Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets?
Abstract
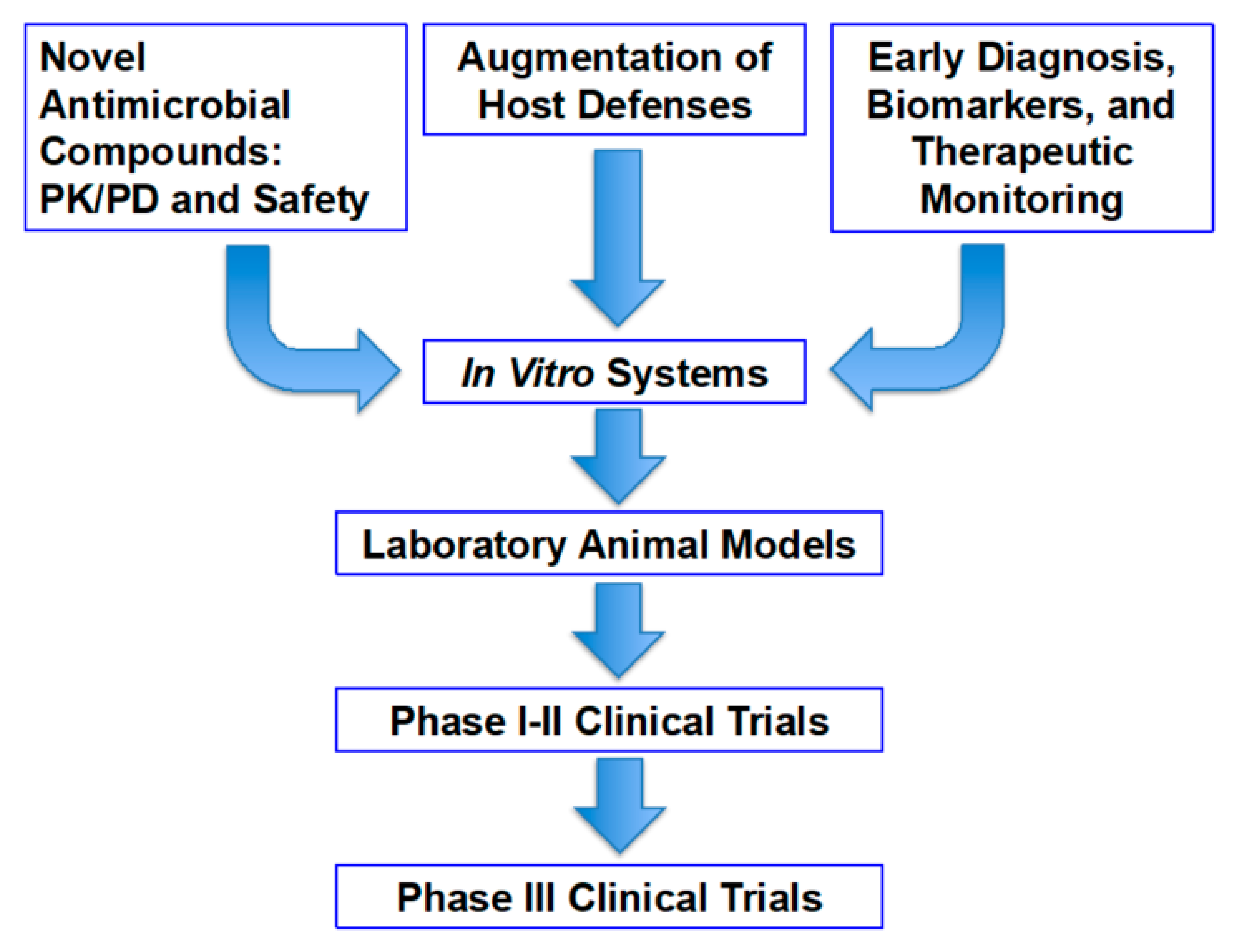
1. Introduction
2. Rabbit Models of Invasive Pulmonary Aspergillosis
2.1. Translational Research Model
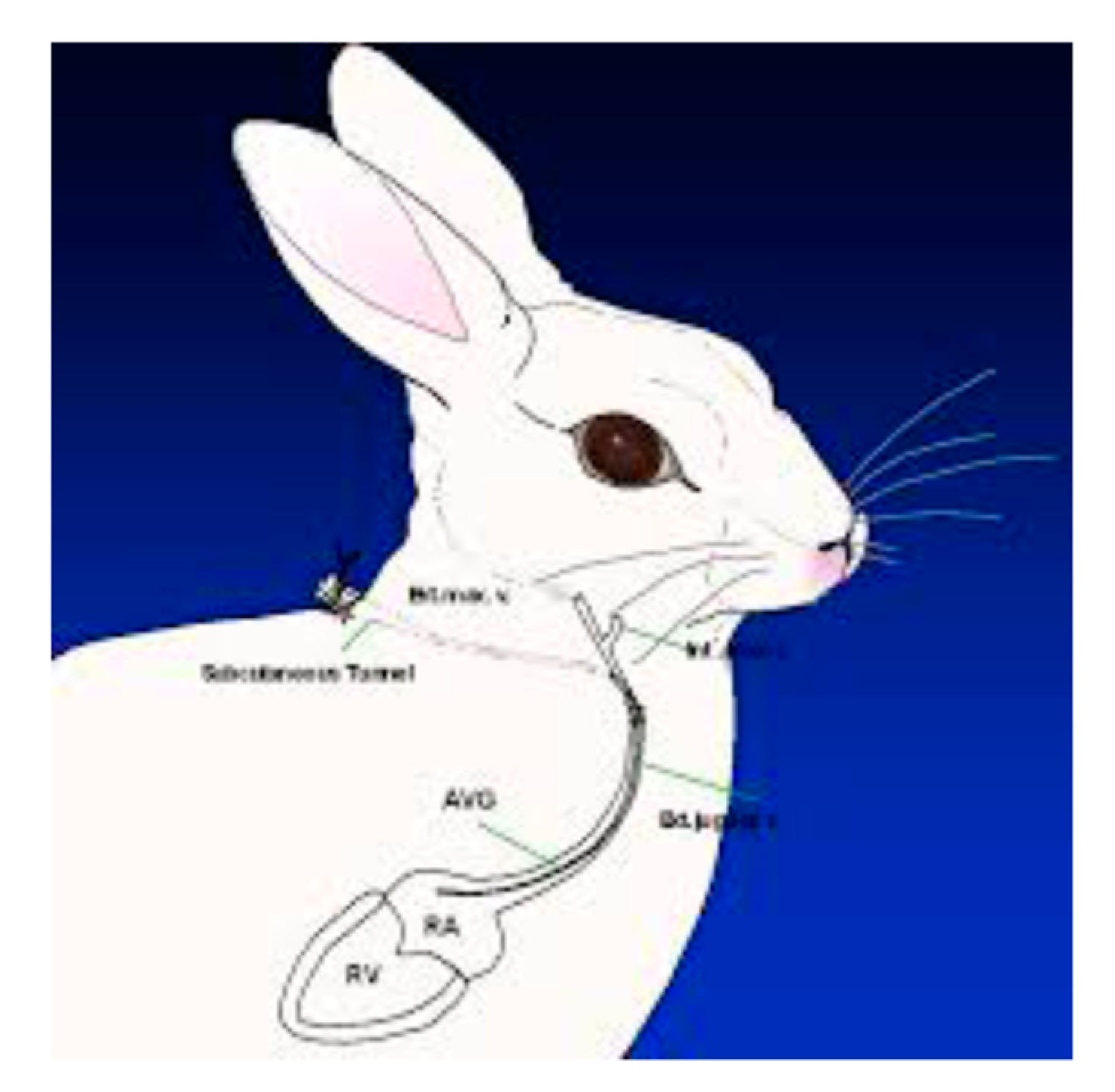
2.2. Methodological Establishment of the Persistently Neutropenic Rabbit Model of Invasive Pulmonary Aspergillosis
2.3. Pathophysiology of Experimental Invasive Pulmonary Aspergillosis
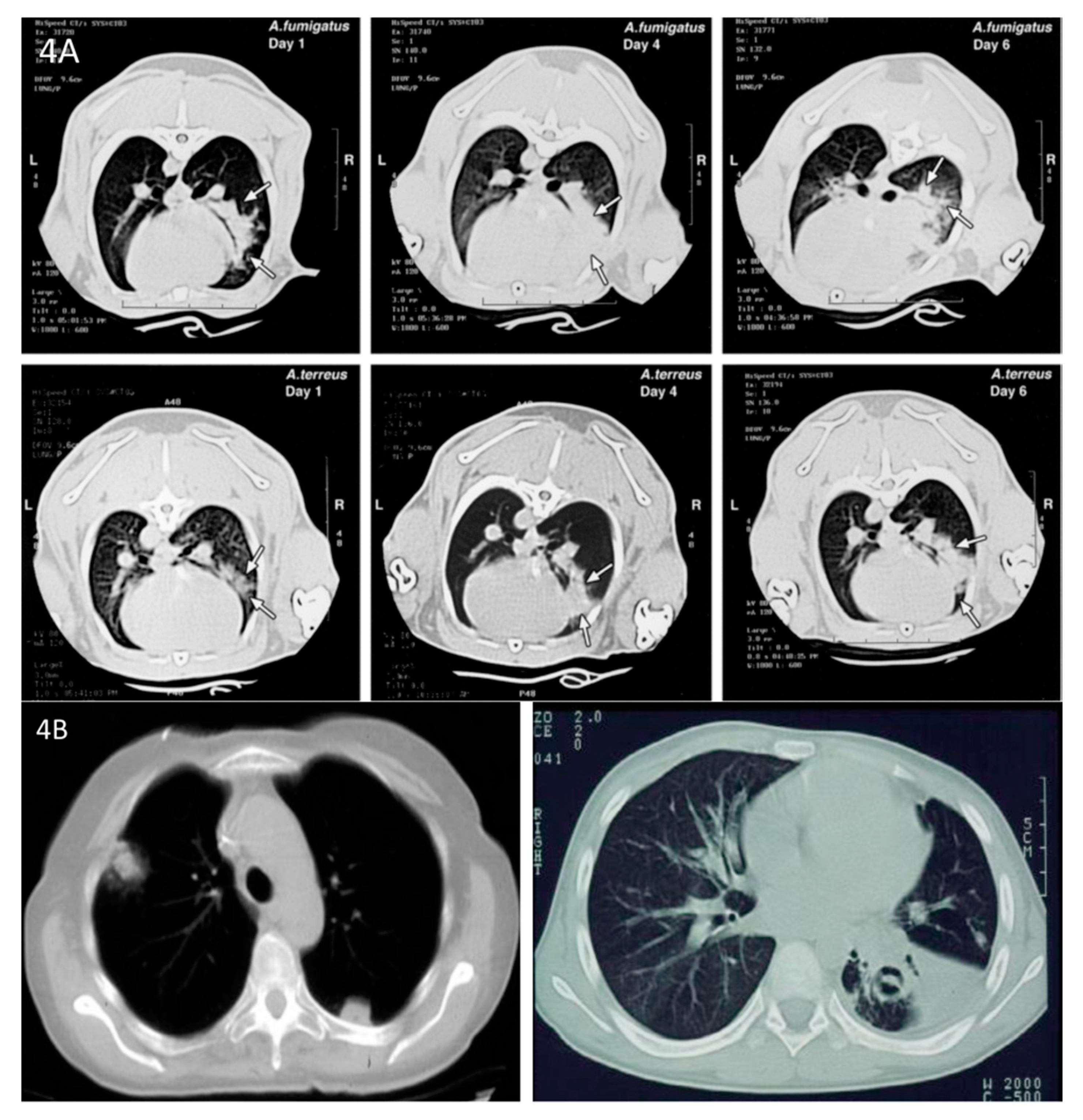
2.4. Clinical Impact of the Rabbit Models of Experimental Invasive Pulmonary Aspergillosis
3. Lipid Formulations of Amphotericin B
4. Antifungal Triazoles
5. Combination Antifungal Therapy
Ibrexafungerp Plus Mould-Active Triazole in Combination Therapy
6. Olorofim
Chronic Invasive Pulmonary Aspergillosis in the Non-Neutropenic Rabbit Model
Author Contributions
Funding
Acknowledgments
Disclosures
Conflicts of Interest
References
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomized-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Lepak, A.J.; Marchillo, K.; Vanhecker, J.; Andes, D.R. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2013, 57, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; Edwards, F.; Andrade, J.; Niki, Y.; Armstrong, D. Comparison of azoles against aspergilli in vitro and in an experimental model of pulmonary aspergillosis. Chemotherapy 1992, 38, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Najvar, L.K.; Vallor, A.C.; Kirkpatrick, W.R.; Bocanegra, R.; Molina, D.; Olivo, M.; Graybill, J.R.; Patterson, T.F. Assessment of serum (1->3)-β-D-glucan concentration as a measure of disease burden in a murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2008, 52, 1176–1178. [Google Scholar] [CrossRef][Green Version]
- Petraitiene, R.; Petraitis, V.; Bacher, J.D.; Finkelman, M.A.; Walsh, T.J. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1→3)-β-D-glucan in experimental invasive pulmonary aspergillosis. Med. Mycol. 2015, 53, 558–568. [Google Scholar] [CrossRef]
- Petraitiene, R.; Petraitis, V.; Groll, A.H.; Sein, T.; Piscitelli, S.; Candelario, M.; Field-Ridley, A.; Avila, N.; Bacher, J.; Walsh, T.J. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: Correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 2001, 45, 857–869. [Google Scholar] [CrossRef]
- Walsh, T.J.; Bacher, J.; Pizzo, P.A. Chronic Silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab. Anim. Sci. 1988, 38, 467–471. [Google Scholar]
- Francis, P.R.; Lee, J.W.; Hoffman, A.G.D.; Peter, J.; Francesconi, A.; Bacher, J.; Shelhamer, J.; Pizzo, P.A.; Walsh, T.J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: The potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 1994, 169, 356–368. [Google Scholar] [CrossRef]
- Hope, W.; Kruhlak, M.J.; Lyman, C.A.; Petraitiene, R.; Petraitis, V.; Francesconi, A.; Kasai, M.; Mickiene, D.; Sein, T.; Peter, J.; et al. Pathogenesis of Aspergillus fumigatus and the kinetics of galactomannan in an in vitro model of early invasive pulmonary aspergillosis: Implications for antifungal therapy. J. Infect. Dis. 2007, 195, 455–466. [Google Scholar] [CrossRef]
- Walsh, T.J.; Garrett, K.; Feurerstein, E.; Girton, M.; Allende, M.; Bacher, J.; Francesconi, A.; Schäufele, R.; Pizzo, P.A. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob. Agents Chemother. 1995, 39, 1065–1069. [Google Scholar] [CrossRef][Green Version]
- Cornely, O.A.; Maertens, J.; Bresnik, M.; Ebrahimi, R.; Ullmann, A.J.; Bouza, E.; Heussel, C.P.; Lortholary, O.; Rieger, C.; Boehme, A.; et al. Liposomal amphotericin B as initial therapy for invasive mold infection: A randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad Trial). Clin. Infect. Dis. 2007, 44, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.E.; Kasai, M.; Francesconi, A.; Petraitis, V.; Petraitiene, R.; Kelaher, A.M.; Sarafandi, A.A.; Walsh, T.J. Development and validation of a quantitative real-time PCR assay using fluorescence resonance energy transfer technology for detection of Aspergillus fumigatus in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 2003, 41, 5676–5682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stergiopoulou, T.; Meletiadis, J.; Roilides, E.; Kleiner, D.E.; Schaufele, R.; Roden, M.; Harrington, S.; Dad, L.; Segal, B.; Walsh, T.J. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am. J. Clin. Pathol. 2007, 127, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Petraitiene, R.; Petraitis, V.; Groll, A.H.; Sein, T.; Schaufele, R.L.; Francesconi, A.; Bacher, J.; Avila, N.A.; Walsh, T.J. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: Pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 2002, 46, 12–23. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Lin, P.; Calis, K.; Kelaher, A.M.; Muray, H.A.; Mya-San, C.; Mickiene, D.; Bacher, J.; Walsh, T.J. Efficacy and safety of generic amphotericin B in experimental pulmonary aspergillosis. Antimicrob. Agents Chemother. 2005, 49, 1642–1645. [Google Scholar] [CrossRef][Green Version]
- Petraitis, V.; Petraitiene, R.; Solomon, J.; Kelaher, A.M.; Murray, H.A.; Mya-San, C.; Bhandary, A.K.; Sein, T.; Avila, N.A.; Basevicius, A.; et al. Multidimensional volumetric imaging of pulmonary infiltrates for measuring therapeutic response to antifungal therapy in experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2006, 50, 1510–1517. [Google Scholar] [CrossRef]
- Hope, W.; Petraitis, V.; Petraitiene, R.; Aghamolla, T.; Bacher, J.; Walsh, T.J. The initial 96 hours of invasive pulmonary aspergillosis: Histopathology, comparative kinetics of galactomannan and (1→3)-β-D-glucan, and consequences of delayed antifungal therapy. Antimicrob. Agents Chemother. 2010, 54, 4879–4886. [Google Scholar] [CrossRef]
- Meletiadis, J.; Petraitis, V.; Petraitiene, R.; Lin, P.; Stergiopoulou, T.; Sein, T.; Bacher, J.; Kelaher, A.M.; Schaufele, R.L.; Walsh, T.J. Triazole? Polyene antagonism in experimental invasive pulmonary aspergillosis: In vitro and in vivo correlation. J. Infect. Dis. 2006, 194, 1008–1018. [Google Scholar] [CrossRef]
- Leenders, A.C.A.P.; Daenen, S.; Jansen, R.L.H.; Hop, W.C.J.; Löwenberg, B.; Wijermans, P.W.; Cornelissen, J.; Herbrecht, R.; Van Der Lelie, H.; Hoogsteden, H.C.; et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br. J. Haematol. 1998, 103, 205–212. [Google Scholar] [CrossRef]
- Walsh, T.J.; Hiemenz, J.W.; Seibel, N.L.; Perfect, J.R.; Horwith, G.; Lee, L.; Silber, J.L.; Di Nubile, M.J.; Reboli, A.; Bow, E.; et al. Amphotericin B lipid complex for invasive fungal infections: Analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 1998, 26, 1383–1396. [Google Scholar] [CrossRef]
- Walsh, T.J.; Seibel, N.L.; Arndt, C.; Harris, R.E.; Di Nubile, M.J.; Reboli, A.; Hiemenz, J.; Chanock, S.J. Amphotericin B lipid complex in pediatric patients with invasive fungal infections. Pediatr. Infect. Dis. J. 1999, 18, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.C.; Lee, J.W.; Francis, P.; Garrett, K.; Dollenberg, H.; Berenguer, J.; Lyman, C.A.; Pizzo, P.A.; Walsh, T.J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob. Agents Chemother. 1994, 38, 518–522. [Google Scholar] [CrossRef] [PubMed]
- White, M.H.; Anaissie, E.J.; Kusne, S.; Wingard, J.R.; Hiemenz, J.W.; Cantor, A.; Gurwith, M.; Du Mond, C.; Mamelok, R.D.; Bowden, R.A. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis. Clin. Infect. Dis. 1997, 24, 635–642. [Google Scholar] [PubMed]
- Anaissie, E.J.; Mattiuzzi, G.N.; Miller, C.B.; Noskin, G.A.; Gurwith, M.J.; Mamelok, R.D.; Pietrelli, L.A. Treatment of invasive fungal infections in renally impaired patients with amphotericin B colloidal dispersion. Antimicrob. Agents Chemother. 1998, 42, 606–611. [Google Scholar] [CrossRef]
- White, M.H.; Bowden, R.A.; Sandler, E.S.; Graham, M.L.; Noskin, G.A.; Wingard, J.R.; Goldman, M.; Van Burik, J.; McCabe, A.; Lin, J.; et al. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clin. Infect. Dis. 1998, 27, 296–302. [Google Scholar] [CrossRef]
- Bowden, R.; Chandrasekar, P.; Pietrelli, L.; Gurwith, M.; Safrin, S.; White, M.H.; Li, X.; Van Burik, J.-A.; Laverdiere, M.; Wingard, J.R. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 2002, 35, 359–366. [Google Scholar] [CrossRef]
- Groll, A.H.; Gonzalez, C.E.; Giri, N.; Kligys, K.; Love, W.; Peter, J.; Feuerstein, E.; Bacher, J.; Piscitelli, S.C.; Walsh, T.J. Liposomal nystatin against experimental pulmonary aspergillosis in persistently neutropenic rabbits: Efficacy, safety and non-compartmental pharmacokinetics. J. Antimicrob. Chemother. 1999, 43, 95–103. [Google Scholar] [CrossRef]
- Offner, F.; Krcmery, V.; Boogaerts, M.; Doyen, C.; Engelhard, D.; Ribaud, P.; Cordonnier, C.; De Pauw, B.; Durrant, S.; Marie, J.-P.; et al. Liposomal nystatin in patients with invasive aspergillosis refractory to or intolerant of amphotericin B. Antimicrob. Agents Chemother. 2004, 48, 4808–4812. [Google Scholar] [CrossRef]
- Maertens, J.; Raad, I.; Petrikkos, G.; Boogaerts, M.; Selleslag, D.; Petersen, F.B.; Sable, C.A.; Kartsonis, N.; Ngai, A.; Taylor, A.; et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 2004, 39, 1563–1571. [Google Scholar] [CrossRef]
- Aliff, T.B.; Maslak, P.; Jurcic, J.G.; Heaney, M.L.; Cathcart, K.; Sepkowitz, K.A.; Weiss, M.A. Refractory Aspergillus pneumonia in patients with acute leukemia. Cancer 2003, 97, 1025–1032. [Google Scholar] [CrossRef]
- Maertens, J.; Glasmacher, A.; Herbrecht, R.; Thiebaut, A.; Cordonnier, C.; Segal, B.H.; Killar, J.; Taylor, A.; Kartsonis, N.; Patterson, T.F.; et al. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 2006, 107, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Hiemenz, J.W.; Raad, I.I.; Maertens, J.A.; Hachem, R.Y.; Saah, A.J.; Sable, C.A.; Chodakewitz, J.A.; Severino, M.E.; Saddier, P.; Berman, R.S.; et al. Efficacy of caspofungin as salvage therapy for invasive aspergillosis compared to standard therapy in a historical cohort. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Teppler, H.; Donowitz, G.R.; Maertens, J.; Baden, L.R.; Dmoszyñska, A.; Cornely, O.A.; Bourque, M.R.; Lupinacci, R.J.; Sable, C.A.; et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 2004, 351, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Al-Saigh, R.J.; Velegraki, A.; Walsh, T.J.; Roilides, E.; Zerva, L. Pharmacodynamic effects of simulated standard doses of antifungal drugs against Aspergillus species in a new in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2011, 56, 403–410. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Hope, W.; Meletiadis, J.; Mickiene, D.; Hughes, J.E.; Cotton, M.P.; Stergiopoulou, T.; Kasai, M.; Francesconi, A.; et al. Combination therapy in treatment of experimental pulmonary aspergillosis: In vitro and in vivo correlations of the concentration- and dose-dependent interactions between anidulafungin and voriconazole by bliss independence drug interaction analysis. Antimicrob. Agents Chemother. 2009, 53, 2382–2391. [Google Scholar] [CrossRef]
- Walsh, T.J.; Adamson, P.C.; Seibel, N.L.; Flynn, P.M.; Neely, M.N.; Schwartz, C.; Shad, A.; Kaplan, S.L.; Roden, M.M.; Stone, J.A.; et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 2005, 49, 4536–4545. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Groll, A.H.; Roussillon, K.; Hemmings, M.; Lyman, C.A.; Sein, T.; Bacher, J.; Bekersky, I.; Walsh, T.J. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 2002, 46, 1857–1869. [Google Scholar] [CrossRef]
- Denning, D.W.; Marr, K.A.; Lau, W.M.; Facklam, D.P.; Ratanatharathorn, V.; Becker, C.; Ullmann, A.J.; Seibel, N.L.; Flynn, P.M.; Van Burik, J.-A.H.; et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J. Infect. 2006, 53, 337–349. [Google Scholar] [CrossRef]
- Kontoyiannis, D.; Ratanatharathorn, V.; Young, J.-A.; Raymond, J.; Laverdière, M.; Denning, D.; Patterson, T.; Facklam, D.; Kovanda, L.; Arnold, L.; et al. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transpl. Infect. Dis. 2009, 11, 89–93. [Google Scholar] [CrossRef]
- Van Burik, J.-A.H.; Ratanatharathorn, V.; Stepan, D.E.; Miller, C.B.; Lipton, J.H.; Vesole, D.H.; Bunin, N.; Wall, D.A.; Hiemenz, J.W.; Satoi, Y.; et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 2004, 39, 1407–1416. [Google Scholar] [CrossRef]
- Santos, R.P.; Sánchez, P.J.; Mejias, A.; Benjamin, D.K.; Walsh, T.J.; Patel, S.; Jafri, H.S. Successful medical treatment of cutaneous aspergillosis in a premature infant using liposomal amphotericin b, voriconazole and micafungin. Pediatr. Infect. Dis. J. 2007, 26, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Petraitis, V.; Petraitiene, R.; Sarafandi, A.A.; Kelaher, A.M.; Lyman, C.A.; Casler, H.E.; Sein, T.; Groll, A.H.; Bacher, J.; Avila, N.A.; et al. Combination therapy in treatment of experimental pulmonary aspergillosis: Synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 2003, 187, 1834–1843. [Google Scholar] [CrossRef]
- Meletiadis, J.; Stergiopoulou, T.; O’Shaughnessy, E.M.; Peter, J.; Walsh, T.J. Concentration-dependent synergy and antagonism within a triple antifungal drug combination against Aspergillus species: Analysis by a new response surface model. Antimicrob. Agents Chemother. 2007, 51, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Seibel, N.L.; Schwartz, C.; Arrieta, A.; Flynn, P.; Shad, A.; Albano, E.; Keirns, J.; Lau, W.M.; Facklam, D.P.; Buell, D.N.; et al. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob. Agents Chemother. 2005, 49, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Petraitis, V.; Petraitiene, R.; Groll, A.H.; Bell, A.; Callender, D.P.; Sein, T.; Schaufele, R.L.; McMillian, C.L.; Bacher, J.; Walsh, T.J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 1998, 42, 2898–2905. [Google Scholar] [CrossRef][Green Version]
- Groll, A.H.; Mickiene, D.; Petraitiene, R.; Petraitis, V.; Lyman, C.A.; Bacher, J.S.; Piscitelli, S.C.; Walsh, T.J. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): Reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 2001, 45, 2845–2855. [Google Scholar] [CrossRef]
- Hope, W.; McEntee, L.; Livermore, J.; Whalley, S.; Johnson, A.; Farrington, N.; Kolamunnage-Dona, R.; Schwartz, J.; Kennedy, A.; Law, D.; et al. Pharmacodynamics of the orotomides against Aspergillus fumigatus: New opportunities for treatment of multidrug-resistant fungal disease. mBio 2017, 8, e01157-17. [Google Scholar] [CrossRef]
- Lee, D.-G.; Lee, H.; Yan, J.L.; Lin, S.S.; Aram, J.A. Efficacy and safety of combination antifungal therapy in Korean haematological patients with invasive aspergillosis. Mycoses 2019, 62, 969–978. [Google Scholar] [CrossRef]
- Denning, D.W.; Lee, J.Y.; Hostetler, J.S.; Pappas, P.; Kauffman, C.A.; Dewsnup, D.H.; Galgiani, J.N.; Graybill, J.R.; Sugar, A.M.; Catanzaro, A.; et al. NIAID mycoses study group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am. J. Med. 1994, 97, 135–144. [Google Scholar] [CrossRef]
- Lamy, T.; Bernard, M.; Courtois, A.; Jacquelinet, C.; Chevrier, S.; Dauriac, C.; Grulois, I.; Guiguen, C.; Le Prise, P. Prophylactic use of itraconazole for the prevention of invasive pulmonary aspergillosis in high risk neutropenic patients. Leuk. Lymphoma 1998, 30, 163–174. [Google Scholar] [CrossRef]
- Ghannoum, M.; Long, L.; Larkin, E.L.; Isham, N.; Sherif, R.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Evaluation of the antifungal activity of the novel oral glucan synthase inhibitor SCY-078, singly and in combination, for the treatment of invasive aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00244-18. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; McCarthy, M.; Meletiadis, J.; Petraitis, V.; Moradi, P.W.; Strauss, G.E.; Fouant, M.M.; Kovanda, L.L.; Petraitiene, R.; Roilides, E.; et al. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob. Agents Chemother. 2014, 58, 6934–6937. [Google Scholar] [CrossRef] [PubMed]
- Elefanti, A.; Mouton, J.W.; Verweij, P.E.; Tsakris, A.; Zerva, L.; Meletiadis, J. Amphotericin B- and voriconazole-echinocandin combinations against Aspergillus spp.: Effect of serum on inhibitory and fungicidal interactions. Antimicrob. Agents Chemother. 2013, 57, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Meletiadis, J.; Melchers, W.J.G.; Rijs, A.J.M.M.; Mouton, J.W.; Verweij, P.E. In vitro interaction of voriconazole and anidulafungin against triazole-resistant Aspergillus fumigatus. Antimicrob. Agents Chemother. 2012, 57, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Brüggemann, R.J.M.; Melchers, W.J.G.; Rijs, A.J.M.M.; Verweij, P.E.; Mouton, J.W. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J. Antimicrob. Chemother. 2012, 68, 385–393. [Google Scholar] [CrossRef][Green Version]
- Caillot, D.; Bassaris, H.; McGeer, A.; Arthur, C.; Prentice, H.G.; Seifert, W.; De Beule, K. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin. Infect. Dis. 2001, 33, e83–e90. [Google Scholar] [CrossRef]
- Lee, J.W.; Amantea, M.A.; Francis, P.A.; Navarro, E.E.; Bacher, J.; Pizzo, P.A.; Walsh, T.J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 1994, 38, 713–718. [Google Scholar] [CrossRef][Green Version]
- Walsh, T.J.; Lutsar, I.; Driscoll, T.; Dupont, B.; Roden, M.; Ghahramani, P.; Hodges, M.; Groll, A.H.; Perfect, J.R. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr. Infect. Dis. J. 2002, 21, 240–248. [Google Scholar] [CrossRef]
- Walsh, T.J.; Pappas, P.; Winston, D.J.; Lazarus, H.M.; Petersen, F.; Raffalli, J.; Yanovich, S.; Stiff, P.J.; Greenberg, R.; Donowitz, G.; et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 2002, 346, 225–234. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T.; et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.-W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Petraitiene, R.; Petraitis, V.; Lyman, C.A.; Groll, A.H.; Mickiene, D.; Peter, J.; Bacher, J.; Roussillon, K.; Hemmings, M.; Armstrong, D.; et al. Efficacy, safety, and plasma pharmacokinetics of escalating dosages of intravenously administered ravuconazole lysine phosphoester for treatment of experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 2004, 48, 1188–1196. [Google Scholar] [CrossRef]
- Groll, A.H.; Walsh, T.J. Antifungal efficacy and pharmacodynamics of posaconazole in experimental models of invasive fungal infections. Mycoses 2006, 49, 7–16. [Google Scholar] [CrossRef]
- Walsh, T.J.; Raad, I.; Patterson, T.F.; Chandrasekar, P.; Donowitz, G.R.; Graybill, R.; Greene, R.; Hachem, R.; Hadley, S.; Herbrecht, R.; et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: An externally controlled trial. Clin. Infect. Dis. 2007, 44, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Selleslag, D.; Mullane, K.; Cornely, O.A.; Hope, W.; Lortholary, O.; Croos-Dabrera, R.; Lademacher, C.; Engelhardt, M.; Patterson, T.F. Impact of unresolved neutropenia in patients with neutropenia and invasive aspergillosis: A post hoc analysis of the SECURE trial. J. Antimicrob. Chemother. 2017, 73, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Krel, M.; Petraitis, V.; Petraitiene, R.; Jain, M.R.; Zhao, Y.; Li, H.; Walsh, T.J.; Perlin, D.S. Host biomarkers of invasive pulmonary aspergillosis to monitor therapeutic response. Antimicrob. Agents Chemother. 2014, 58, 3373–3378. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Petraitiene, R.; Petraitis, V.; Walsh, T.J.; Desai, A.; Bonate, P.; Hope, W. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: Implications for clinical breakpoints. J. Antimicrob. Chemother. 2016, 71, 1885–1891. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Moradi, P.W.; Strauss, G.E.; Katragkou, A.; Kovanda, L.L.; Hope, W.; Walsh, T.J. Pharmacokinetics and concentration-dependent efficacy of isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2016, 60, 2718–2726. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Katragkou, A.; Maung, B.B.W.; Naing, E.; Kavaliauskas, P.; Barat, S.; Borroto-Esoda, K.; Azie, N.; Angulo, D.; et al. Combination therapy with ibrexafungerp (formerly SCY-078), a first-in-class triterpenoid inhibitor of (1->3)-β-D-glucan synthesis, and isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Hope, W.; Walsh, T.J. Endpoint assessment in rabbit models of invasive pulmonary aspergillosis and mucormycosis. Methods Mol. Biol. 2017, 1625, 259–277. [Google Scholar] [CrossRef]
- Al-Nakeeb, Z.; Petraitis, V.; Goodwin, J.; Petraitiene, R.; Walsh, T.J.; Hope, W. Pharmacodynamics of amphotericin B deoxycholate, amphotericin B lipid complex, and liposomal amphotericin B against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 2735–2745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Francesconi, A.; Kasai, M.; Petraitiene, R.; Petraitis, V.; Kelaher, A.M.; Schaufele, R.; Hope, W.; Shea, Y.R.; Bacher, J.; Walsh, T.J. Characterization and comparison of galactomannan enzyme immunoassay and quantitative real-time PCR assay for detection of Aspergillus fumigatus in bronchoalveolar lavage fluid from experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 2006, 44, 2475–2480. [Google Scholar] [CrossRef]
- Walsh, T.J.; Wissel, M.C.; Grantham, K.J.; Petraitiene, R.; Petraitis, V.; Kasai, M.; Francesconi, A.; Cotton, M.P.; Hughes, J.E.; Greene, L.; et al. Molecular detection and species-specific identification of medically important Aspergillus species by real-time PCR in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 2011, 49, 4150–4157. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, D.A.; De Torre-Minguela, C.; Wang, H.; Devor, C.B.; Munson, P.J.; Ying, S.-X.; Kern, S.J.; Petraitiene, R.; Levens, D.L.; Walsh, T.J.; et al. Protein expression profiles distinguish between experimental invasive pulmonary aspergillosis and Pseudomonas pneumonia. Proteomics 2010, 10, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Petraitis, V.; Petraitiene, R.; Field-Ridley, A.; Sutton, D.; Ghannoum, M.; Sein, T.; Schaufele, R.; Peter, J.; Bacher, J.; et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: Pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 2003, 188, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Schock, K.; Marino, S.; Andriole, V.T. Efficacies of two new antifungal agents, the triazole ravuconazole and the echinocandin LY-303366, in an experimental model of invasive aspergillosis. Antimicrob. Agents Chemother. 2000, 44, 3381–3388. [Google Scholar] [CrossRef]
- Jeans, A.R.; Howard, S.J.; Al-Nakeeb, Z.; Goodwin, J.; Gregson, L.; Warn, P.A.; Hope, W. Combination of voriconazole and anidulafungin for treatment of triazole-resistant Aspergillus fumigatus in an in vitro model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2012, 56, 5180–5185. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; McCarthy, M.W.; Kovanda, L.L.; Zaw, M.H.; Hussain, K.; Shaikh, N.; Maung, B.B.W.; Sekhon, N.K.; Hope, W.W.; et al. Combination therapy with isavuconazole and micafungin for treatment of experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2017, 61, e00305-17. [Google Scholar] [CrossRef]
- Stevens, V.M.; Mueller, S.W.; Reynolds, P.M.; MacLaren, R.; Kiser, T.H. Extrapolating antifungal animal data to humans—Is it reliable? Curr. Fungal. Infect. Rep. 2020, 14, 50–62. [Google Scholar] [CrossRef]
- Siopi, M.; Siafakas, N.; Vourli, S.; Mouton, J.W.; Zerva, L.; Meletiadis, J. Dose optimization of voriconazole/anidulafungin combination against Aspergillus fumigatus using an in vitro pharmacokinetic/pharmacodynamic model and response surface analysis: Clinical implications for azole-resistant aspergillosis. J. Antimicrob. Chemother. 2016, 71, 3135–3147. [Google Scholar] [CrossRef][Green Version]
- O’Shaughnessy, E.M.; Meletiadis, J.; Stergiopoulou, T.; Demchok, J.P.; Walsh, T.J. Antifungal interactions within the triple combination of amphotericin B, caspofungin and voriconazole against Aspergillus species. J. Antimicrob. Chemother. 2006, 58, 1168–1176. [Google Scholar] [CrossRef]
- Marr, K.A.; Schlamm, H.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination antifungal therapy for invasive aspergillosis. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Ali, N.M.; Allende, M.C.; Lee, J.; Garrett, K.; Battaglia, S.; Piscitelli, S.C.; Rinaldi, M.G.; Pizzo, P.A.; Walsh, T.J. Itraconazole for experimental pulmonary aspergillosis: Comparison with amphotericin B, interaction with cyclosporin A, and correlation between therapeutic response and itraconazole concentrations in plasma. Antimicrob. Agents Chemother. 1994, 38, 1303–1308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berenguer, J.; Allende, M.C.; Lee, J.W.; Garrett, K.; Lyman, C.; Ali, N.M.; Bacher, J.; Pizzo, P.A.; Walsh, T.J. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 1995, 152, 1079–1086. [Google Scholar] [CrossRef] [PubMed]

| Antifungal Agent (References) | Markers of In Vivo Therapeutic Response | |||||||
|---|---|---|---|---|---|---|---|---|
| RFB | PH-Score | Lung-Wt | Survival | Serum GMI | Serum BDG | CTS Score | PCR | |
| Deoxycholate amphotericin B (DAMB) [11,12,13,14,15,16] | √ | √ | √ | √ | √ | √ | √ | |
| Liposomal amphotericin B (LAMB) [4,7,11,17,18] | √ | √ | √ | √ | √ | √ | √ | √ |
| Amphotericin B lipid complex ABLC) [11,19,20] | √ | √ | √ | √ | √ | |||
| Amphotericin B colloidal dispersion (ABCD) [21,22,23,24,25] | √ | √ | √ | √ | ||||
| Liposomal nystatin [26,27] | √ | √ | √ | √ | ||||
| Caspofungin [13,28,29,30,31,32,33] | √ | √ | √ | √ | √ | |||
| Micafungin [34,35,36,37,38,39,40,41] | √ | √ | √ | √ | √ | √ | ||
| Anidulafungin [42,43,44,45,46] | √ | √ | √ | √ | √ | |||
| Itraconazole [12,47,48,49,50] | √ | √ | √ | √ | √ | |||
| Voriconazole [42,51,52,53,54,55,56,57,58,59] | √ | √ | √ | √ | √ | √ | √ | |
| Ravuconazole [17,34,60,61,62] | √ | √ | √ | √ | √ | √ | √ | |
| Posaconazole [5,12,63,64] | √ | √ | √ | √ | √ | √ | √ | |
| Isavuconazole [35,65,66,67,68] | √ | √ | √ | √ | √ | √ | ||
| Ibrexafungerp [65] | √ | √ | √ | √ | √ | √ | ||
| Olorofim (F901318) [69] | √ | √ | √ | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, T.J.; Petraitiene, R.; Petraitis, V. Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets? J. Fungi 2020, 6, 198. https://doi.org/10.3390/jof6040198
Walsh TJ, Petraitiene R, Petraitis V. Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets? Journal of Fungi. 2020; 6(4):198. https://doi.org/10.3390/jof6040198
Chicago/Turabian StyleWalsh, Thomas J., Ruta Petraitiene, and Vidmantas Petraitis. 2020. "Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets?" Journal of Fungi 6, no. 4: 198. https://doi.org/10.3390/jof6040198
APA StyleWalsh, T. J., Petraitiene, R., & Petraitis, V. (2020). Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets? Journal of Fungi, 6(4), 198. https://doi.org/10.3390/jof6040198





