Prediction of Conserved Peptides of Paracoccidioides for Interferon-γ Release Assay: The First Step in the Development of a Lab-Based Approach for Immunological Assessment during Antifungal Therapy
Abstract
1. Introduction
2. Material and Methods
2.1. Exploration of Candidate Proteins
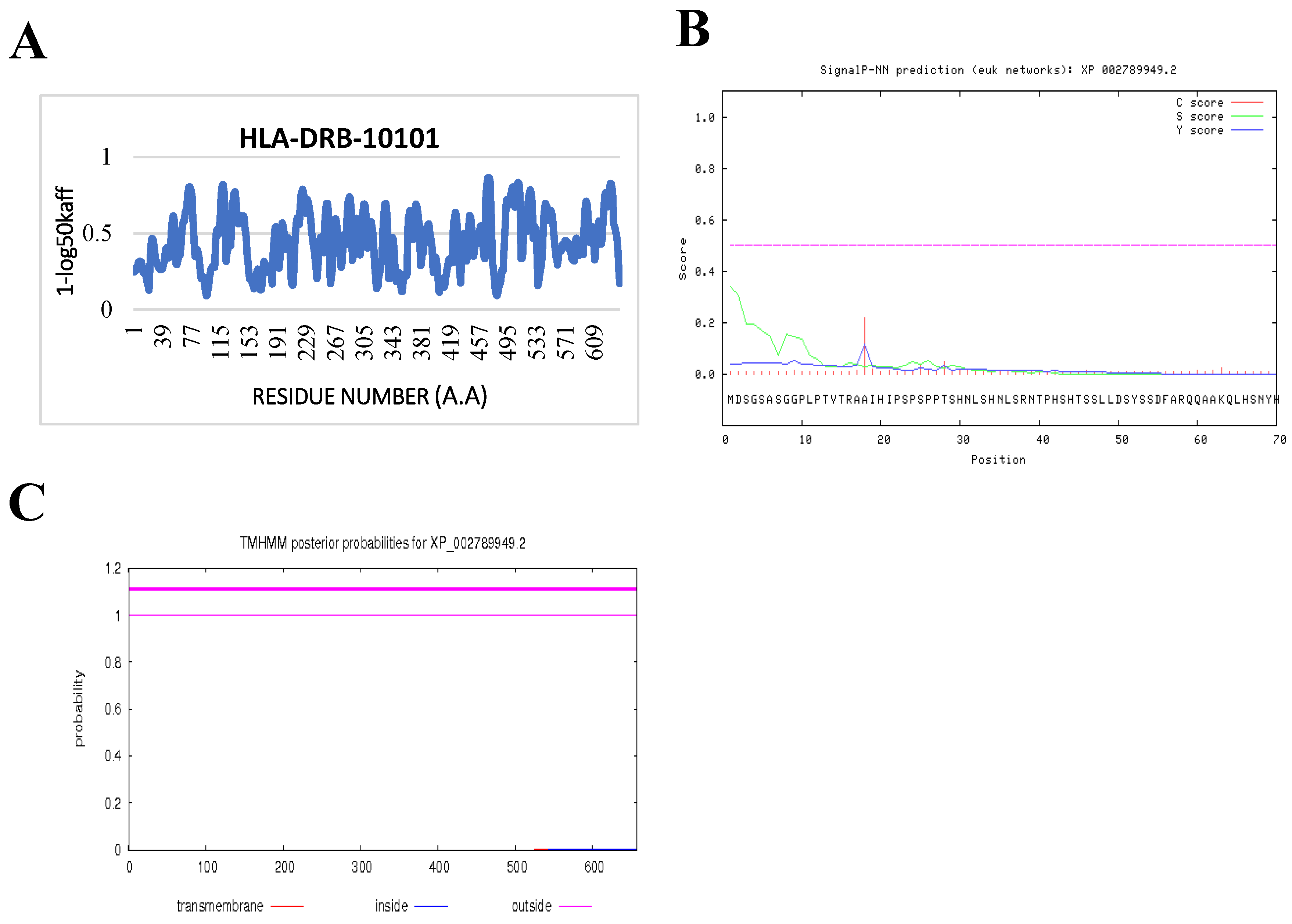
2.2. Bioinformatics Prediction Programs
2.3. Investigation of Amino Acid Sequences
2.4. Alignment of the Amino Acid Sequences of P. brasiliensis and P. lutzii
2.5. Identification of Conserved Peptides from Protein Sequences of P. brasiliensis and P. lutzii
2.6. Prediction of Antigenic and Immunogenic T Cell Epitope
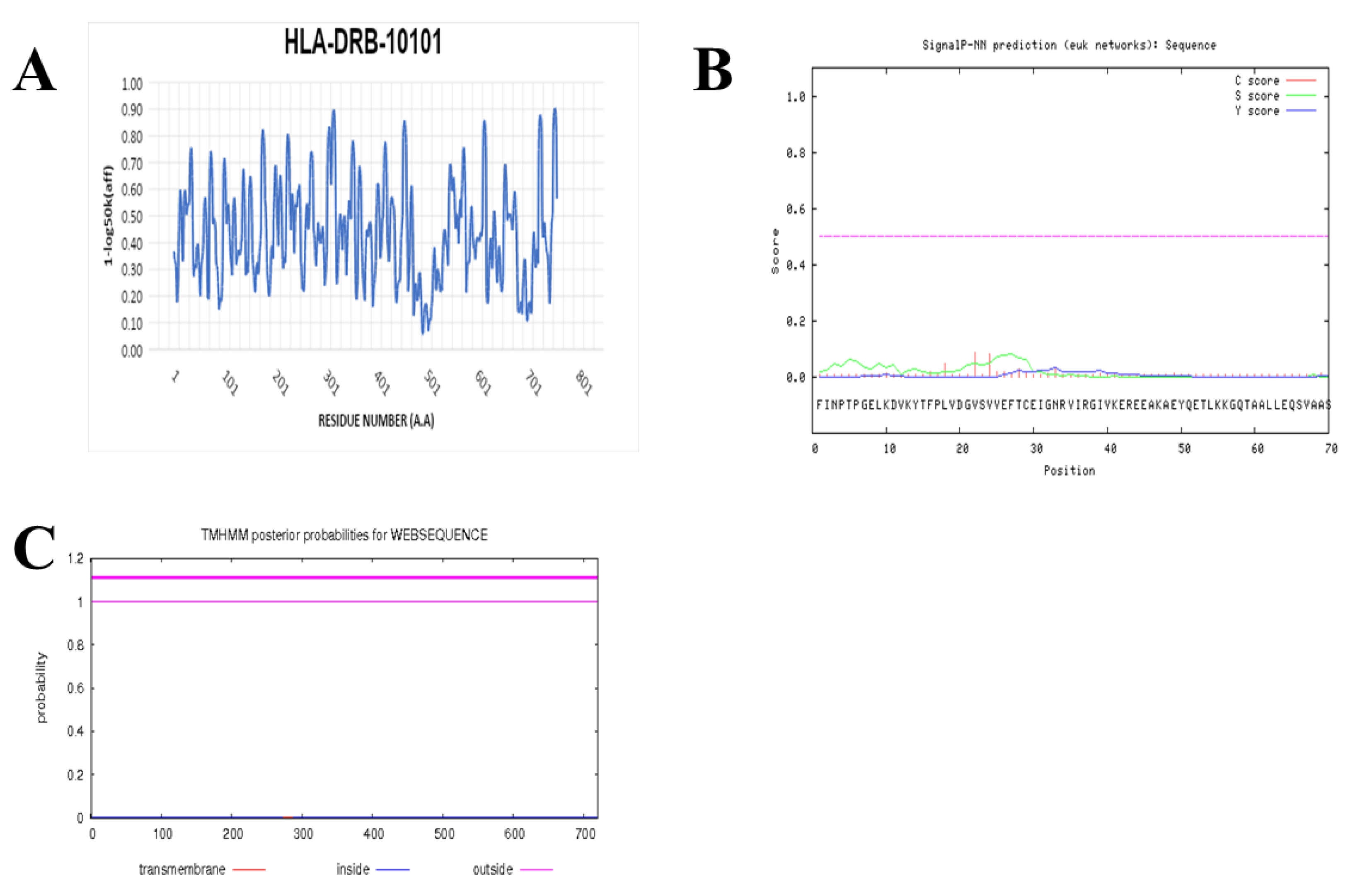
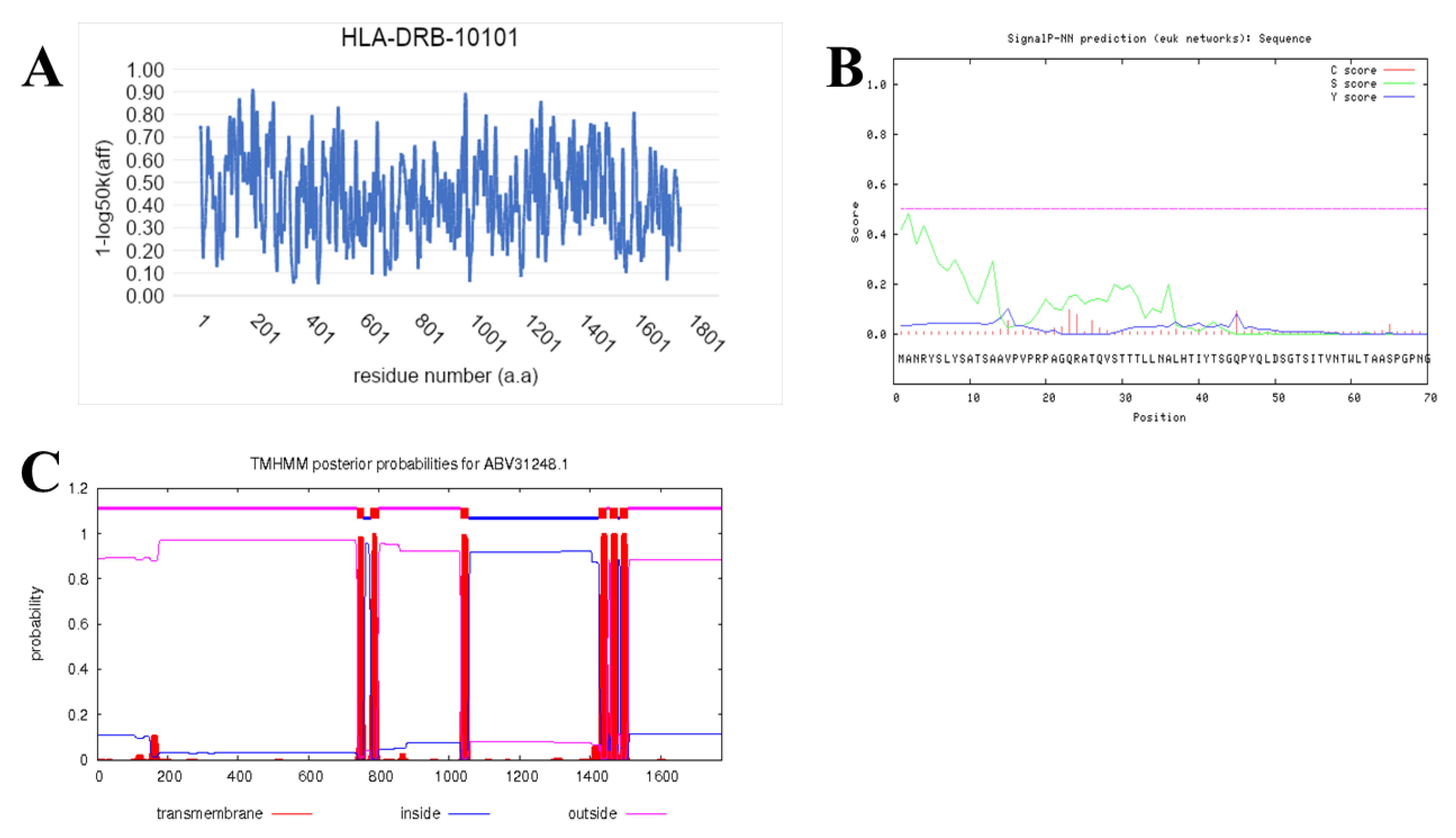
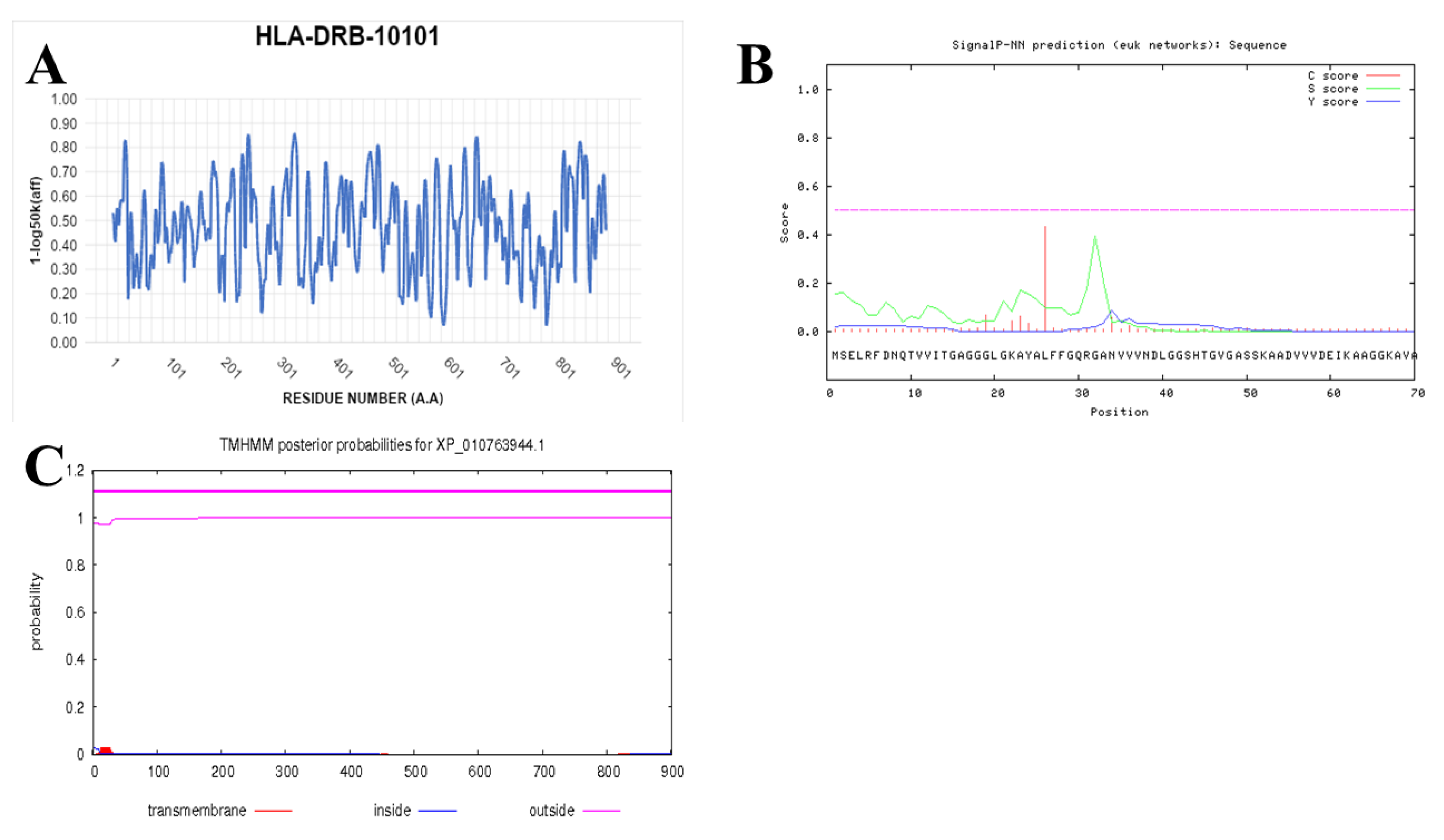
2.7. Prediction of IFN-γ-inducing MHC Class II Binders
2.8. Validation Screening
3. Results
3.1. Selected Articles and Protein Inclusion Criteria
3.2. Analysis of Proteins Based on Their Conserved Domain Database
3.3. Similarity Analysis between the Proteins Selected for P. brasiliensis and P. lutzii
3.4. Prediction of IFN-γ inducing MHC Class II Binders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendes, R.P.; de Souza Cavalcante, R.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; de Fátima da Silva, J.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol J. 2017, 11, 224–282. [Google Scholar] [CrossRef]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef]
- De Melo Teixeira, M.; Theodoro, R.C.; de Oliveira, F.F.M.; Machado, G.C.; Hahn, R.C.; Bagagli, E.; San-Blas, G.; Soares Felipe, M.S. Paracoccidioides lutzii sp. nov.: Biological and clinical implications. Med. Mycol. 2014, 52, 19–28. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; de Queiroz Telles, F.; Kono, A.; Paniago, A.M.M.; Nathan, A.; do Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of Paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, Z.F.; da Silva, D.; Lazéra, M.; Petri, V.; de Oliveira, R.M.; Sabroza, P.C.; Wanke, B. Paracoccidioidomycosis mortality in Brazil (1980–1995) Mortalidade por paracoccidioidomicose no Brasil (1980–1995). Cad. Saúde Pública 2002, 18, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Carmo, J.P.M.; Dias-Melicio, L.A.; Calvi, S.A.; Peraçoli, M.T.S.; Soares, A.M.V.C. TNF-α activates human monocytes for Paracoccidioides brasiliensis killing by an H2O2-dependent mechanism. Med. Mycol. 2006, 44, 363–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Damme, P.A.; Bierenbroodspot, F.; Telgt, D.S.C.; Kwakman, J.M.; De Wilde, P.C.M.; Meis, J.F.G.M. A case of imported paracoccidioidomycosis: An awkward infection in the Netherlands. Med. Mycol. 2006, 44, 13–18. [Google Scholar] [CrossRef][Green Version]
- De Castro, L.F.; Ferreira, M.C.; da Silva, R.M.; de Souza Lima Blotta, M.H.; Longhi, L.N.A.; Mamoni, R.L. Characterization of the immune response in human paracoccidioidomycosis. J. Infect. 2013, 67, 470–485. [Google Scholar] [CrossRef]
- Venturini, J.; Cavalcante, R.S.; de Assis Golim, M.; Marchetti, C.M.; De Azevedo, P.Z.; Amorim, B.C.; De Arruda, M.S.P.; Mendes, R.P. Phenotypic and functional evaluations of peripheral blood monocytes from chronic-form paracoccidioidomycosis patients before and after treatment. BMC Infect. Dis. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Amorim, B.C.; Pereira-Latini, A.C.; de Assis Golim, M.; Ruiz Júnior, R.L.; Yoo, H.H.B.; de Arruda, M.S.P.; Tavares, A.H.; de Souza Cavalcante, R.; Mendes, R.P.; Pontillo, A.; et al. Enhanced expression of NLRP3 inflammasome components by monocytes of patients with pulmonary paracoccidioidomycosis is associated with smoking and intracellular hypoxemia. Microbes Infect. 2020, 22, 137–143. [Google Scholar] [CrossRef]
- Benard, G.; Romano, C.C.; Cacere, C.R.; Juvenale, M.; Mendes-Giannini, M.J.; Duarte, A.J. Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine 2001, 13, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Benard, G.; Hong, M.A.; Del Negro, G.M.; Batista, L.; Shikanai-Yasuda, M.A.; Duarte, A.J. Antigen-specific immunosuppression in paracoccidioidomycosis. Am. J. Trop. Med. Hyg. 1996, 54, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The global prevalence of latent tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef] [PubMed]
- Takwoingi, Y.; Whitworth, H.; Rees-Roberts, M.; Badhan, A.; Partlett, C.; Green, N.; Boakye, A.; Lambie, H.; Marongiu, L.; Jit, M.; et al. Interferon gamma release assays for diagnostic evaluation of active tuberculosis (IDEA): Test accuracy study and economic evaluation. Health Technol. Assess. 2019, 23, 1–152. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Vir, P.; Raghava, G.P.S. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Nielsen, H. From Sequence to Sorting: Prediction of Signal Peptides. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 1999. [Google Scholar]
- Reynolds, S.M.; Käll, L.; Riffle, M.E.; Bilmes, J.A.; Noble, W.S. Transmembrane topology and signal peptide prediction using dynamic Bayesian networks. PLoS Comput. Biol. 2008, 4. [Google Scholar] [CrossRef]
- Fleri, W.; Paul, S.; Dhanda, S.K.; Mahajan, S.; Xu, X.; Peters, B.; Sette, A. The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Front. Immunol. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Worning, P.; Lise, S.; Øller, L.; Lamberth, K.; Buus, S.; Brunak, S.; Lund, O.L.E. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003, 12, 1007–1017. [Google Scholar] [CrossRef]
- Ruhwald, M.; De Thurah, L.; Kuchaka, D.; Zaher, M.R.; Salman, A.M.; Abdel-Ghaffar, A.R.; Shoukry, F.A.; Michelsen, S.W.; Soborg, B.; Blauenfeldt, T.; et al. Introducing the ESAT-6 free IGRA, a companion diagnostic for TB vaccines based on ESAT-6. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alegre, A.C.; Oliveira, A.F.; Dos Reis Almeida, F.B.; Roque-Barreira, M.C.; Hanna, E.S. Recombinant paracoccin reproduces the biological properties of the native protein and induces protective Th1 immunity against Paracoccidioides brasiliensis infection. PLoS Negl. Trop. Dis. 2014, 4, e2788. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, C.C.; Fernandes, G.F.; Rodrigues, A.M.; Burger, E.; de Camargo, Z.P. Proteomic analysis of Paracoccidioides brasiliensis complex isolates: Correlation of the levels of differentially expressed proteins with in vivo virulence. PLoS ONE 2019, 14, e0218013. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.S.; de Sousa Lima, P.; Baeza, L.C.; Parente, A.F.A.; Melo Bailão, A.; Borges, C.L.; de Almeida Soares, C.M. Employing proteomic analysis to compare Paracoccidioides lutzii yeast and mycelium cell wall proteins. Biochim. Biophys. Acta. Proteins Proteom. 2017, 1865, 1304–1314. [Google Scholar] [CrossRef]
- Araújo, D.S.; Pereira, M.; Portis, I.G.; De Castro Moreira Dos Santos, A.; Fontes, W.; De Sousa, M.V.; Do Prado Assunção, L.; Baeza, L.C.; Bailão, A.M.; Ricart, C.A.O.; et al. Metabolic peculiarities of Paracoccidioides brasiliensis dimorphism as demonstrated by iTRAQ labeling proteomics. Front. Microbiol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baeza, L.C.; da Mata, F.R.; Pigosso, L.L.; Pereira, M.; de Souza, G.H.M.F.; Coelho, A.S.G.; de Almeida Soares, C.M. Differential Metabolism of a Two-Carbon Substrate by Members of the Paracoccidioides Genus. Front. Microbiol. 2017, 8, 2308. [Google Scholar] [CrossRef]
- Castilho, D.G.; Chaves, A.F.; Xander, P.; Zelanis, A.; Kitano, E.S.; Serrano, S.M.; Tashima, A.K.; Batista, W.L. Exploring potential virulence regulators in Paracoccidioides brasiliensis isolates of varying virulence through quantitative proteomics. J. Proteome Res. 2014, 13, 4259–4271. [Google Scholar] [CrossRef]
- Chaves, E.G.A.; Parente-Rocha, J.A.; Baeza, L.C.; Araujo, D.S.; Borges, C.L.; de Oliveira, M.A.P.; Soares, C.M.D.A. Proteomic Analysis of Paracoccidioides brasiliensis During Infection of Alveolar Macrophages Primed or Not by Interferon-Gamma. Front. Microbial. 2019, 10, 96. [Google Scholar] [CrossRef]
- De Curcio, J.S.; Silva, M.G.; Silva Bailão, M.G.; Báo, S.N.; Casaletti, L.; Bailão, A.M.; de Almeida Soares, C.M. Identification of membrane proteome of Paracoccidioides lutzii and its regulation by zinc. Future Sci. OA 2017, 3, FSO232. [Google Scholar] [CrossRef]
- Chaves, A.F.; Castilho, D.G.; Navarro, M.V.; Oliveira, A.K.; Serrano, S.M.; Tashima, A.K.; Batista, W.L. Phosphosite-specific regulation of the oxidative-stress response of Paracoccidioides brasiliensis: A shotgun phosphoproteomic analysis. Microbes Infect. 2017, 19, 34–46. [Google Scholar] [CrossRef]
- Desjardins, C.A.; Champion, M.D.; Holder, J.W.; Muszewska, A.; Goldberg, J.; Bailao, A.M.; Brigido, M.M.; da Silva Ferreira, M.E.; Garcia, A.M.; Grynberg, M.; et al. Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genet. 2011, 7, e1002345. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.R.; Oliveira, L.N.; Chaves, E.G.A.; Weber, S.S.; Bailao, A.M.; Parente-Rocha, J.A.; Baeza, L.C.; de Almeida Soares, C.M.; Borges, C.L. Characterization of extracellular proteins in members of the Paracoccidioides complex. Fungal Biol. 2018, 122, 738–751. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Lima, P.; Casaletti, L.; Bailão, A.M.; de Vasconcelos, A.T.; de Rocha Fernandes, G.; Soares, C.M. Transcriptional and proteomic responses to carbon starvation in Paracoccidioides. PLoS Negl. Trop. Dis. 2014, 8, e2855. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Farrer, R.A.; Desjardins, C.A.; Gallo, J.E.; Sykes, S.; Sakthikumar, S.; Misas, E.; Whiston, E.A.; Bagagli, E.; Soares, C.M.; et al. Genome Diversity, Recombination, and Virulence across the Major Lineages of Paracoccidioides. mSphere 2016, 1, e00213-16. [Google Scholar] [CrossRef]
- Oliveira, L.N.; Casaletti, L.; Báo, S.N.; Borges, C.L.; de Sousa Lima, P.; de Almeida Soares, C.M. Characterizing the nuclear proteome of Paracoccidioides spp. Fungal Biol. 2016, 120, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.F.; Bailão, A.M.; Borges, C.L.; Parente, J.A.; Magalhães, A.D.; Ricart, C.A.; Soares, C.M. Proteomic analysis reveals that iron availability alters the metabolic status of the pathogenic fungus Paracoccidioides brasiliensis. PLoS ONE 2011, 6, e22810. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.F.; de Rezende, T.C.; de Castro, K.P.; Bailão, A.M.; Parente, J.A.; Borges, C.L.; Silva, L.P.; Soares, C.M. A proteomic view of the response of Paracoccidioides yeast cells to zinc deprivation. Fungal Biol. 2013, 117, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Parente-Rocha, J.A.; Parente, A.F.; Baeza, L.C.; Bonfim, S.M.; Hernandez, O.; McEwen, J.G.; Bailão, A.M.; Taborda, C.P.; Borges, C.L.; Soares, C.M. Macrophage Interaction with Paracoccidioides brasiliensis Yeast Cells Modulates Fungal Metabolism and Generates a Response to Oxidative Stress. PLoS ONE 2015, 10, e0137619. [Google Scholar] [CrossRef]
- Lacerda Pigosso, L.; Parente, A.F.; Coelho, A.S.; Silva, L.P.; Borges, C.L.; Bailão, A.M.; Soares, C.M. Comparative proteomics in the genus Paracoccidioides. Fungal Genet. Biol. 2013, 60, 87–100. [Google Scholar] [CrossRef]
- Lacerda Pigosso, L.; Baeza, L.C.; Vieira Tomazett, M.; Batista Rodrigues Faleiro, M.; Brianezi Dignani de Moura, V.M.; Melo Bailao, A.; Borges, C.L.; Alves Parente Rocha, J.; Rocha Fernandes, G.; Gauthier, G.M.; et al. Paracoccidioides brasiliensis presents metabolic reprogramming and secretes a serine proteinase during murine infection. Virulence 2017, 8, 1417–1434. [Google Scholar] [CrossRef]
- Rezende, T.C.; Borges, C.L.; Magalhães, A.D.; de Sousa, M.V.; Ricart, C.A.; Bailão, A.M.; Soares, C.M. A quantitative view of the morphological phases of Paracoccidioides brasiliensis using proteomics. J. Proteomics 2011, 75, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, D.; Muñoz, J.F.; Lopez, Á.; Urán, M.; Herrera, J.; Borges, C.L.; Restrepo, Á.; Soares, C.M.; Taborda, C.P.; Almeida, A.J.; et al. Identification and Analysis of the Role of Superoxide Dismutases Isoforms in the Pathogenesis of Paracoccidioides spp. PLoS Negl. Trop. Dis. 2016, 10, e0004481. [Google Scholar] [CrossRef] [PubMed]
- Tashima, A.K.; Castilho, D.G.; Chaves, A.F.; Xander, P.; Zelanis, A.; Batista, W.L. Data in support of quantitative proteomics to identify potential virulence regulators in Paracoccidioides brasiliensis isolates. Data Brief. 2015, 5, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tomazett, M.V.; Baeza, L.C.; Paccez, J.D.; Parente-Rocha, J.A.; Ribeiro-Dias, F.; de Almeida Soares, C.M. Identification and characterization of Paracoccidioides lutzii proteins interacting with macrophages. Microbes Infect. 2019, 21, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Tristão, G.B.; do Prado Assunção, P.; dos Santos, L.P.A.; Borges, C.L.; Silva-Bailão, M.G.; Soares, C.M.; Cavallaro, G.; Bailão, A.M. Predicting copper-, iron-, and zinc-binding proteins in pathogenic species of the Paracoccidioides genus. Front. Microbiol. 2015, 5, 761. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.S.; Parente, A.F.; Borges, C.L.; Parente, J.A.; Bailão, A.M.; de Almeida Soares, C.M. Analysis of the secretomes of Paracoccidioides mycelia and yeast cells. PLoS ONE 2012, 7, e52470. [Google Scholar] [CrossRef]
- Parente-Rocha, J.A.; Tomazett, M.V.; Pigosso, L.L.; Bailao, A.M.; Ferreira de Souza, A.; Paccez, J.D.; Baeza, L.C.; Pereira, M.; Silva Bailao, M.G.; Borges, C.L.; et al. In vitro, ex vivo and in vivo models: A comparative analysis of Paracoccidioides spp. proteomic studies. Fungal Biol. 2018, 122, 505–513. [Google Scholar] [CrossRef]
- San-Blas, G.; San-Blas, F. Paracoccidioides brasileensis: Cell wall structure and virulence—A review. Mycopathologia 1977, 62, 77–86. [Google Scholar] [CrossRef]
- Seider, K.; Heyken, A.; Lüttich, A.; Miramón, P.; Hube, B. Interaction of pathogenic yeasts with phagocytes: Survival, persistence and escape. Curr. Opin. Microbiol. 2010, 13, 392–400. [Google Scholar] [CrossRef]
- Camacho, E.; Niño-Vega, G.A. Paracoccidioides Spp.: Virulence Factors and Immune-Evasion Strategies. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Bocca, A.L.; Silva, M.F.; Silva, C.L.; Cunha, F.Q.; Figueiredo, F. Macrophage expression of class II major histocompatibility complex gene products in Paracoccidioides brasiliensis-infected mice. Am. J. Trop. Med. Hyg. 1999, 61, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Rehill, J.; Moffitt, K.; Douglas, L.; Stuart Elborn, J.; Jones, A.; Lorraine Martin, S. Sputum trypsin-like protease activity relates to clinical outcome in cystic fibrosis. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tomazett, P.K.; da Silva Castro, N.; Lenzi, H.L.; de Almeida Soares, C.M.; Pereira, M. Response of Paracoccidioides brasiliensis Pb01 to stressor agents and cell wall osmoregulators. Fungal Biol. 2011, 115, 62–69. [Google Scholar] [CrossRef]
- Jabes, D.L.; de Freitas Oliveira, A.C.; Alencar, V.C.; Menegidio, F.B.; Reno, D.L.S.; Santos, D.S.; Barbosa, D.A.; Vilas Boas, R.O.; de Oliveira Rodrigues Cunha, R.L.; Rodrigues, T.; et al. Thioridazine inhibits gene expression control of the cell wall signaling pathway (CWI) in the human pathogenic fungus Paracoccidioides brasiliensis. Mol. Genet. Genomics 2016, 291, 1347–1362. [Google Scholar] [CrossRef]
- Souza, A.C.O.; Favali, C.; Soares, N.C.; Tavares, N.M.; Jerônimo, M.S.; Junior, P.H.V.; Marina, C.L.; Santos, C.; Brodskyn, C.; Bocca, A.L. New role of P. Brasiliensis α-glucan: Differentiation of non-conventional dendritic cells. Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Mou, Z.; Li, J.; Boussoffara, T.; Kishi, H.; Hamana, H.; Ezzati, P.; Hu, C.; Yi, W.; Liu, D.; Khadem, F.; et al. Identification of broadly conserved cross-species protective Leishmania antigen and its responding CD4+ T cells. Sci. Transl. Med. 2015, 7, 1–13. [Google Scholar] [CrossRef]
- Sonah, H.; Deshmukh, R.K.; Bélanger, R.R. Computational prediction of effector proteins in fungi: Opportunities and challenges. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Nielsen, H.; Von Heijne, G.; Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004, 340, 783–795. [Google Scholar] [CrossRef]
- Niu, L.N.; Fu, T.T.; Chen, M.L.; Dong, Y.Y.; Tu, J.C.; Wang, Z.H.; Wang, S.Q.; Zhao, X.; Hou, N.X.; Chen, Q.; et al. Prediction of T cell and B cell epitopes of the 22-, 47-, 56-, and 58-kDa proteins of Orientia tsutsugamushi. Asian Pac. J. Trop. Biomed. 2019, 9, 443–448. [Google Scholar] [CrossRef]
- Da Silva, T.A.; Roque-Barreira, M.C.; Casadevall, A.; Almeida, F. Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.R.; Freitas, K.O.; Silva, R.C.; Pires, S.P.C.; Pratte-Santos, R. Frequency of classes i and II HLA alleles in deceased donors of solid organs in Espirito Santo, Brazil. J. Bras. Patol. Med. Lab. 2017, 53, 298–304. [Google Scholar] [CrossRef]
- Boquett, J.A.; Bisso-Machado, R.; Zagonel-Oliveira, M.; Schüler-Faccini, L.; Fagundes, N.J.R. HLA diversity in Brazil. Hla 2019. [Google Scholar] [CrossRef] [PubMed]

| Study, Year (Reference) | Study of Methodology | Upregulated Proteins |
|---|---|---|
| Alegre et al. [23] 2014 | Genome | Glycosyl hydrolase. |
| Do Amaral et al. [24] 2019 | LC–MS/MS | Ligand RNA, fructose bisphosphate aldolase, nucleic acid ligand, phosphoglycerate kinase, perixosomal catalase. |
| Araújo et al. [25] 2017 | NanoUPLC–MS | Shock protein SSB1, glucan synthase. |
| Araújo et al. [26] 2019 | NanoUPLC–MS | Gp 43, pyruvate dehydrogenase, ATP-citrate synthase, succinyl CoA ligase, Shock protein (Hsp 90, HPs 70, Hps 88, Hps 30, Hsp 7, Hsp 70, Hsp 75), alcohol dehydrogenase, pyruvate dehydrogenase. Cap 20, progesterone ligand, regulatory myosin cdc 4, mitochondrial perodoxin PRX1, DNA ligand, shock protein 88, serine phosphatase, threonine, protein F beta acin, enolase, triosphosphate isomerase, carbonic anhydrase, vacuolar protease 4, perodoxin, acetamidase. |
| Baeza, et al. [27] 2017 | NanoUPLC–MS | GlucosaminE-6-phosphate-deaminase, phosphoacetilglucosamina mutase, isocitrate mutase, isocitrate liase, malate synthase, 3-hydroxybutyryl-CoA dehydrogenase, 3-Cetoacyl-CoA thiolase, Acyl-CoA dehydrogenase, Acyl-Coenzyme A oxidase, Enoyl-CoA hydratase, short-chain dehydrogenase, carnitine, adenosylomocysteinase, threonine dehydrogenase, methylcrotoyl-CoA carboxilase beta, methylmalonate semialdehyde dehydrogenase, catalase. |
| Castilho et al. [28] 2014 | LC–MS/MS | RAC, vacuolar protease, 26 S regulatory subunit, palmitoyl thioesterase, protein associated with a pathogenesis. Cap 20, ligante de progesterona, regulatory myosin cdc 4, mitochondrial perodoxin PRX1, DNA ligand, shock protein 88, phosphatase serine treonine, actin F protein beta, enolase, triophosphate isomerase, carbonic anhydrase, vacuolar protease 4, perodoxin, acetamidase. |
| Chaves et al. [29] 2019 | NanoUPLC–MS | Thioredoxin, superoxide dismutase (SOD Fe+, SOD Cu +), serina protease, mitochondrial acetonitate hydratase, histidin kinase, perixossomal hydratase, shock protein (Hsp 90, Hsp 88, Hsp 70, Hsp 30). |
| De Curcio et al. [30] 2017 | NanoUPLC–MS | Carrier ATP/ADP, Plasma ATPpase, YOP1 protein, phosphate mitochondrial, osmosensor protein, ologossacaryl transferase, alpha-1,2-mannosyltransferase KTR1, dolichol-phosphato aminotransferase, phosphoinositide phosphate, oxoisovalerate dehydrogenase beta, serine-3-dehydrogenase. |
| Chaves et al. [31] 2017 | NanoUPLC–MS | Carrier ATP/ADP, plasma ATPase, YOP1 protein, mitochondrial phosphate, osmosis protein, oligosaccaccharyl transferase, alpha-1.2-mannosyltransferase KTR1, dolichol-phosphate aminotransferase, phosphoinositide phosphate, oxoisovalerate dehydrogenase 3 |
| Desjardins et al. [32] 2011 | Genome | Chitin synthase, Shock protein (Hsp 60, Hsp, 88, Hsp 90, Hsp 7, Hsp 70), mycoserosic acid synthase, acyl transferase, Tioredoxina, perixosomal hydratase, histidine kinase, MATA_HMG-box, Interalpha-trypsin, methyltransferase, N-acetyltransferase, peptidase, transketolase, glucoamylase, PADG_11448, PADG_01788. |
| De Oliveira, et al. [33] 2018 | LC–MS/MS | Phosphoglycerato kinase, glucose-6-phosphasto isomerase, phosphomannomutase, triosephosphate isomerase, homogentified 1,2-dioxigenase, alpha-ATPase subunit, 12-oxophytodienoate reductase, PAAG_00340, ATP citrate synthase. |
| Lima et al. [34] 2014 | NanoUPLC–MS | 4-hydroxylphenolpyruvate dehydrogenase, alanine-glyoxylate aminotransferase, cysteine dioxygenase, aspartate aminotransferase, choline dehydrogenase, glutamate decarboxylase, methylcrotonoyl-CoA carboxylase, sorbitol SOU2, inosine-dehydrogenase-5-monophosphate, fumarylacetoacetate hydrolase, WD40, ATPase, calnexin, complet T subunit T epsilon, tetraclycine. |
| Munhoz, et al. [35] 2016 | Genome | 3-hydroxyantaranilate 3,4 dioxygenase, 1,3 glucanase, BUD 32 kinase, glucan 1,3 glucosidase, aminopeptidase M18, serine protease, LOL, cation efflux, RING, phosphatidyl inositol, PADG_00954, thioredoxin, amino acid permease, acyl-CoA dehydrogenase, 3-ketoacyl reductase, transferrin peptidase. |
| Oliveira et al. [36] 2016 | NanoUPLC–MS | Epsilon protein, Rad24, ARF GTPase. |
| Parente, et al. [37] 2011 | LC–MS/MS | 2-nitropropane dioxygenase, hydroxyacilglutathione hydrolase, L-threonine-3-dehydrogenase, spermidine synthase, glucokinase, pyruvate dehydrogenase, component X protein, nucleoside-diphosphate epimerase, thioredoxin, pentafunctional AROM, adenisulfate kinase, cytochromo c, anikirina, ubiquitin E1, citocromo C, phosophoglycerate kinase, D-hexose-6-phosphate epimerase, hydrolipoyl dehydrogenase, carnitine O acetyltransferase, perixosomal catalase. |
| Parente, et al. [38] 2013 | MALDI–MS/MS | Thioredoxin, protein Y20, aldehyde dehydrogenase, shock protein (Hsp 30, Hsp 30, Hsp 70, Hsp 88, SSB1), malate dehydrogenase, methylcitrate synthase, co-chaperone mitochondrial GrpE, 6-phosphogluconolactonase, xanthine phosophoribosyl transferase, aldehyde dehydrogenase, aminoacyl-tRNA-synthase, oxidoreductase, oxalocrotonate tautomerase, metalloproteins, formamidase. |
| Parente-Rocha et al. [39] 2015 | Nano-ESI-UPLC–MS | Phosphoglucomutase, hydrolipoyl-dehydrogenase, succinate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, 4-hydroxyfenylpyruvate dioxigenase, vacuolar aminopeptidase, carboxypeptidase Y, aspartyl protease, protepian Y20, monitiol glutaredoxin, thioredoxin, cytochromo c peroxidase, Superoxido dismutase Cu/Zn, glucosamine-frutose-6-phosphato, ATP synthase F1F0, NADP glutamato dehydrogenase, gama glutamyltranspeptidase. |
| Pigosso et al. [40] 2013 | MALDI- MS/MS | L-threonine-3-dehydrogenase, 1,2 diyidroxi-3-keto-5-methyllopententene-dioxigenase, 2,5-diceto-D-gluconic acid reductase A, glutathione reductase, 27 KDa, glycoprotein mitochondrial, DNA ligand, corusmate mutase, formamidase fator-1-alpha, phosphoenolpiruvato carboxikinase, 12-oxophitodienate reductase, citrate synthase, gi295666522. |
| Pigosso et al. [41] 2017 | NanoUPLC–MS | Formamidase, carnityl-CoA dehydratase, acil-CoA dehydratase, GABA permease, integral membrane protein, 12-oxophytodinoate reductase, ABB effux, dienetaelone hydrolase, cysteine protease PalB, xanthine dehydrogenase, phosphotransferase, PADG_00675, treonina dehydrogenase, glioxilase, acil-CoA dehydrogenase, metallohydrolase. |
| Rezende et al. [42] 2011 | MALDI- MS/MS | Mitochondrial peroxiredoxin, mannitol-1-phosphato-5-dehydrogenase, aldehyde dehydrogenase, ciclofilin, cofilin, protein G, trompomyosin, hydrolase, phosphoglycerate kinase, cobalamin, phosphoglucomutase, acetylmomoserine(tiol)-liase. |
| Tamayo et al. [43] 2016 | Genoma | Superoxide dismutase (SOD, SOD Cu/Zn). |
| Tashima et al. [44] 2015 | LC–MS/MS | Alpha -tubulin. |
| Tomazetti, et al. [45] 2019 | LC–MS/MS | 1,6 Glyceradeído-3-phosphate-dehydrogenase, Aqualysin, Fructose-1,6-biphosphate aldolase, enolase, Superoxide dismutase Fe+, thioredoxin reductase, succinil-CoA ligase subunidade beta, methyl-2-citrate-synthase, cytochromo c1 mitochondrial heme, Hsp 30, Hsp 70, cytocromo C peroxidase, oxirredutase, Ras-2, methylcitrate dehydrogenase, thioredoxin, 5-aminolevulinato synthase, serine protease, RPA3, ATPase, Profilin, reductase ferric, S-adenosylmethionine dehydrogenase-dependent-2-methyltransferase beta, pyruvate dehydrogenase subunit E1 beta, dyhydrolipoamide acetyl-transferase, glucosamine-fructose-6-phosphate, GTPase RhoA, receptor endossomal Erp3, Shock protein (Hsp020, Hsp 70), perixosomal, citochromo c peroxidase, phosphoesterase, cofilin, estrictosidin synthase, destrin, RRM-Srp1p. |
| Tristão, et al. [46] 2015 | Proteomic | Superoxide dismutase (SOD, SOD Cu/Zn, SOD cytosolic, SOD cytosolic Cu/Zn), laccase 1, L-ascorbitate oxidase, laccase IV, cupredoxin, alcohol dehydrogenase, class II aldolase, PADG_00743, mannose-6-phosphate isomerase, peptidase M1, copper carrier ATPase, heavy metal ATPase, urease, D-arabinose-1-dehydrogenase, calcium carrier, carrier CCC1, calcium carrier, iron and magnesium, cytochromo, ypt5 binding GTPase, mitochondrial porin, SEC62 protein, chpA protein, binding GTPase ypt7, chaperone, ECM33 precursor protein, extracellular matrix component, GTPase sar1, G2/M RNA ligand, iron carrier, vacuolar protein, Ras, clatrin, beta-glucosidase, endosome carrier. |
| Weber, et al. [47] 2012 | MALDI-Q-TOF MS | Aminotransferase, fumarylacetoacetase, beta-glycosidase, glycosyl hydrolase, Grp1p, peptidyl-propyl-cis-trans-isomerase A2, disulfide isomerase Pdi1. Enolase, Hsp 10, malate dehydrogenase, serine hydroxylmethyltransferase. |
| Protein | Identification | E-Value | Identity | |
|---|---|---|---|---|
| Pb18 | Pl 01 | |||
| Carrier ADP/ATP | PAAG_08620 | 0.0 | 100% | 97% |
| Acyl-CoA dehydrogenase | PAAG_03116 | 0.0 | 98% | 100% |
| Acyl-CoA dehydrogenase | PADG_06805 | 0.0 | 100% | 98% |
| Acyl CoA dehydrogenase | PADG_07604 | 0.0 | 100% | 97% |
| Acyl CoA hydratase | PAAG_06309 | 0.0 | 97% | 100% |
| Actin F protein subunit uptake protein | PADG_07756 | 0.0 | 100% | 100% |
| Alcohol dehydrogenase | PADG_01174 | 0.0 | 100% | 97% |
| Alpha-1,2 mannosyltransferase | PAAG_02462 | 0.0 | 100% | 98% |
| Alpha-1,2 mannosyltransferase KTR1 | PAAG_07238 | 0.0 | 97% | 100% |
| Adenosillomocysteine | PADG_02859 | 0.0 | 99% | 100% |
| Aminotransferase | PAAG_03045 | 0.0 | 100% | 98% |
| Aminotransferase | PAAG_00053 | 0.0 | 98% | 100% |
| ATP citrate synthase | PADG_04993 | 0.0 | 100% | 98% |
| ATP_dependent 26S on proteasome regulation | PAAG_01926 | 0.0 | 99% | 100% |
| Calnexin | PAAG_07037 | 0.0 | 95% | 100% |
| Carrier ADP/ATP | PAAG_08620 | 0.0 | 100% | 97% |
| Carrier calcium | PAAG_07762 | 0.0 | 100% | 98% |
| Carnitil CoA dehydrogenase | PADG_05773 | 0.0 | 100% | 97% |
| Catalase | PAAG_01454 | 0.0 | 100% | 98% |
| Catalase | PADG_00324 | 0.0 | 98% | 100% |
| Catalase | PAAG_01943 | 0.0 | 99% | 99% |
| Chitin synthase VII class | ABV31248.1 | 0.0 | 98% | 99% |
| 1,3 ketoacyl-CoA lyase | PADG_07365 | 0.0 | 100% | 98% |
| 3-ketoacyl reductase | PADG_01943 | 0.0 | 100% | 95% |
| Citrate synthase | PAAG_08075 | 0.0 | 98% | 100% |
| Colin acetyltransferase | PADG_07023 | 0.0 | 100% | 98% |
| Gamma subunit complex T | PAAG_07165 | 0.0 | 99% | 100% |
| Corismate mutase | PAAG_05198 | 0.0 | 98% | 100% |
| Dehydrogenase | PADG_07369 | 0.0 | 100% | 98% |
| Dihydrolopiol | PAAG_03330 | 0.0 | 97% | 100% |
| Dihydrolopiol dehydrogenase | PAAG_06494 | 0.0 | 100% | 97% |
| Dolichol-phosphate mannosyltransferase | PAAG_01874 | 0.0 | 98% | 100% |
| Enoyl coenzyme crontonase | PADG_01209 | 0.0 | 100% | 97% |
| Formamidase | PAAG_03333 | 0.0 | 99% | 98% |
| Formamidase | PADG_06490 | 0.0 | 100% | 97% |
| Fumarylacetoacetoato hydrolase | PAAG_00869 | 0.0 | 98% | 100% |
| Glutamyl transferase range | PADG_01479 | 0.0 | 97% | 100% |
| Glutamate dehydrogenase | PADG_04516 | 0.0 | 100% | 98% |
| Glucan synthase | PADG_07373 | 0.0 | 97% | 100% |
| Glycosamine-6-phosphate deaminase | PADG_00401 | 0.0 | 100% | 97% |
| Hydratase mitochondrial acetones | PAAG_00845 | 0.0 | 100% | 99% |
| Hydroacylgluthatione hydrolase | PAAG_02548 | 0.0 | 98% | 100% |
| 4-Hydroxyfenylpyruvate dioxygenase | PAAG_08468 | 0.0 | 99% | 100% |
| Hexose-6-phosphato epimerase | PADG_03243 | 0.0 | 98% | 100% |
| Hydratase | PADG_11845 | 0.0 | 99% | 100% |
| Hydroxyl-CoA dehydrogenase | PADG_01228 | 0.0 | 100% | 98% |
| Hydroacylglutathione hydrolase | PAAG_02548 | 0.0 | 98% | 100% |
| Histidin kinase | PADG_11468 | 0.0 | 100% | 96% |
| Hypothetical protein | PAAG_02761 | 0.0 | 94% | 100% |
| Hypothetical protein | PADG_01788 | 0.0 | 98% | 100% |
| Hypothetical protein | PADG_01788 | 0.0 | 98% | 100% |
| Interalpha trypsin | PADG_06178 | 0.0 | 100% | 96% |
| Inosine-S-monophosphate dehydrogenase MD2 | ||||
| Isocitrate lyase | PAAG_04542 | 0.0 | 98% | 99% |
| Isocitrate lyase | PAAG_06951 | 0.0 | 100% | 98% |
| 3-isopropyl dehydrogenase | PAAG_05328 | 0.0 | 97% | 100% |
| L-threonine dehydrogenase | PAAG_00966 | 0.0 | 99% | 100% |
| Laccase | PADG_06196 | 0.0 | 100% | 96% |
| Mallate dehydrogenase | PAAG_00053 | 0.0 | 98% | 100% |
| Mallate synthase, glyoxysomal | PADG_04702 | 0.0 | 99% | 97% |
| Mallate synthase, glyoxysomal | PADG_04702 | 0.0 | 99% | 97% |
| Mannitol-1-phosphate dehydrogenase | PAAG_06473 | 0.0 | 100% | 98% |
| Methyltransferase | PADG_01183 | 0.0 | 100% | 94% |
| Methyltransferase | PAAG_09014 | 0.0 | 94% | 100% |
| Methylcronotonoil-CoA carboxylase | PAAG_04103 | 0.0 | 98% | 100% |
| Methilmalonate-semialdehyde dehydrogenase | PAAG_07036 | 0.0 | 98% | 100% |
| Metalo hydrolase | PADG_03136 | 0.0 | 100% | 97% |
| Mitochondrial | PAAG_05350 | 0.0 | 97% | 100% |
| Mitochondrial acetonate hydratase | PAAG_00845 | 0.0 | 100% | 99% |
| NADP-especific glutamate dehydrogenase | PADG_04516 | 0.0 | 98% | 100% |
| 2-nitropropane dioxygenase | PAAG_06693 | 0.0 | 98% | 100% |
| Osmosensor | PAAG_04025 | 0.0 | 100% | 97% |
| Oligosaccharide transferase | PAAG_04719 | 0.0 | 98% | 100% |
| Oxoisovalerate dehydrogenase beta subunit | PAAG_01194.2 | 0.0 | 99% | 100% |
| Oxoisovalerate dehydrogenase alpha subunit | PAAG_01310 | 0.0 | 98% | 100% |
| Oxirreductase | PADG_06082 | 0.0 | 90% | 100% |
| Plasma ATPase | PAAG_08082 | 0.0 | 99% | 100% |
| Phosphatase ser/thre | PADG_03544 | 0.0 | 100% | 100% |
| Plasma ATPase | PAAG_08082 | 0.0 | 99% | 100% |
| Perixosomal catalase | PADG_01943 | 0.0 | 100% | 95% |
| Perixosomal catalase | PADG_00686 | 0.0 | 100% | 96% |
| Perixosomal hydratase | PADG_08651 | 0.0 | 100% | 97% |
| Pyruvate dehydrogenase component E1 beta E1 | PAAG_01534 | 0.0 | 98% | 100% |
| Pyruvate dehydrogenase | PADG_00246 | 0.0 | 100% | 98% |
| Shock protein SSVB1 | PAAG_07775 | 0.0 | 98% | 100% |
| Serine 3 dehydrogenase | PAAG_02354 | 0.0 | 97% | 100% |
| Serine hydroxymethyltransferase | PAAG_07412 | 0.0 | 99% | 100% |
| Sorbitol SOU2 | PAAG_04184 | 0.0 | 98% | 100% |
| Succinate dehydrogenase | PADG_06494 | 0.0 | 97% | 100% |
| Succinate dehydrogenase | PADG_08013 | 0.0 | 100% | 98% |
| Succinyl CoA ligase | PADG_02260 | 0.0 | 99% | 100% |
| Shock protein 90 | PADG_02785 | 0.0 | 100% | 98% |
| Shock protein 98 | PADG_00765 | 0.0 | 100% | 99% |
| Shock protein SSVB1 | PAAG_07775 | 0.0 | 98% | 100% |
| Transferrin | PADG_00686 | 0.0 | 100% | 98% |
| Transketolase | PADG_00246 | 0.0 | 100% | 98% |
| Tetracycline transporter | PAAG_07990 | 0.0 | 97% | 100% |
| Thioredoxin | PADG_03161 | 0.0 | 100% | 97% |
| Thioredoxin | PADG_01551 | 0.0 | 100% | 97% |
| Urease | PADG_03874 | 0.0 | 100% | 97% |
| Urease | PADG_00954 | 0.0 | 100% | 97% |
| Xaa-Pro aminopeptidase | PAAG_07500 | 0.0 | 100% | 100% |
| Pep_H1 MSAFSRMTASLGFSK (PADG_06178) | |||
|---|---|---|---|
| Peptide | Allele | Method Used | Percentage Rank (%) |
| MSAFSRMTASLGFSK | HLA-DRB1 * 07:01 | Consensus (comb.lib./smm/nn) | 1.11 |
| HLA-DRB3 * 02:02 | NetMHCIIpan | 2.37 | |
| HLA-DRB5 * 01:01 | Consensus (smm/nn/sturniolo) | 2.92 | |
| HLA-DRB1 * 15:01 | Consensus (smm/nn/sturniolo) | 14.19 | |
| HLA-DRB1 * 03:01 | Consensus (smm/nn/sturniolo) | 14.96 | |
| HLA-DRB3 * 01:01 | Consensus (comb.lib./smm/nn) | 20.72 | |
| HLA-DRB4 * 01:01 | Consensus (comb.lib./smm/nn) | 32.06 | |
| Pep_SQ2FDVFYYLLTSASTPA (>ABV31248.1) | |||
| FDVFYYLLTSASTPA | HLA-DRB1 * 07:01 | Consensus (comb.lib./smm/nn) | 1.67 |
| HLA-DRB3 * 01:01 | Consensus (smm/nn/sturniolo) | 3.95 | |
| HLA-DRB1 * 15:01 | Consensus (smm/nn/sturniolo) | 3.58 | |
| HLA-DRB3 * 02:02 | NetMHCIIpan | 4.02 | |
| HLA-DRB5 * 01:01 | Consensus (smm/nn/sturniolo) | 4.34 | |
| HLA-DRB3 * 01:01 | Consensus (comb.lib./smm/nn) | 40.25 | |
| HLA-DRB4 * 01:01 | Consensus (comb.lib./smm/nn) | 42.57 | |
| Pep_PH3RAYALLFSKLGAAVV (PADG_08651) | |||
| HLA-DRB5 * 01:01 | Consensus (smm/nn/sturniolo) | 3.19 | |
| HLA-DRB1 * 15:01 | Consensus (smm/nn/sturniolo) | 3.37 | |
| HLA-DRB1 * 07:01 | Consensus (smm/nn/sturniolo) | 4.79 | |
| HLA-DRB3 * 02:02 | NetMHCIIpan | 11.93 | |
| HLA-DRB1 * 03:01 | Consensus (smm/nn/sturniolo) | 17.05 | |
| HLA-DRB4 * 01:01 | Consensus (comb.lib./smm/nn) | 23.65 | |
| HLA-DRB3 * 01:01 | Consensus (comb.lib./smm/nn) | 32.36 | |
| Pep_ SQ4ERVSIIANPAVASLY (PAAG_08203) | |||
| HLA-DRB3 * 02:02 | NetMHCIIpan | 0.01 | |
| HLA-DRB1 * 15:01 | Consensus (smm/nn/sturniolo) | 2.60 | |
| HLA-DRB1 * 03:01 | Consensus (smm/nn/sturniolo) | 11.00 | |
| HLA-DRB1 * 07:01 | Consensus (comb.lib./smm/nn) | 15.00 | |
| HLA-DRB4 * 01:01 | Consensus (comb.lib./smm/nn) | 19.00 | |
| HLA-DRB3 * 01:01 | Consensus (comb.lib./smm/nn) | 7.70 | |
| HLA-DRB5 * 01:01 | Consensus (comb.lib./smm/nn) | 43.00 | |
| Epitope | Method | IFN-ɤ | Score IFN-ɤ | Score Other Cytokines | Score Random IFN-ɤ |
|---|---|---|---|---|---|
| Epi 1—MSAFSRMTASLGFSK | Scan/MSV | Negative | −0.14 | 0.49 | 0.81 |
| Epi 2—FDVFYYLLTSASTPA | Scan/MSV | Negative | −0.56 | 0.44 | −0.00 |
| Epi 3—RAYALLFSKLGAAVV | Scan/MSV | Positive | −0.16 | 0.61 | 0.00 |
| Epi 4—ERVSIIANPAVASLY | Scan/MSV | Positive | 0.15 | 0.51 | −0.38 |
| ESAT-6—QWNFAGIEAAASAIQ | San/MSV | Positive | 0.30 | 0.88 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, S.B.A.; Csordas, B.G.; do Valle Leone de Oliveira, S.M.; Ribeiro dos Santos, A.; Paniago, A.M.M.; Venturini, J. Prediction of Conserved Peptides of Paracoccidioides for Interferon-γ Release Assay: The First Step in the Development of a Lab-Based Approach for Immunological Assessment during Antifungal Therapy. J. Fungi 2020, 6, 379. https://doi.org/10.3390/jof6040379
Rosa SBA, Csordas BG, do Valle Leone de Oliveira SM, Ribeiro dos Santos A, Paniago AMM, Venturini J. Prediction of Conserved Peptides of Paracoccidioides for Interferon-γ Release Assay: The First Step in the Development of a Lab-Based Approach for Immunological Assessment during Antifungal Therapy. Journal of Fungi. 2020; 6(4):379. https://doi.org/10.3390/jof6040379
Chicago/Turabian StyleRosa, Sarah Brena Aparecida, Bárbara Guimarães Csordas, Sandra Maria do Valle Leone de Oliveira, Amanda Ribeiro dos Santos, Anamaria Mello Miranda Paniago, and James Venturini. 2020. "Prediction of Conserved Peptides of Paracoccidioides for Interferon-γ Release Assay: The First Step in the Development of a Lab-Based Approach for Immunological Assessment during Antifungal Therapy" Journal of Fungi 6, no. 4: 379. https://doi.org/10.3390/jof6040379
APA StyleRosa, S. B. A., Csordas, B. G., do Valle Leone de Oliveira, S. M., Ribeiro dos Santos, A., Paniago, A. M. M., & Venturini, J. (2020). Prediction of Conserved Peptides of Paracoccidioides for Interferon-γ Release Assay: The First Step in the Development of a Lab-Based Approach for Immunological Assessment during Antifungal Therapy. Journal of Fungi, 6(4), 379. https://doi.org/10.3390/jof6040379






