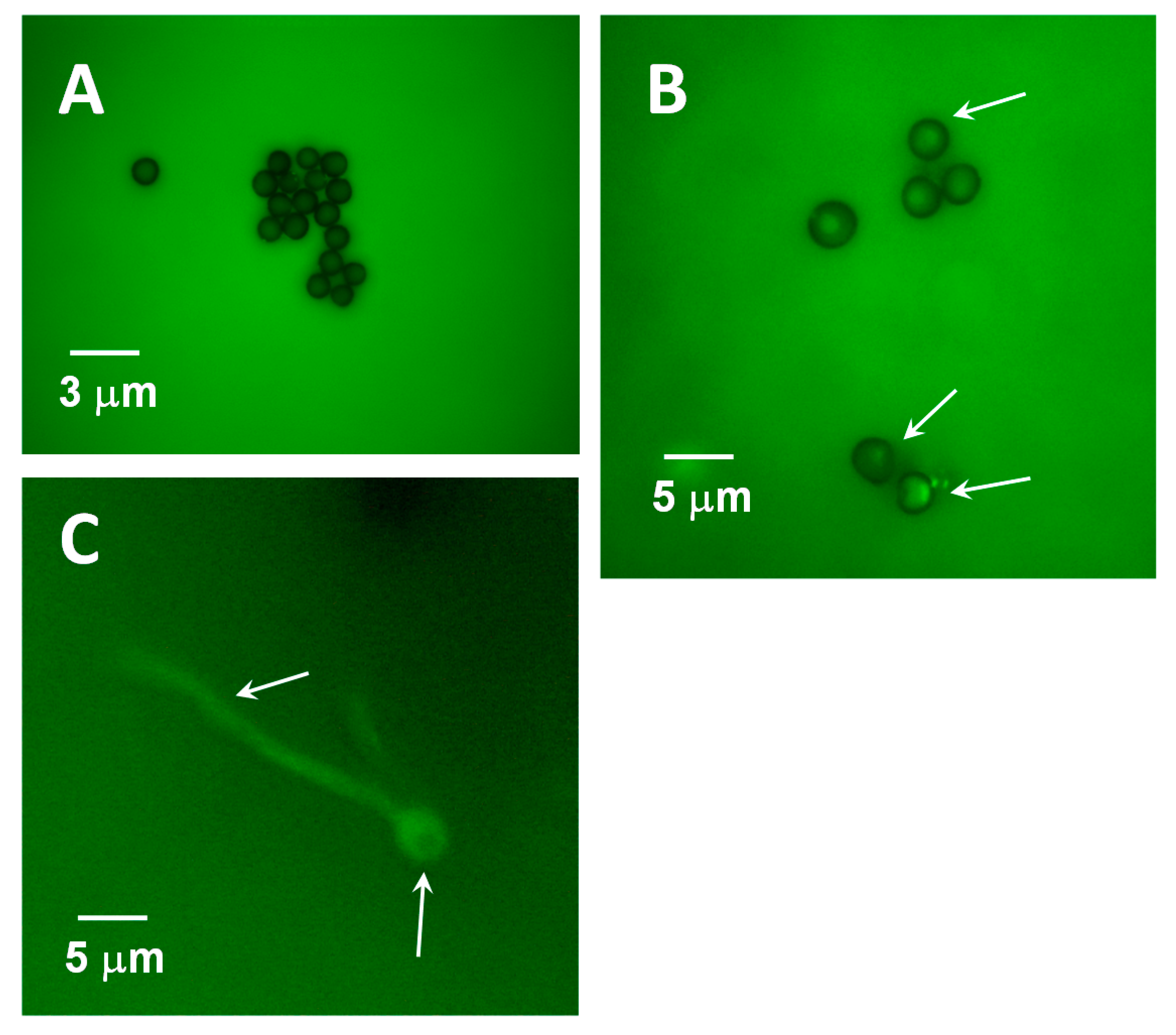

 : myosin II,
: myosin II,  : actin filaments.
: actin filaments.
 : myosin II,
: myosin II,  : actin filaments.
: actin filaments.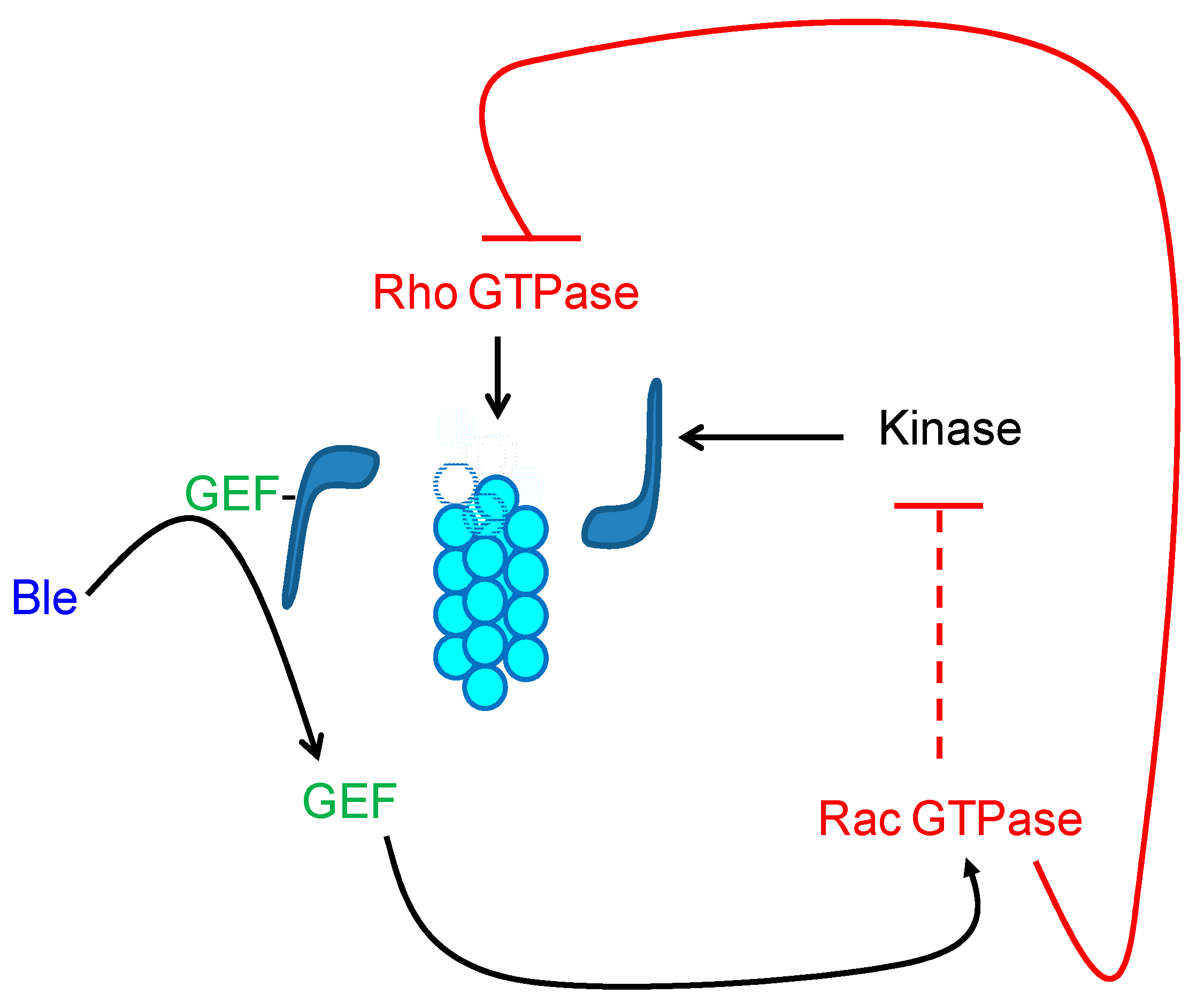
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, C.; Legaz, M.-E.; Sánchez-Elordi, E. Physiological Basis of Smut Infectivity in the Early Stages of Sugar Cane Colonization. J. Fungi 2021, 7, 44. https://doi.org/10.3390/jof7010044
Vicente C, Legaz M-E, Sánchez-Elordi E. Physiological Basis of Smut Infectivity in the Early Stages of Sugar Cane Colonization. Journal of Fungi. 2021; 7(1):44. https://doi.org/10.3390/jof7010044
Chicago/Turabian StyleVicente, Carlos, María-Estrella Legaz, and Elena Sánchez-Elordi. 2021. "Physiological Basis of Smut Infectivity in the Early Stages of Sugar Cane Colonization" Journal of Fungi 7, no. 1: 44. https://doi.org/10.3390/jof7010044
APA StyleVicente, C., Legaz, M.-E., & Sánchez-Elordi, E. (2021). Physiological Basis of Smut Infectivity in the Early Stages of Sugar Cane Colonization. Journal of Fungi, 7(1), 44. https://doi.org/10.3390/jof7010044





