Unveiling the Hidden Diversity of Rock-Inhabiting Fungi: Chaetothyriales from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Sampling
2.2. Isolation of Fungi
2.3. Morphology
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Alignment and Phylogenetic Analyses
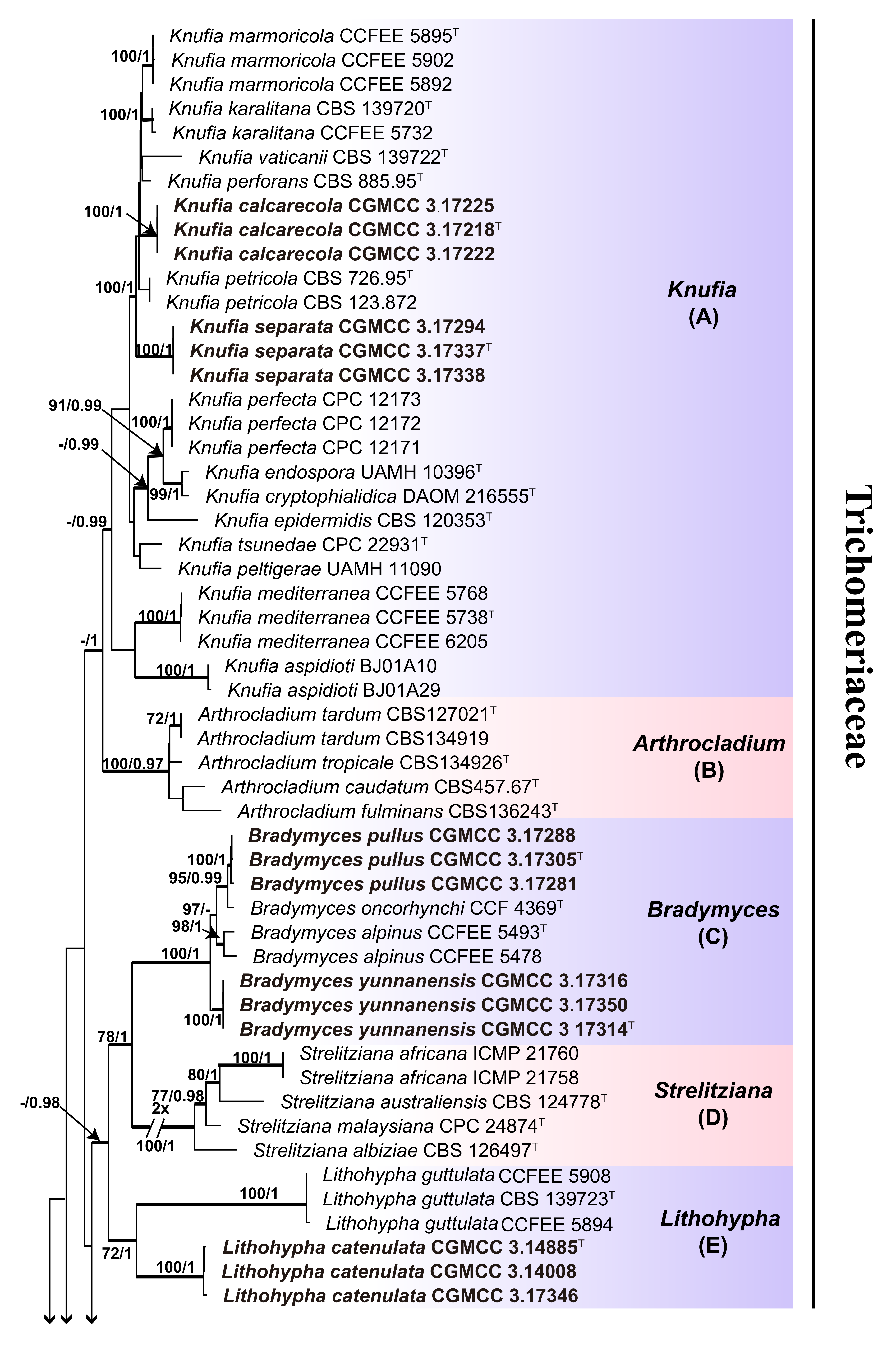
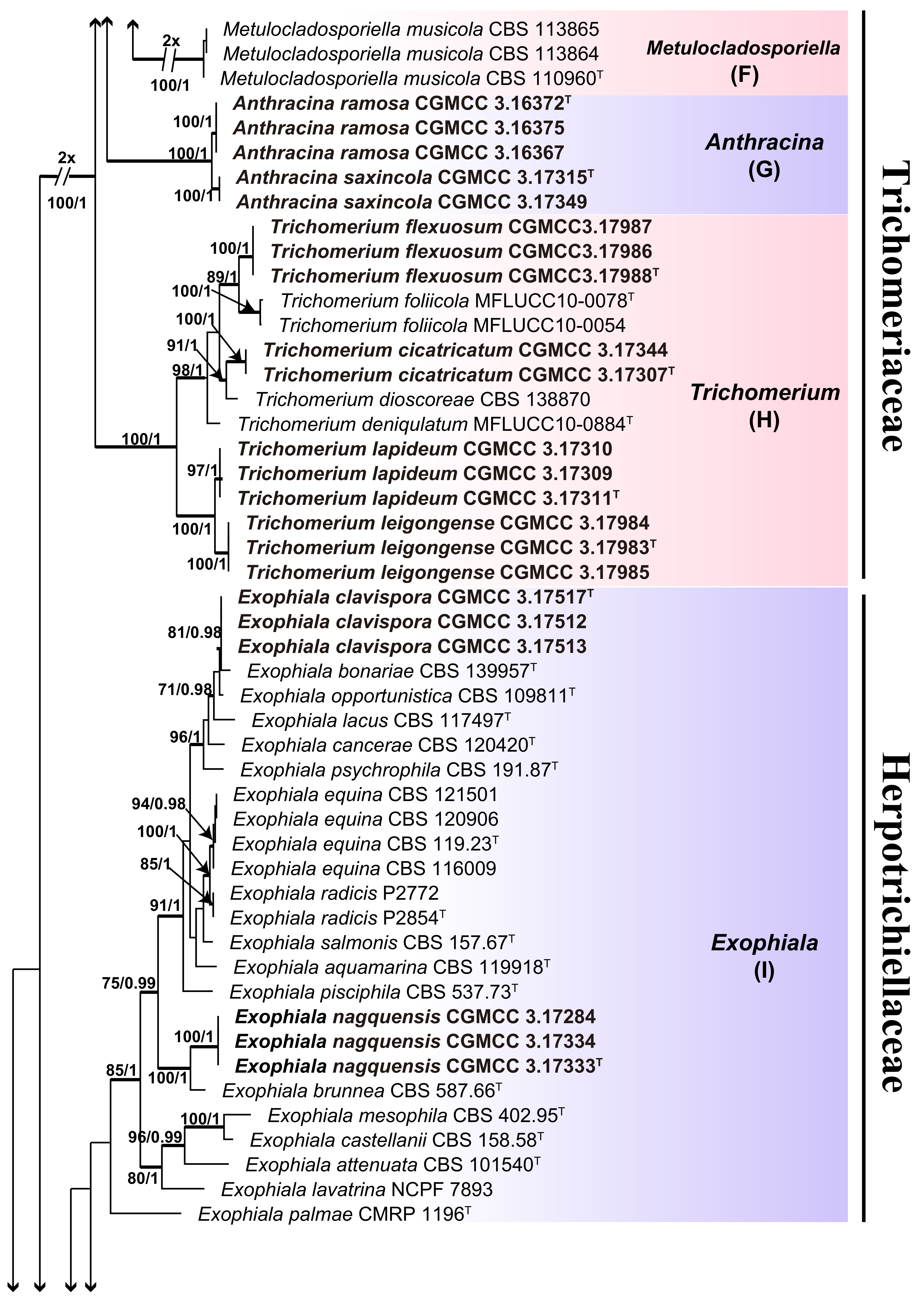
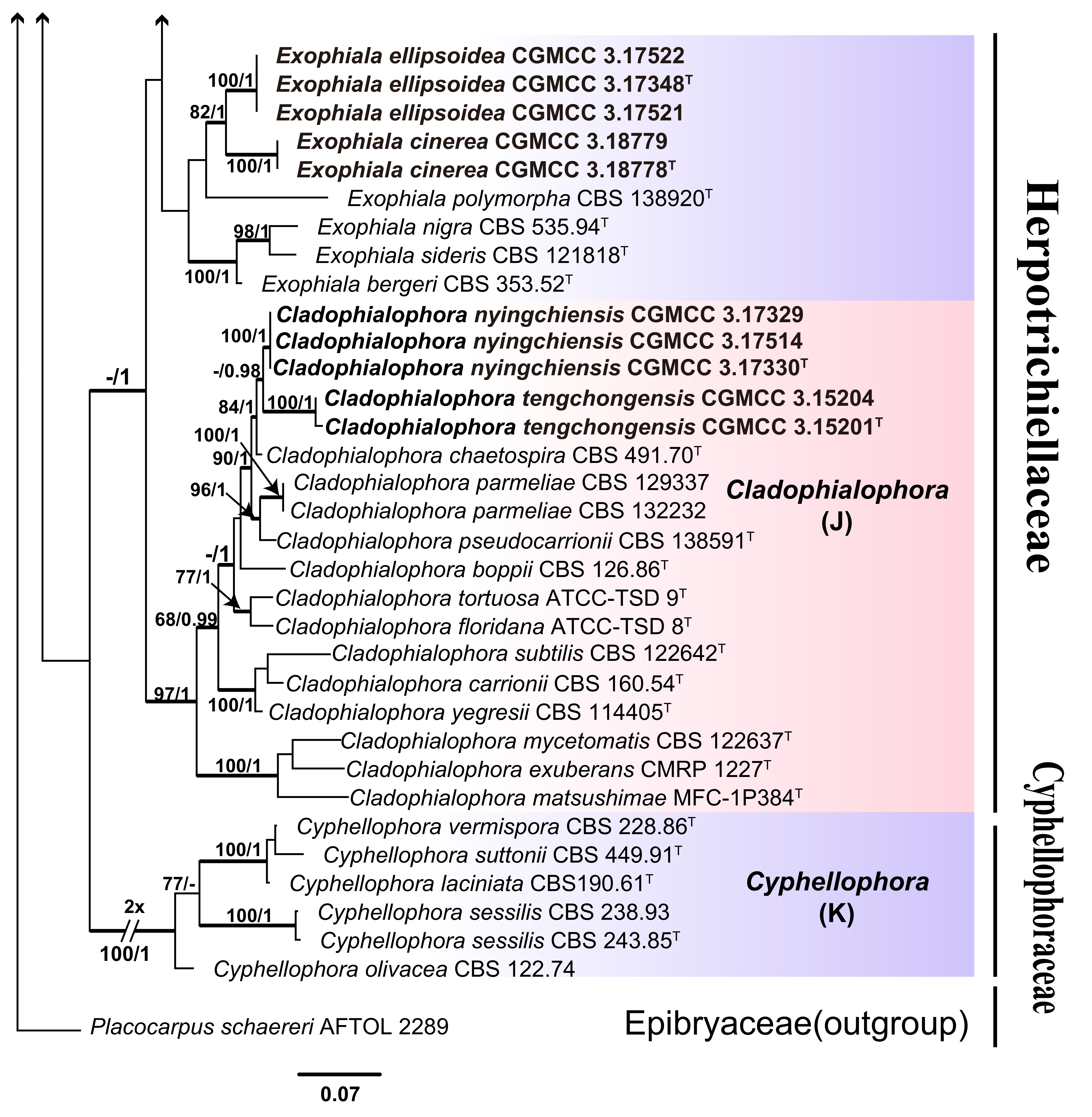
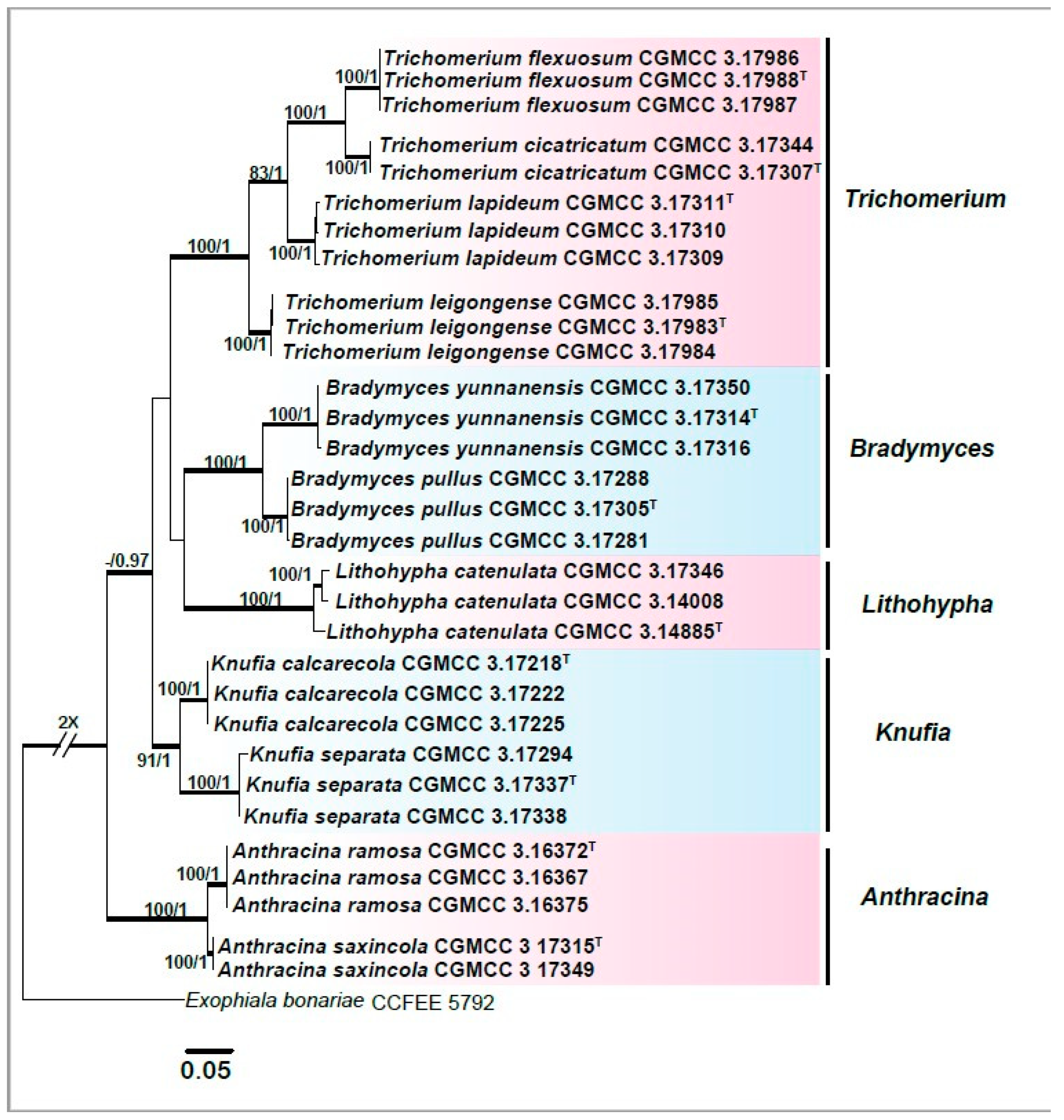
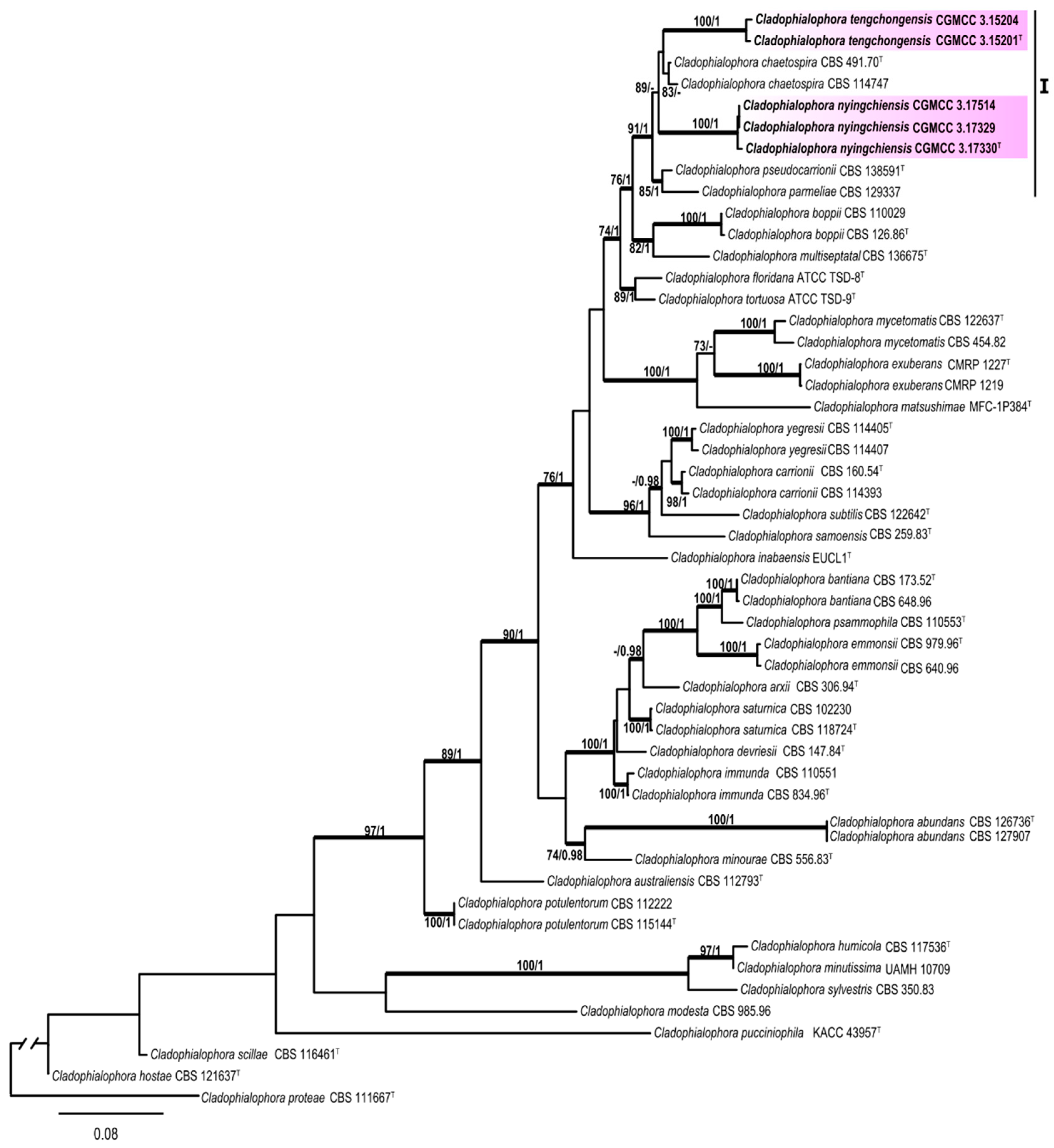
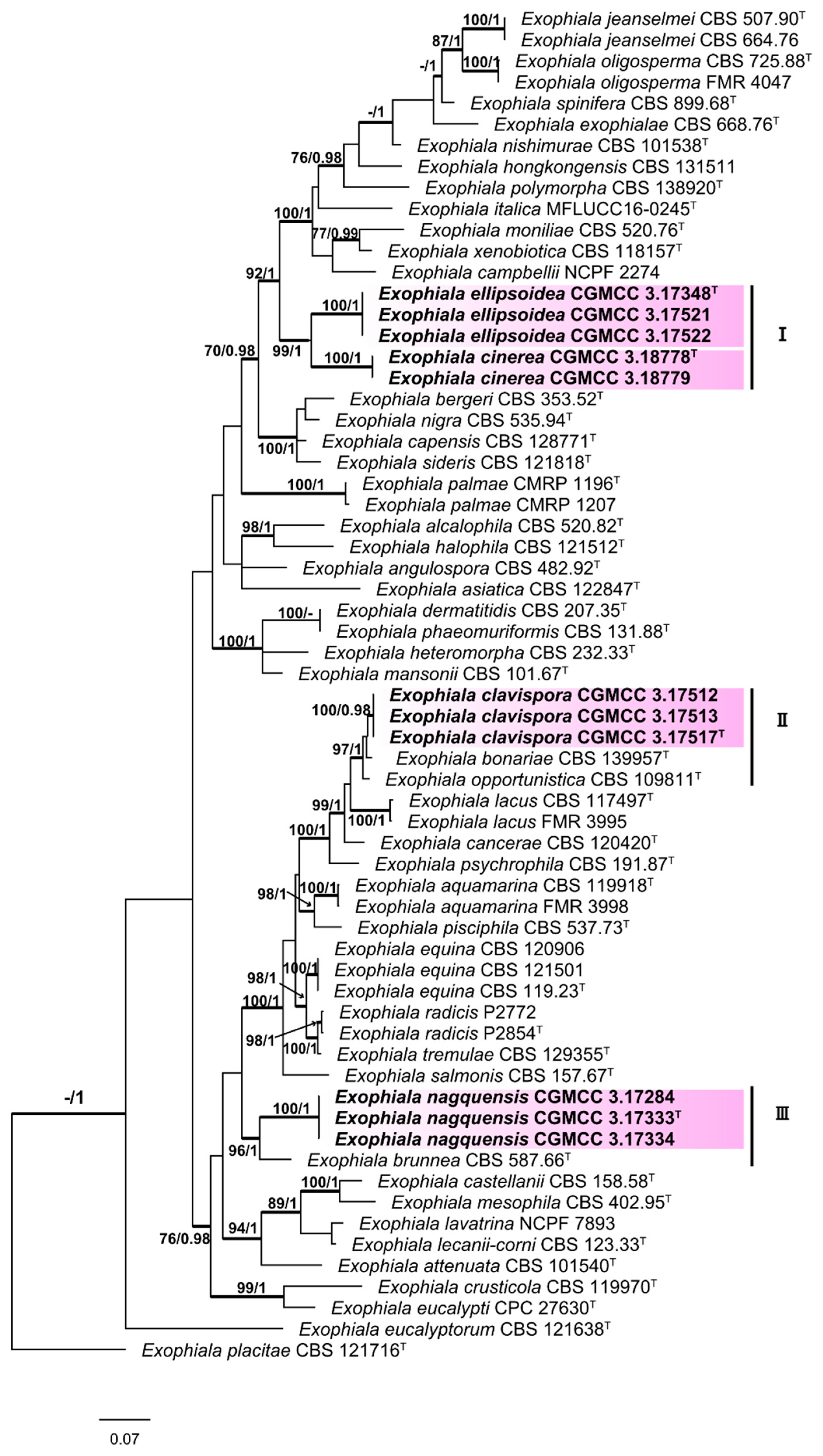
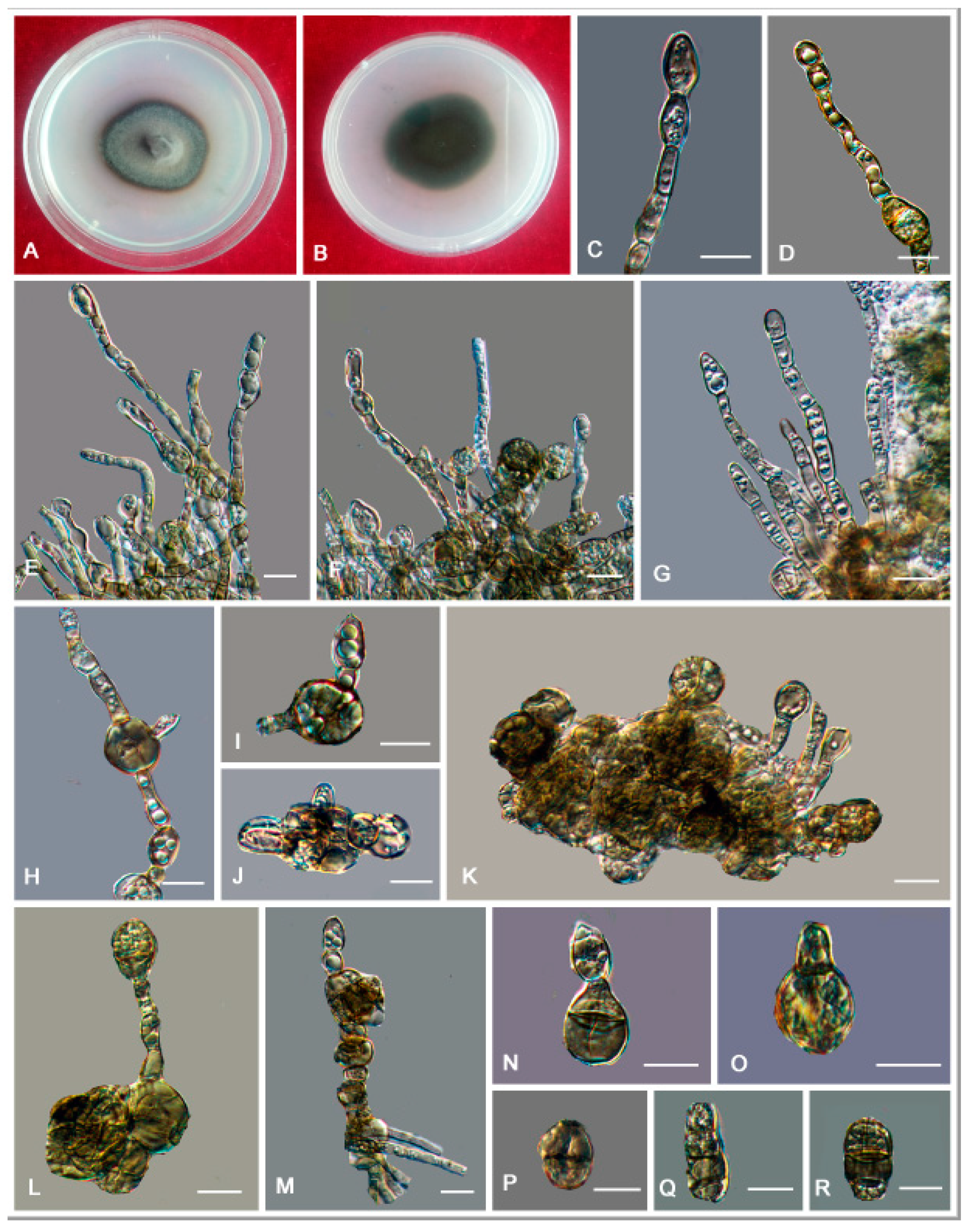
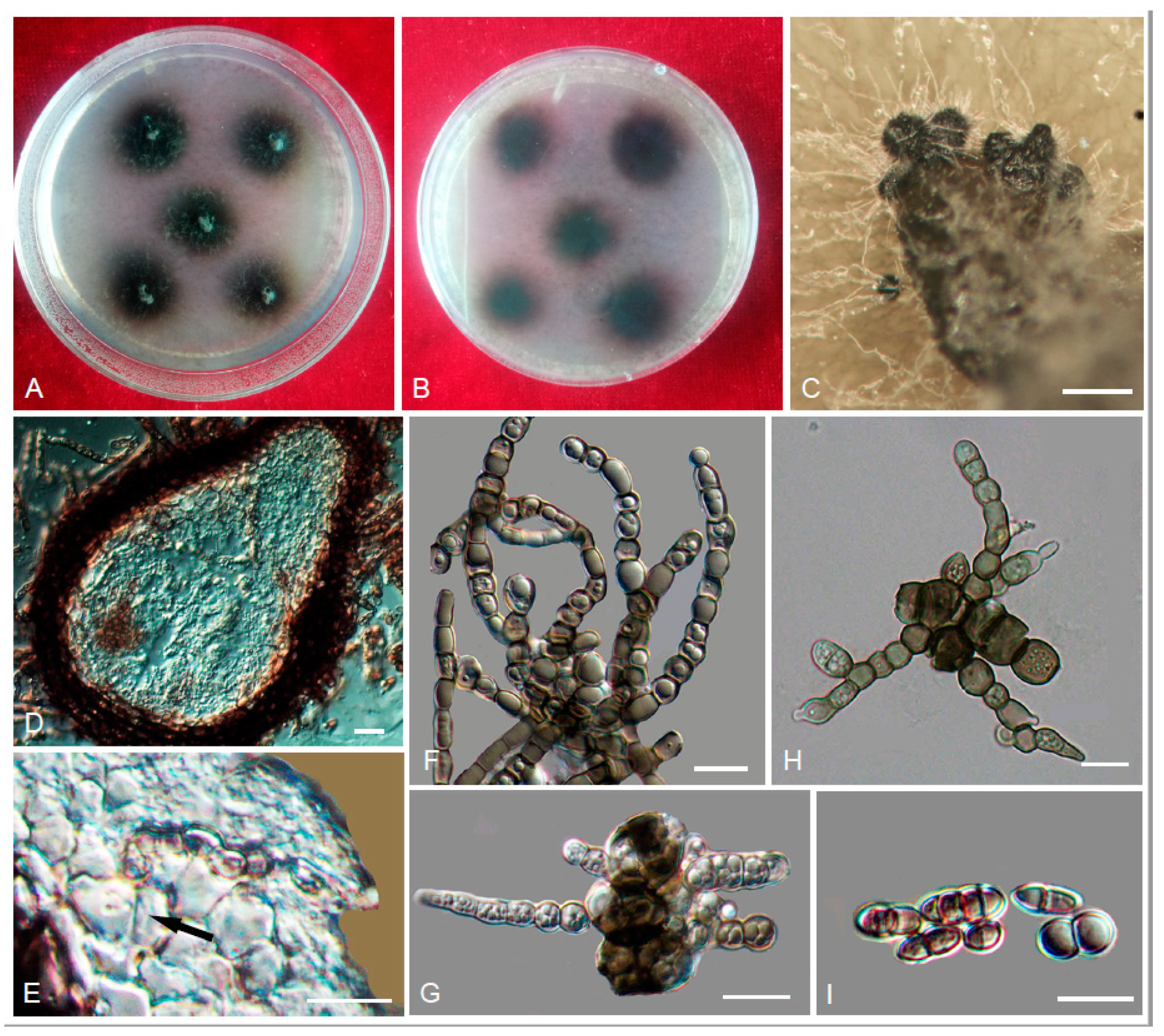
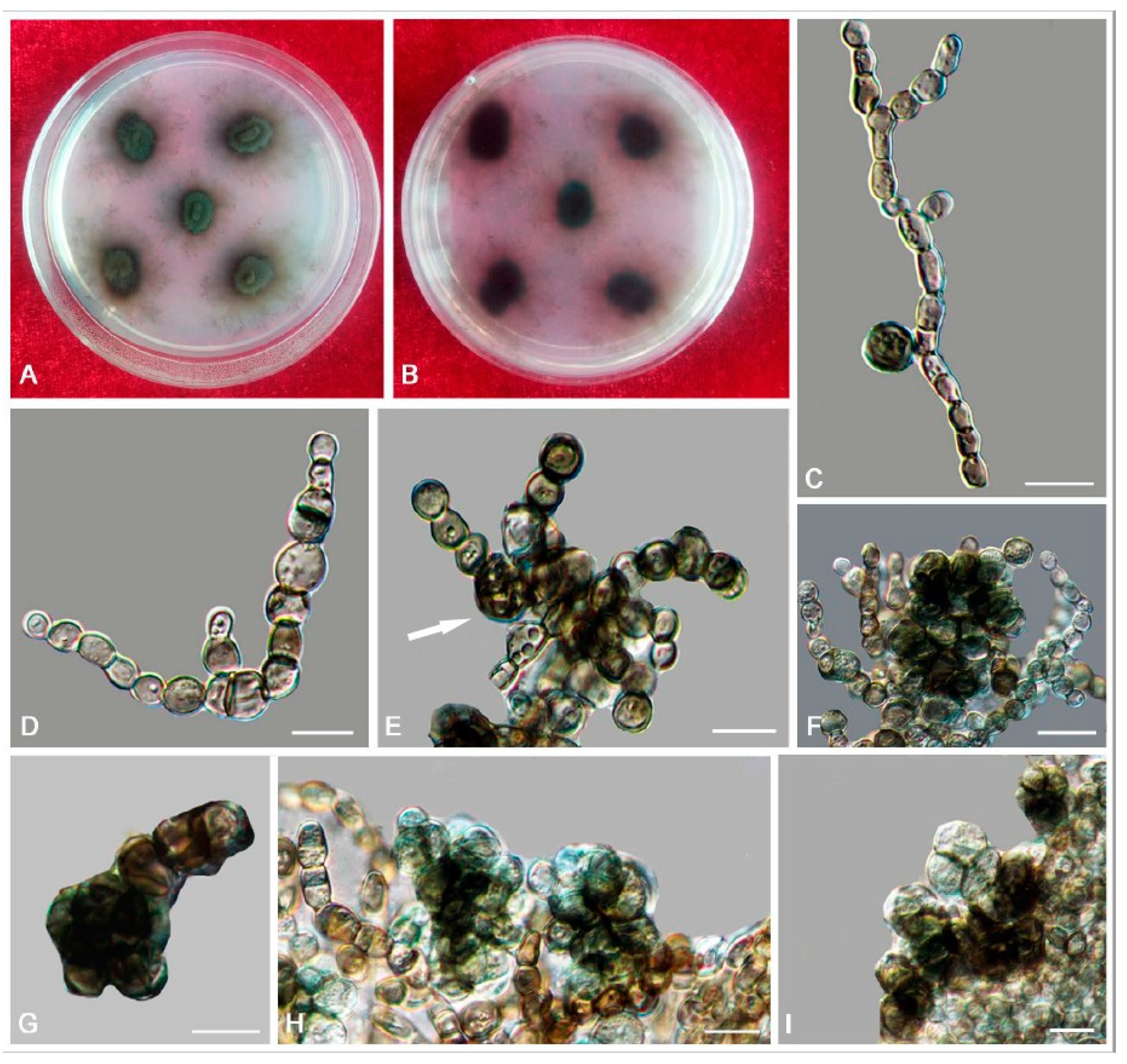
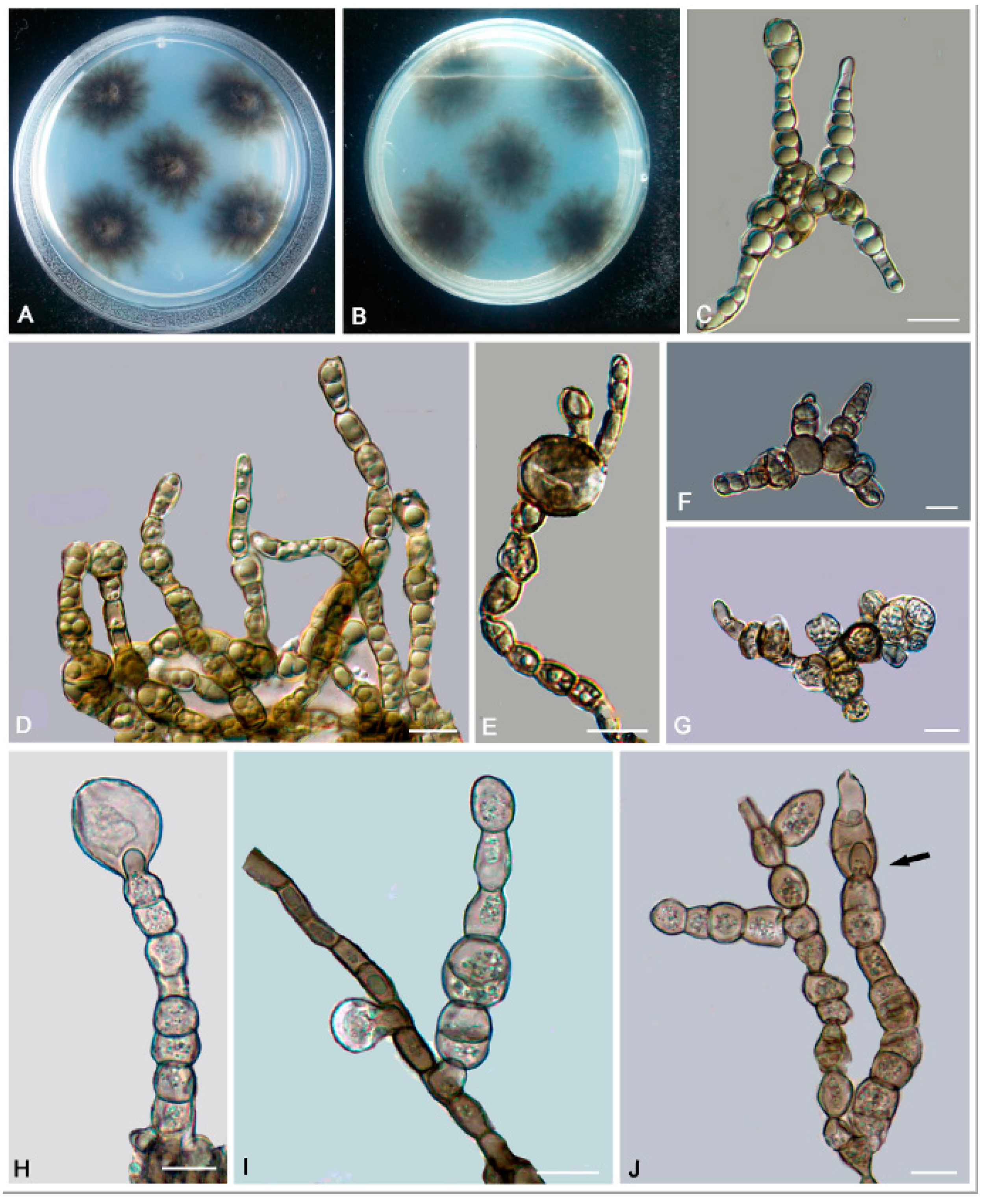
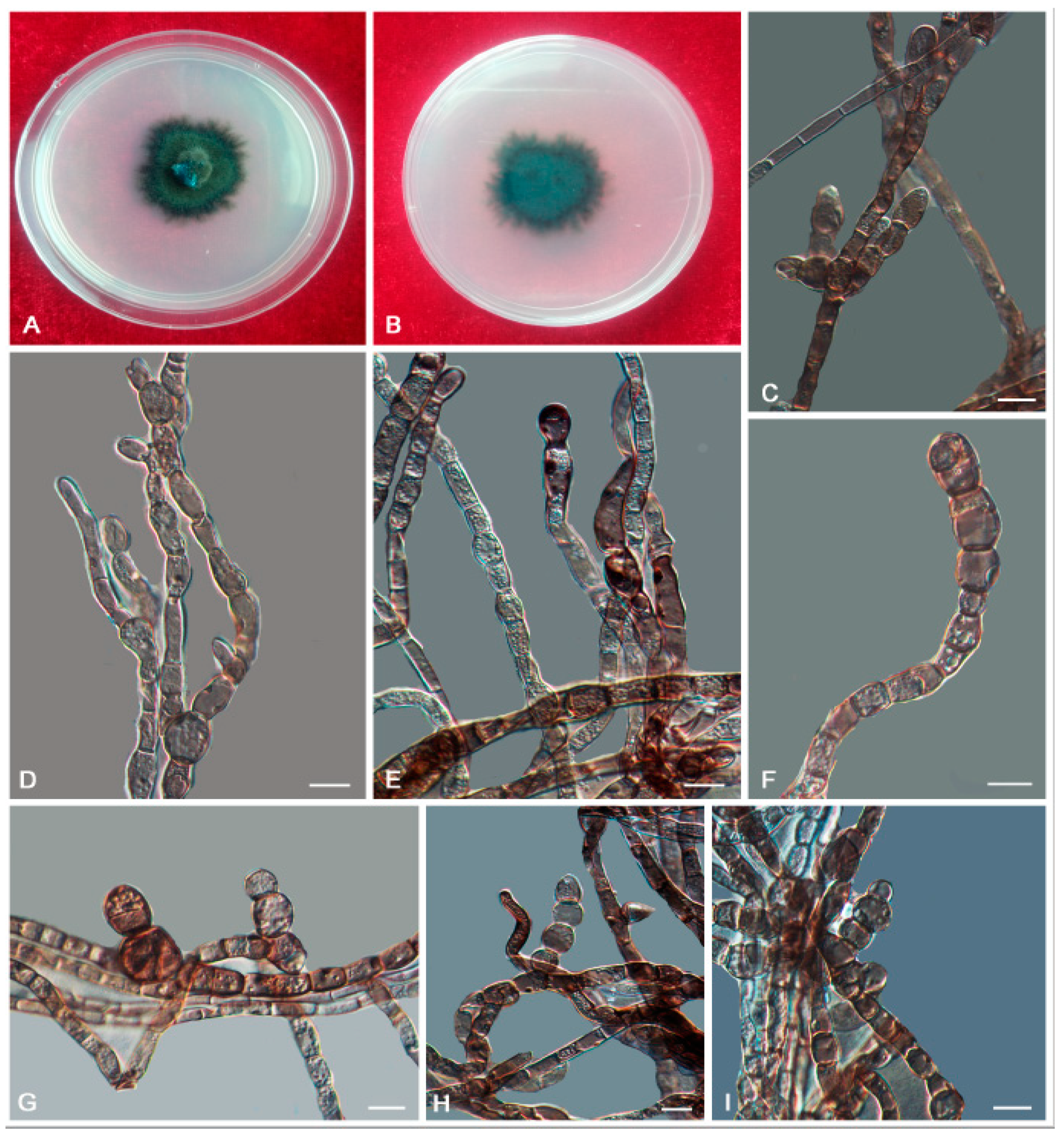
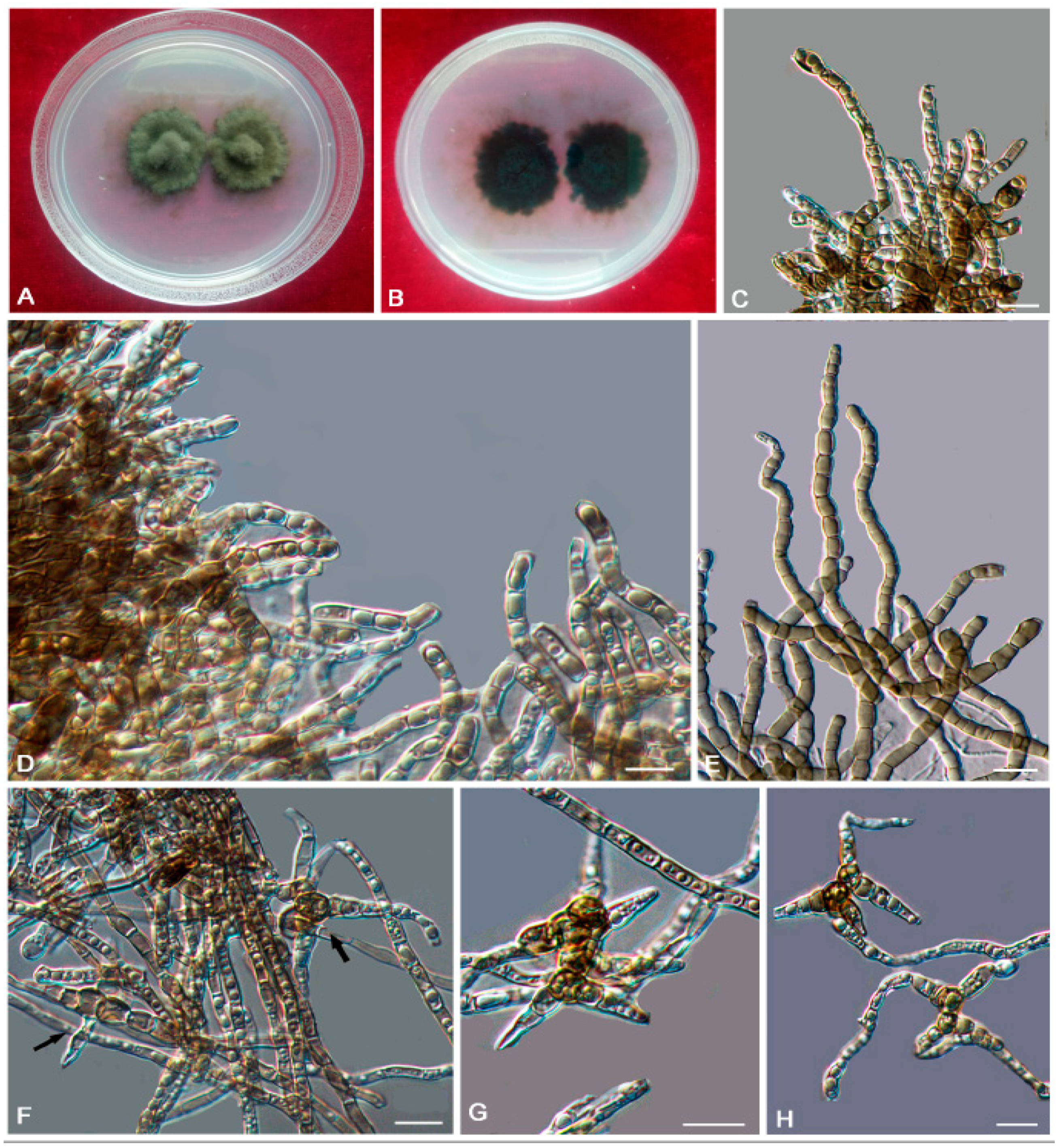
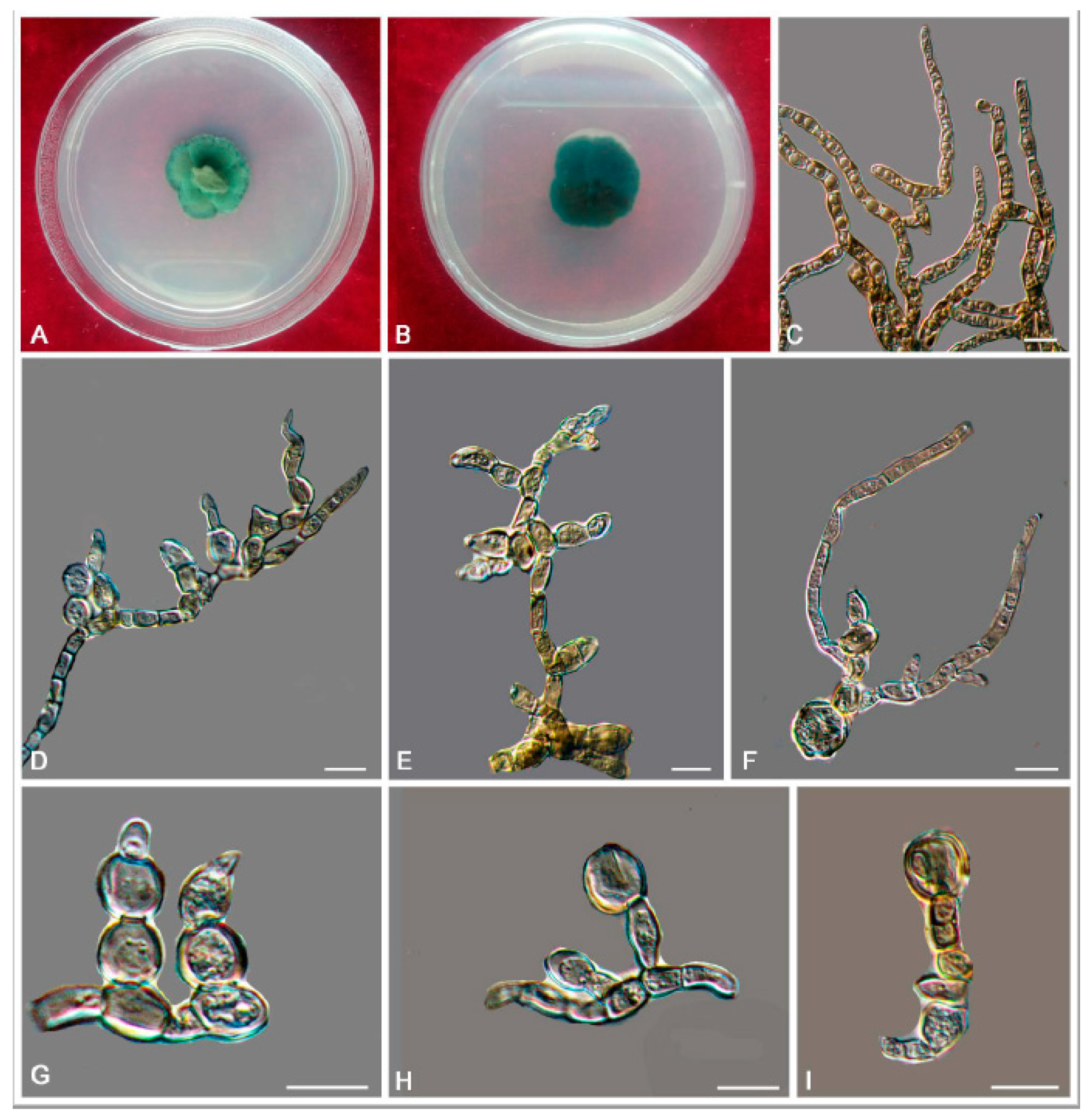
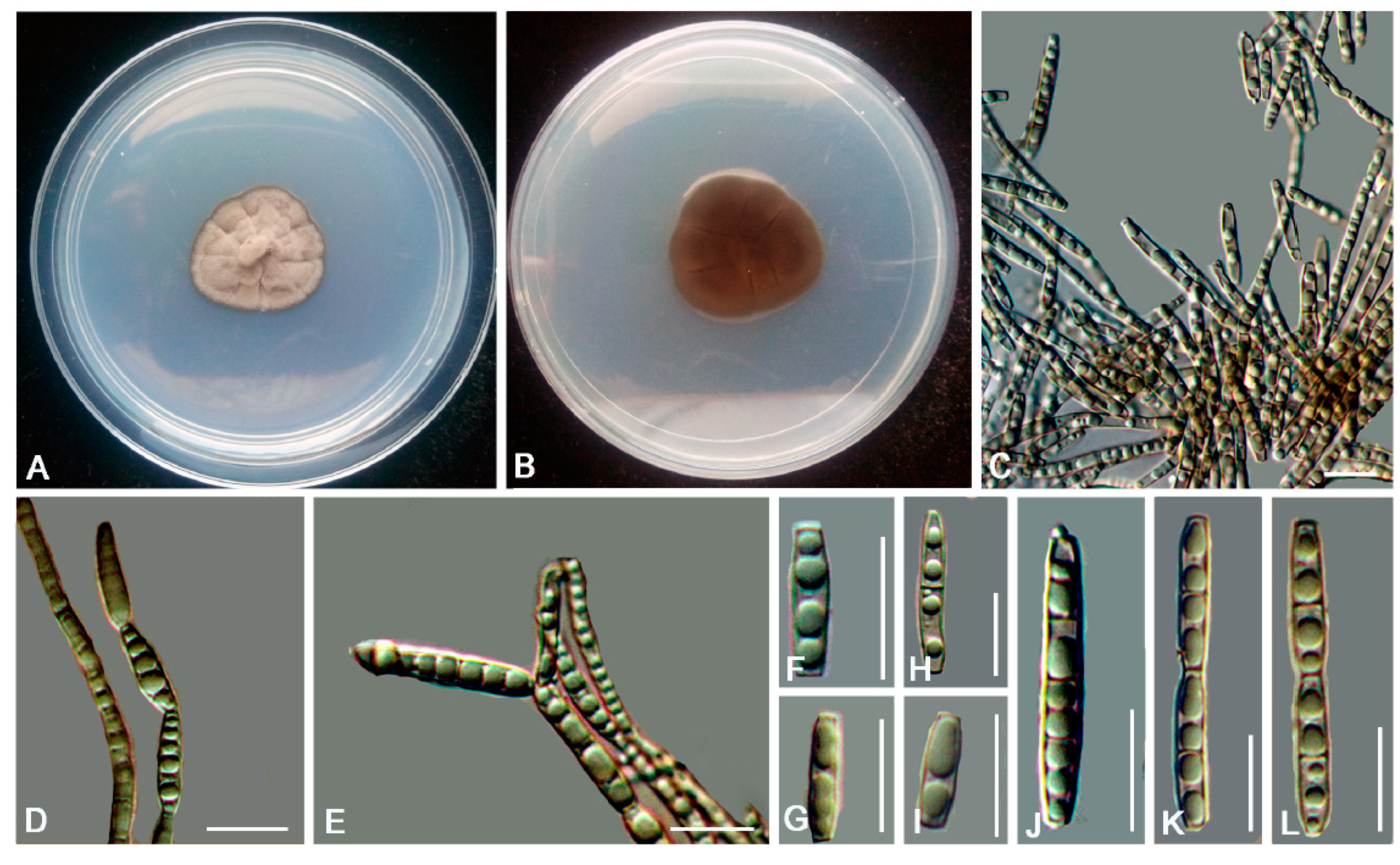
3. Results
3.1. Multi-Locus Phylogeny
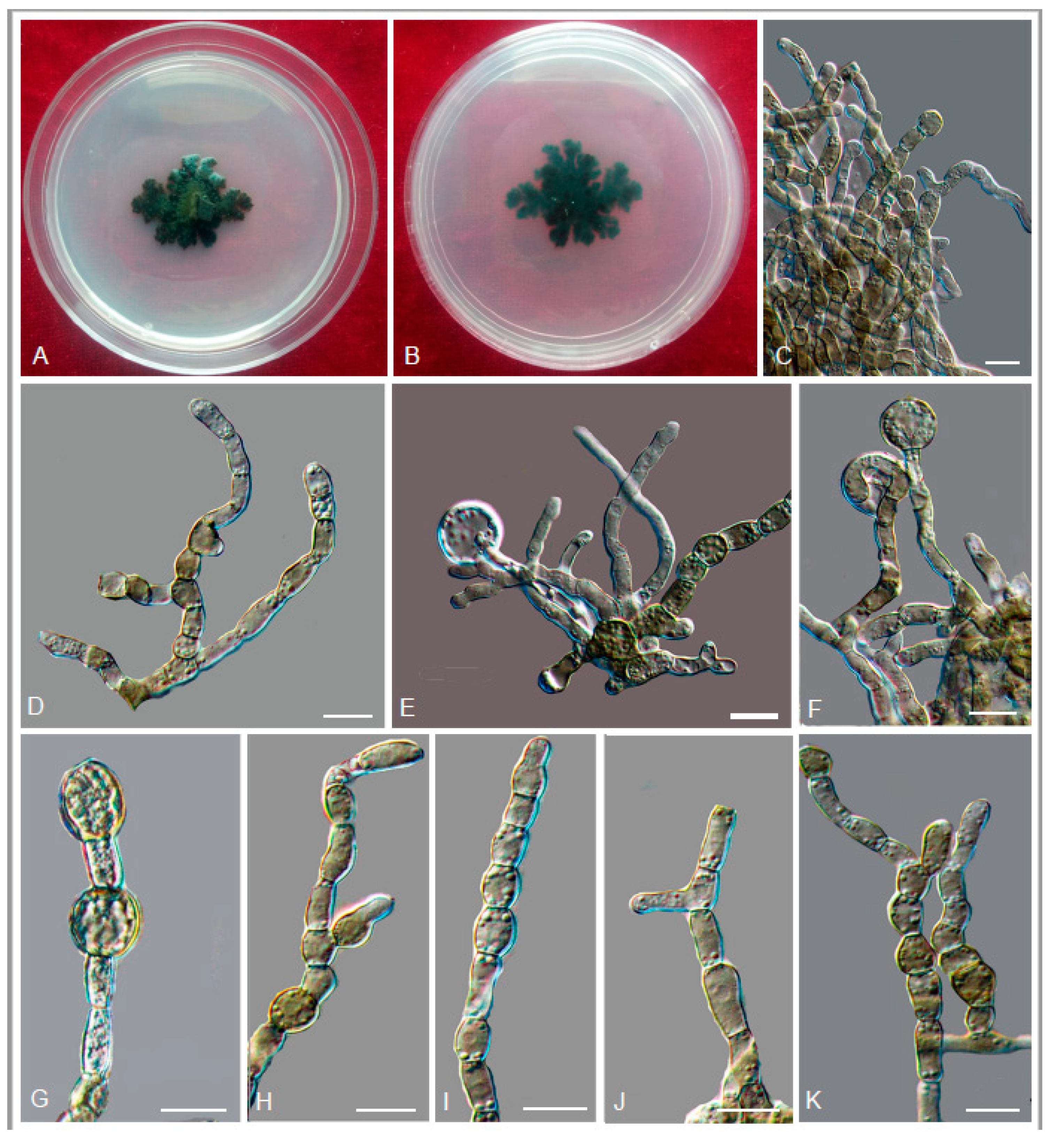
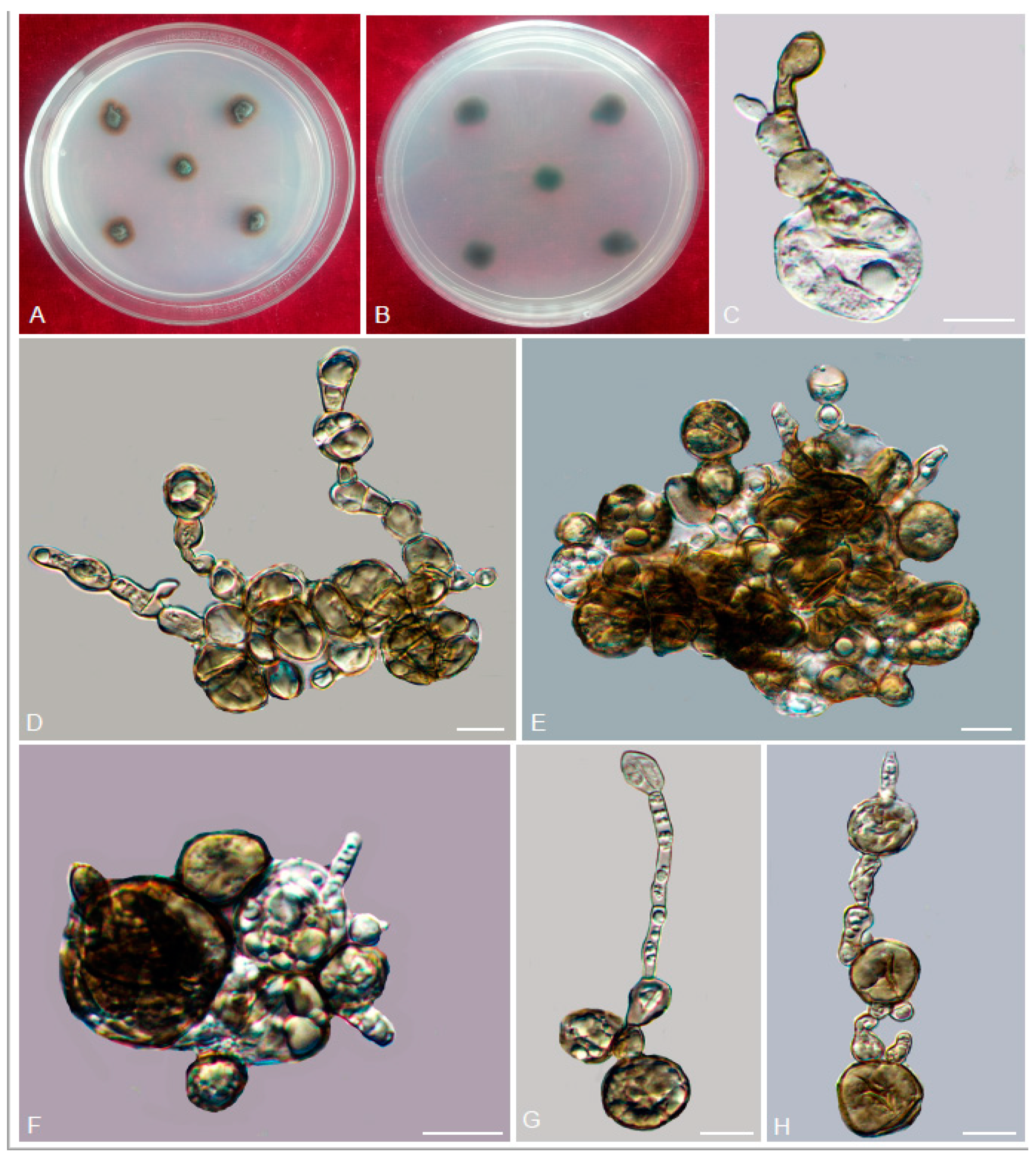
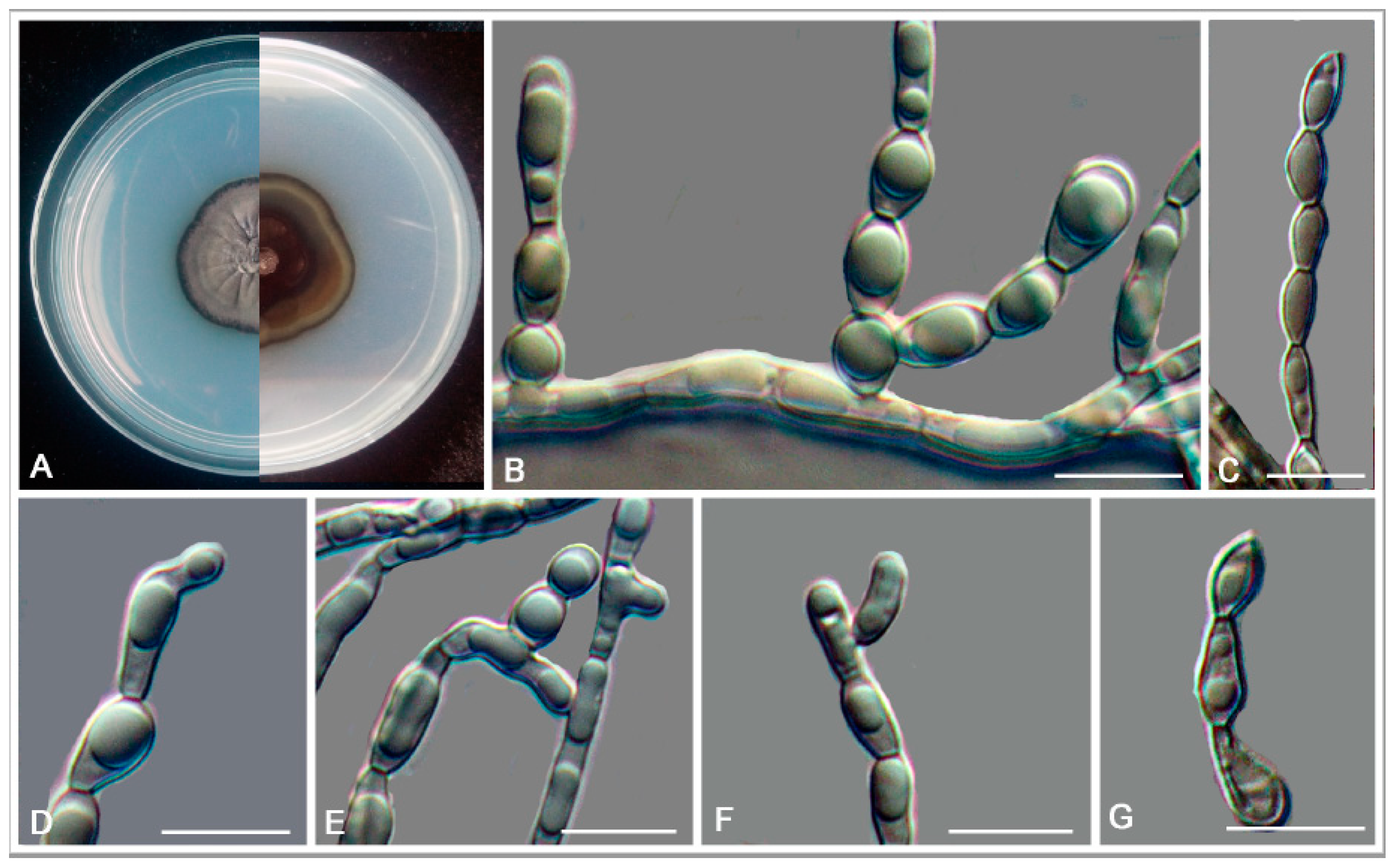
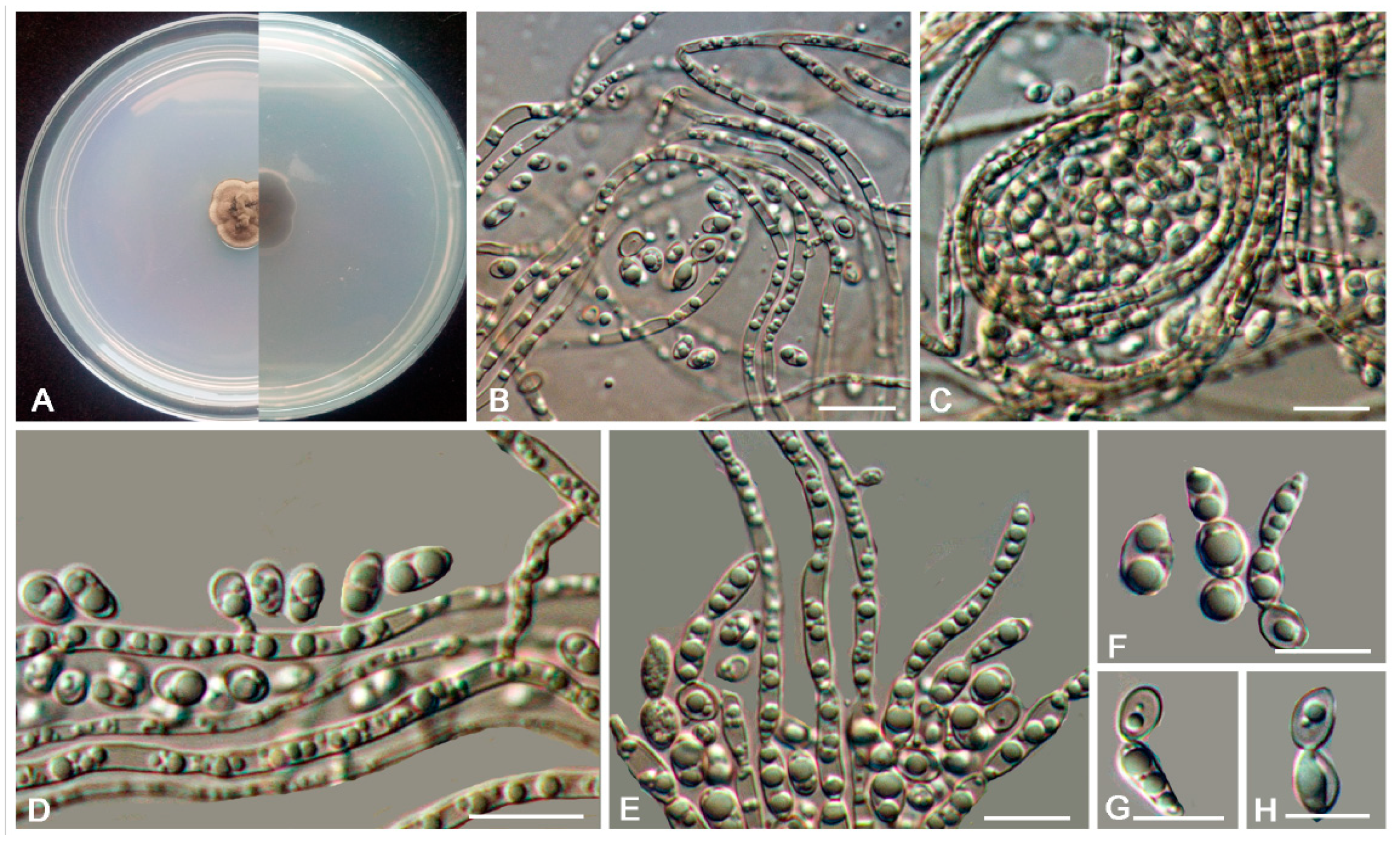
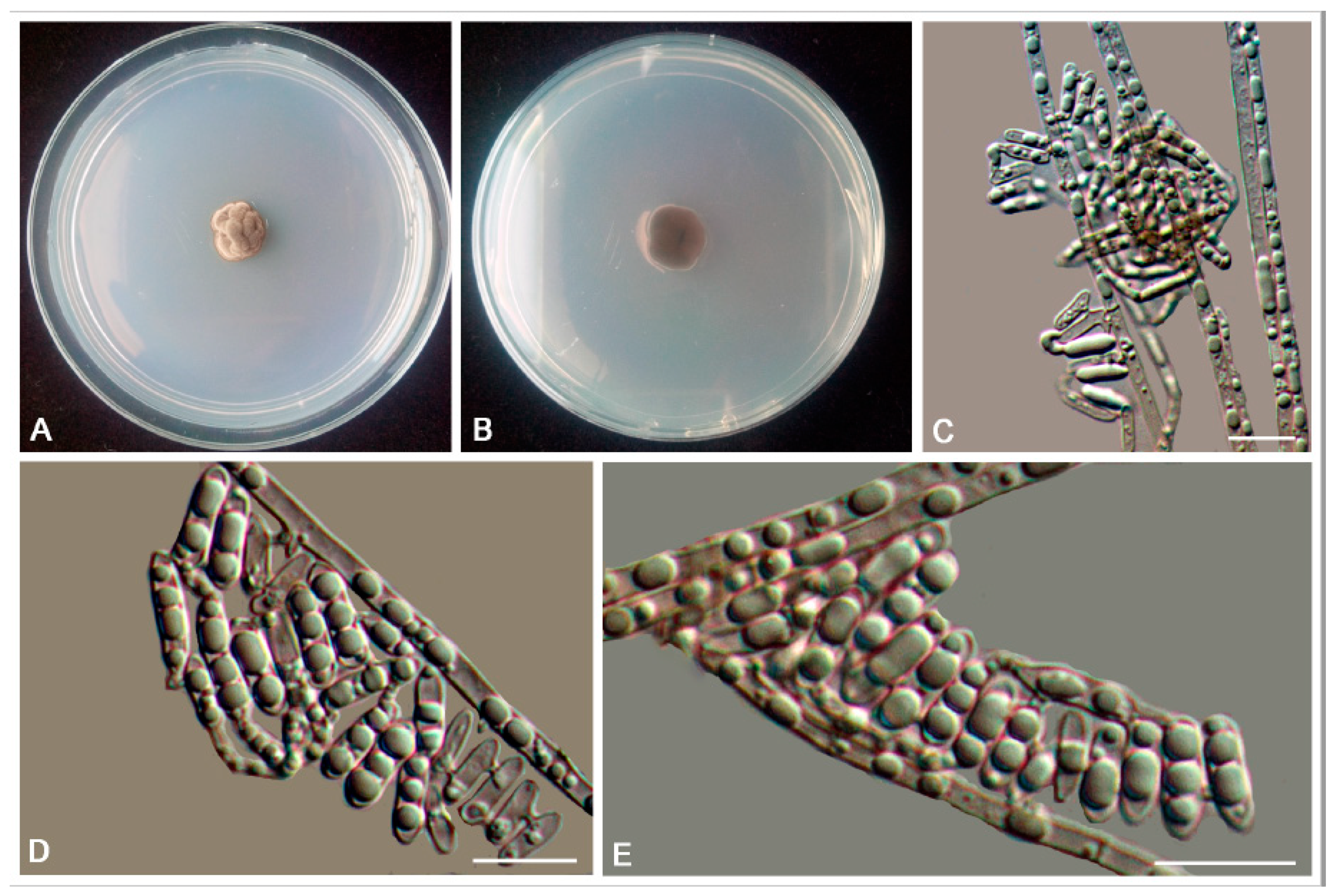
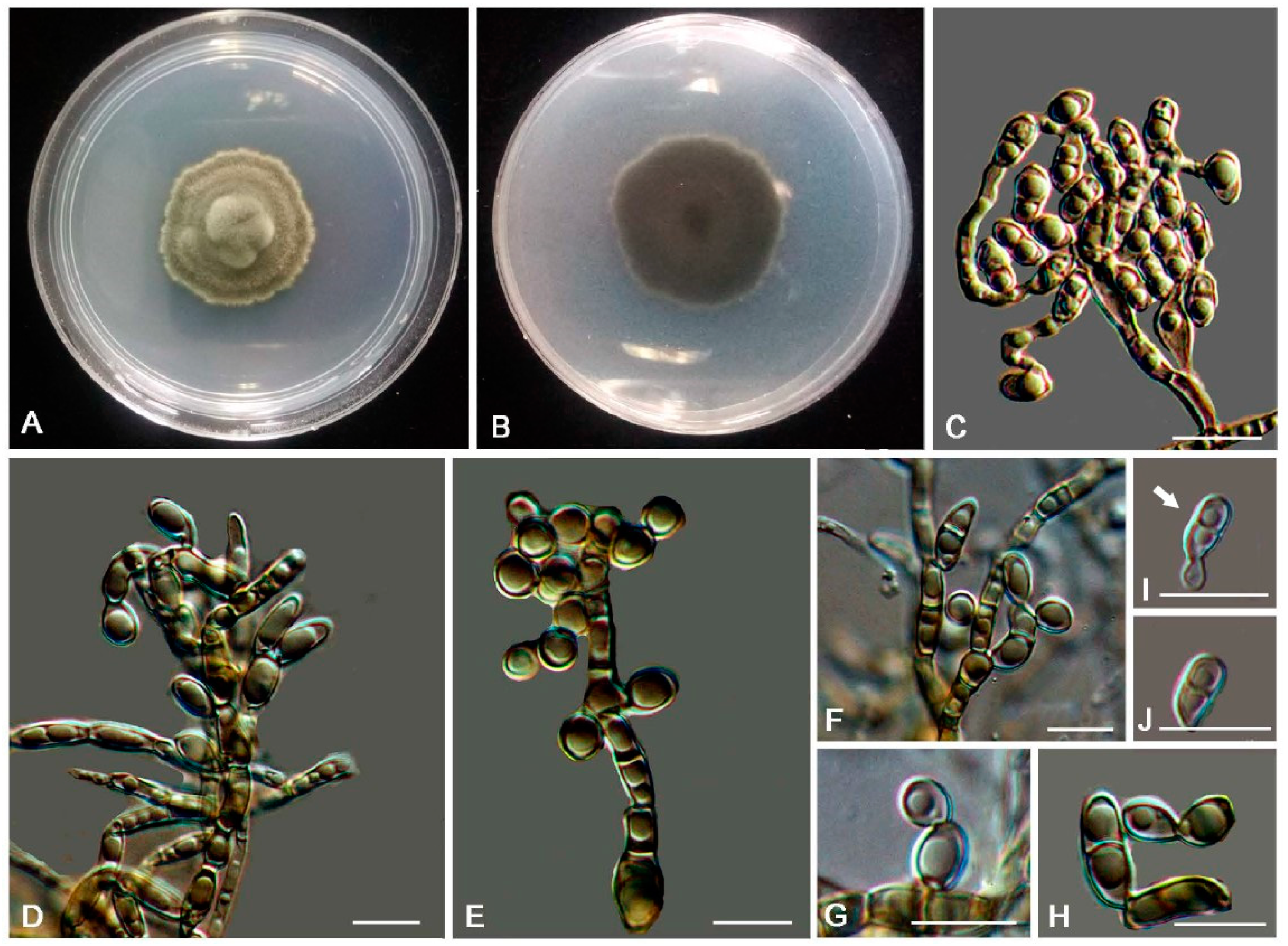
3.2. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuhlman, K.R.; Fusco, W.G.; La Duc, M.T.; Anderson, R.C.; Erickson, I.K.; Stuecker, T.; Benardini, J.; Strap, J.L.; Crawford, R.L. Diversity of microorganisms within rock varnish in the Whipple Mountains, California. Appl. Environ. Microbiol. 2006, 72, 1708–1715. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Gueidan, C.; Villasenor, C.R.; de Hoog, G.S.; Gorbushina, A.A.; Untereiner, W.A. A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud. Mycol. 2008, 61, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gostincar, C.; Grube, M.; de Hoog, S.; Zalar, P.; Gunde-Cimerman, N. Extremotolerance in fungi: Evolution on the edge. FEMS Microbiol. Ecol. 2010, 71, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Gostincar, C.; Muggia, L.; Grube, M. Polyextrernotolerant black fungi: Oligotrophisrn, adaptive potential, and a link to lichen symbioses. Front. Microbiol. 2012, 3, 390. [Google Scholar] [CrossRef]
- Sterflinger, K.; Tesei, D.; Zakharova, K. Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecol. 2012, 5, 453–462. [Google Scholar] [CrossRef]
- Friedmann, E.I. Endolithic microorganisms in the Antarctic cold desert. Science 1982, 215, 1045–1053. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Krumbein, W.E.; Hamman, C.H.; Panina, L.; Soukharjevski, S.; Wollenzien, U. Role of black fungi in color change and biodeterioration of antique marbles. Geomicrobiol. J. 1993, 11, 205–221. [Google Scholar] [CrossRef]
- Sert, H.B.; Sümbül, H.; Sterflinger, K. Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey). Antonie Van Leeuwenhoek 2007, 91, 217–227. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Isola, D.; Zucconi, L.; Onofri, S.; Caneva, G.; de Hoog, G.S.; Selbmann, L. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Divers. 2016, 76, 75–96. [Google Scholar] [CrossRef]
- Sterflinger, K.; Krumbein, W.E. Multiple stress factors affecting growth of rock-inhabiting black fungi. Plant Biol. 1995, 108, 490–496. [Google Scholar] [CrossRef]
- Onofri, S.; Selbmann, L.; de Hoog, G.S.; Mrube, M.; Barreca, D.; Ruisi, S.; Zucconi, L. Evolution and adaptation of fungi at boundaries of life. Adv. Space Res. 2007, 40, 1657–1664. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Kotlova, E.R.; Sherstneva, O.A. Cellular responses of microcolonial rock fungi to long-term desiccation and subsequent rehydration. Stud. Mycol. 2008, 61, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, K.; Tesei, D.; Marzban, G.; Dijksterhuis, J.; Wyatt, T.; Sterflinger, K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia 2013, 175, 537–547. [Google Scholar] [CrossRef]
- Ruibal, C.; Platas, G.; Bills, G.F. Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycol. Prog. 2005, 4, 23–38. [Google Scholar] [CrossRef]
- Sert, H.B.; Sümbül, H.; Sterflinger, K. A new species of Capnobotryella from monument surfaces. Mycol. Res. 2007, 111, 1235–1241. [Google Scholar] [CrossRef]
- Sert, H.B.; Sümbül, H.; Sterflinger, K. Sarcinomyces sideticae, a new black yeast from historical marble monuments in Side (Antalya, Turkey). Bot. J. Linnean Soc. 2007, 154, 373–380. [Google Scholar] [CrossRef]
- Marvasi, M.; Donnarumma, F.; Frandi, A.; Mastromei, G.; Sterflinger, K.; Tiano, P.; Perito, B. Black microcolonial fungi as deteriogens of two famous marble statues in Florence, Italy. Int. Biodeterior. Biodegrad. 2012, 68, 36–44. [Google Scholar] [CrossRef]
- Onofri, S.; Zucconi, L.; Isola, D.; Selbmann, L. Rock-inhabiting fungi and their role in deterioration of stone monuments in the Mediterranean area. Plant Biosyst. 2014, 148, 384–391. [Google Scholar] [CrossRef]
- Ruibal, C.; Gueidan, C.; Selbmann, L.; Gorbushina, A.A.; Crous, P.W.; Groenewald, J.Z.; Muggia, L.; Grube, M.; Isola, D.; Schoch, C.L.; et al. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud. Mycol. 2009, 64, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, A.; Hambleton, S.; Currah, R.S. The anamorph genus Knufia and its phylogenetically allied species in Coniosporium, Sarcinomyces, and Phaeococcomyces. Botany 2011, 89, 523–536. [Google Scholar] [CrossRef]
- Réblová, M.; Untereiner, W.A.; Réblová, K. Novel evolutionary lineages revealed in the Chaetothyriales (fungi) based on multigene phylogenetic analyses and comparison of its secondary structure. PLoS ONE 2013, 8, e63547. [Google Scholar] [CrossRef] [PubMed]
- Hubka, V.; Reblova, M.; Rehulka, J.; Selbmann, L.; Isola, D.; de Hoog, G.S.; Kolařík, M. Bradymyces gen. nov. (Chaetothyriales, Trichomeriaceae), a new ascomycete genus accommodating poorly differentiated melanized fungi. Antonie Van Leeuwenhoek 2014, 106, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Gueidan, C.; Ruibal, C.; de Hoog, G.S.; Schneider, H. Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol. 2011, 115, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Gueidan, C.; Aptroot, A.; Caceres, M.E.D.; Badali, H.; Stenroos, S. A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes, Ascomycota). Mycol. Prog. 2014, 13, 1027–1039. [Google Scholar] [CrossRef]
- Chomnunti, P.; Bhat, D.J.; Jones, E.G.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Trichomeriaceae, a new sooty mould family of Chaetothyriales. Fungal Divers. 2012, 56, 63–76. [Google Scholar] [CrossRef]
- Chomnunti, P.; Ko, T.W.K.; Chukeatirote, E.; Hyde, K.D.; Cai, L.; Jones, E.B.G.; Kodsueb, R.; Hassan, B.A. Phylogeny of Chaetothyriaceae in northern Thailand including three new species. Mycologia 2012, 104, 382–395. [Google Scholar] [CrossRef]
- Spegazzini, C.L. Notas mycological. Physics 1918, 4, 281–295. [Google Scholar]
- Batista, A.C.; Ciferri, R. Capnodiales, 2nd ed.; Saccardoa Press: Japan, 1963. [Google Scholar]
- Hughes, S.J. Sooty Molds. Mycologia 1976, 68, 693–820. [Google Scholar] [CrossRef]
- Chomnunti, P.; Schoch, C.L.; Aguirre-Hudson, B.; Koko, T.W.; Hongsanan, S.; Jones, E.B.G.; Kodsueb, R.; Phookamsak, R.; Chukeatirote, E.; Bahkali, A.H.; et al. Capnodiaceae. Fungal Divers. 2011, 51, 103–134. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Selbmann, L.; Sharifynia, S.; Ai-Hatmi, A.M.S.; Voglmayr, H.; Vicente, V.A.; Deng, S.; Kargl, A.; Moussa, T.A.A.; Al-Zahrani, H.S.; et al. Arthrocladium, an unexpected human opportunist in Trichomeriaceae (Chaetothyriales). Fungal Biol. 2016, 120, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Hyde, K.D.; Rajeshkumar, K.C.; Hawksworth, D.L.; Madrid, H.; Kirk, P.M.; Braun, U.; Singh, R.V.; Crous, P.W.; Kukwa, M.; et al. Notes for genera: Ascomycota. Fungal Divers. 2017, 86, 1–594. [Google Scholar] [CrossRef]
- Badali, H.; Gueidan, C.; Najafzadeh, M.J.; Bonifaz, A.; van den Ende, A.G.; de Hoog, G.S. Biodiversity of the genus Cladophialophora. Stud. Mycol. 2008, 61, 175–191. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Zeng, J.S.; Harrak, M.J.; Sutton, D.A. Exophiala xenobiotica sp. nov.; an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie Van Leeuwenhoek 2006, 90, 257–268. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Vicente, V.A.; Najafzadeh, M.J.; Harrak, M.J.; Badali, H.; Seyedmousavi, S. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 2011, 27, 46–72. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.W. Cerebral mycetoma of trout due to a Phialophora-like fungus. Sabouraudia: J. Med. Vet. Mycol. 1966, 5, 120–123. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; de Hoog, G.S.; Wang, X.; Wan, Z.; Yu, J.; Liu, W.; Li, R. Biodiversity and human-pathogenicity of Phialophora verrucosa and relatives in Chaetothyriales. Persoonia 2017, 38, 1–19. [Google Scholar] [CrossRef]
- Feng, P.Y.; de Hoog, G.S.; Najafzadeh, M.J.; Gerrits van den Ende, A.H.G.; Stielow, J.B.; Badali, H.; Boeger, W.A.; Vicente, V.A. Cladophialophora abundans, a novel species of Chaetothyriales isolated from the natural environment. Mycol. Prog. 2014, 13, 381–391. [Google Scholar] [CrossRef]
- Selbmann, L.; de Hoog, G.S.; Mazzaglia, A.; Friedann, E.I. Fungi at the edge of life: Cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 2005, 51, 1–32. [Google Scholar]
- Selbmann, L.; de Hoog, G.S.; Zucconi, L.; Isola, D.; Ruisi, S.; Gerrits van den Ende, A.H.G.; Ruibal, C.; de Leo, F.; Urzi, C.; Onofri, S. Drought meets acid: Three new genera in a dothidealean clade of extremotolerant fungi. Stud. Mycol. 2008, 61, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Standard, P.G.; Padhye, A.A.; Kaufman, L. Exoantigen test for the rapid identification of Exophiala spinifera. J. Med. Vet. Mycol. 1991, 29, 273–277. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Gueho, E.; Masclaux, F.; van den Ende, A.H.G.; Kwon-Chung, K.J.; McGinnis, M.R. Nutritional physiology and taxonomy of human-pathogenic Cladosporium-Xylohypha species. J. Med. Vet. Mycol. 1995, 33, 339–347. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Gerrits van den Ende, A.H.G.; Uijthof, J.M.J.; Untereiner, W.A. Nutritional Physiology of Type Isolates of Currently Accepted Species of Exophiala and Phaeococcomyces. Antonie Van Leeuwenhoek 1995, 68, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tintelnot, K.; von Hunnius, P.; de Hoog, G.S.; Polak-Wyss, A.; Gueho, E.; Masclaux, F. Systemic mycosis caused by a new Cladophialophora species. J. Med. Vet. Mycol. 1995, 33, 349–354. [Google Scholar] [CrossRef]
- Su, L.; Guo, L.Y.; Hao, Y.; Xiang, M.C.; Cai, L.; Liu, X.Z. Rupestriomyces and Spissiomyces, two new genera of rock-inhabiting fungi from China. Mycologia 2015, 107, 831–844. [Google Scholar] [CrossRef]
- Coetzee, J.C.; Eicker, A. A simple slide culture technique facilitating the viewing of growing fungi. Phytophylactica 1990, 22, 361–362. [Google Scholar]
- Riddell, R.W. Permanent stained mycological preparations obtained by slide culture. Mycologia 1950, 42, 265–270. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, S.; Liu, X.Z.; Wen, H.A.; Wang, M. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett. Appl. Microbiol. 2010, 51, 114–118. [Google Scholar] [CrossRef]
- Voigt, K.; Wostemeyer, J. Reliable amplification of actin genes facilitates deep-level phylogeny. Microbiol. Res. 2000, 155, 179–195. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innes, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Zoller, S.; Scheidegger, C.; Sperisen, C. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of gliocladium analyzed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B.; Liu, Y.J.; Ammirati, J.F.; Hall, B.D. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am. J. Bot. 2002, 89, 688. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Phylogenet. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Stamatakis, A.; Ludwig, T.; Meier, H. RAxML-III: A fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 2005, 21, 456–463. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Zhaxybayeva, O.; Gogarten, J.P. Bootstrap, Bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genomics 2002, 3, 4. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Schumacher, R.K.; Summerell, B.A.; Giraldo, A.; Gené, J.; Guarro, J.; Wanasinghe, D.N.; Hyde, K.D.; Camporesi, E.; et al. Fungal Planet description sheets: 281-319. Persoonia 2014, 33, 212–289. [Google Scholar] [CrossRef] [PubMed]
- Diederich, P.; Ertz, D.; Lawrey, J.D.; Sikaroodi, M.; Untereiner, W.A. Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales). Fungal Divers. 2013, 58, 61–72. [Google Scholar] [CrossRef]
- Madrid, H.; Hernandez-Restrepo, M.; Gene, J.; Cano, J.; Guarro, J.; Silva, V. New and interesting chaetothyrialean fungi from Spain. Mycol. Prog. 2016, 15, 1179–1201. [Google Scholar] [CrossRef]
- Crous, P.W.; Schubert, K.; Braun, U.; de Hoog, G.S. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Stud. Mycol. 2007, 58, 185–217. [Google Scholar] [CrossRef]
- Borman, A.M.; Fraser, M.; Szekely, A.; Larcombe, D.E.; Johnson, E.M. Rapid identification of clinically relevant members of the genus Exophiala by matrix-assisted laser desorption ionization-time of flight mass spectrometry and description of two novel species, Exophiala campbellii and Exophiala lavatrina. J. Clin. Microbiol. 2017, 55, 1162–1176. [Google Scholar] [CrossRef]
- Staley, J.T.; Palmer, F.; Adams, J.B. Microcolonial fungi: Common inhabitants on desert rocks? Science 1982, 215, 1093–1095. [Google Scholar] [CrossRef]
- Ruibal, C.; Platas, G.; Bills, G.F. High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 2008, 21, 93–110. [Google Scholar] [CrossRef]
- Sert, H.B.; Sterflinger, K. A new Coniosporium species from historical marble monuments. Mycol. Prog. 2010, 9, 353–359. [Google Scholar] [CrossRef]
- Egidi, E.; de Hoog, G.S.; Isola, D.; Onofri, S.; Quaedvlieg, W.; de Vries, M.; Verkley, G.J.M.; Stielow, J.B.; Zucconi, L.; Selbmann, L. Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Divers. 2014, 65, 127–165. [Google Scholar] [CrossRef]
- Bogomolova, E.V.; Minter, D.W. A new microcolonial rock-inhabiting fungus from marble in Chersonesos (Crimea, Ukraine). Mycotaxon 2003, 86, 195–204. [Google Scholar]
- Braun, U.; Feiler, U. Cladophialophora and its teleomorph. Microbiol. Res. 1995, 150, 81–91. [Google Scholar] [CrossRef]
- Davey, M.L.; Currah, R.S. A new species of Cladophialophora (hyphomycetes) from boreal and montane bryophytes. Mycol. Res. 2007, 111, 106–116. [Google Scholar] [CrossRef]
- McGinnis, M.R.; Ajello, L. A new species of Exophiala isolated from channel catfish. Mycologia 1974, 66, 518–520. [Google Scholar] [CrossRef]
- De Leo, F.; Urzì, C. Molecular techniques applied to the taxonomy of black meristematic fungi. Coalit. Newsletters 2002, 5, 2–3. [Google Scholar]
- Sterflinger, K. Black Yeasts and Meristematic Fungi: Ecology, Diversity and Identification, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zeng, J.S.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Harrak, M.J.; de Hoog, G.S. Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 2007, 45, 3713–3720. [Google Scholar] [CrossRef]
- Untereiner, W.A.; Gueidan, C.; Orr, M.J.; Diederich, P. The phylogenetic position of the lichenicolous ascomycete Capronia peltigerae. Fungal Divers. 2011, 49, 225–233. [Google Scholar] [CrossRef]
- Wollenzien, U.; de Hoog, G.S.; Krumbein, W.E.; Urzi, C. On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Sci. Total Environ. 1995, 167, 287–294. [Google Scholar] [CrossRef]
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531. [Google Scholar] [CrossRef]
- Selbmann, L.; Isola, D.; Zucconi, L.; Onofri, S. Resistance to UV-B induced DNA damage in extreme-tolerant cryptoendolithic Antarctic fungi: Detection by PCR assays. Fungal Biol. 2011, 115, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Ruibal, C. Isolation and Characterization of Melanized, Slow-Growing Fungi from Semiarid Rock Surfaces of Central Spain and Mallorca. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2004. [Google Scholar]
- Coleine, C.; Masonjones, S.; Sterflinger, K.; Onofri, S.; Selbmann, L.; Stajich, J.E. Peculiar genomic traits in the stress-adapted cryptoendolithic Antarctic fungus Friedmanniomyces endolithicus. Fungal Biol. 2020, 124, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Ametrano, C.G.; Muggia, L.; Grube, M. Extremotolerant Black Fungi from Rocks and Lichens. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S., Grube, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 119–143. [Google Scholar]




| Locus | Primer | Annealing Temperature (°C) | Orientation | Literature |
|---|---|---|---|---|
| ACT | Act1 | 52 | Forward | [51] |
| ACT | Act5ra | 52 | Reverse | [51] |
| ITS | ITS5 | 54 | Forward | [52] |
| ITS | ITS4 | 54 | Reverse | [52] |
| mtSSU | mrSSU1 | 55 | Forward | [53] |
| mtSSU | mrSSU3R | 55 | Reverse | [53] |
| nucLSU | LROR | 52 | Forward | [54] |
| nucLSU | LR5 | 52 | Reverse | [55] |
| RPB1 | RPB1-Af | 51 | Forward | [56] |
| RPB1 | RPB1-Cr | 51 | Reverse | [56] |
| SSU | NS1 | 52 | Forward | [52] |
| SSU | NS4 | 52 | Reverse | [52] |
| TEF | EF1-728F | 57 | Forward | [57] |
| TEF | EF1-986R | 57 | Reverse | [57] |
| TUB | Bt2a | 52 | Forward | [58] |
| TUB | Bt2b | 52 | Reverse | [58] |
| TUB | T1 | 52 | Forward | [59] |
| TUB | T22 | 52 | Reverse | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Su, L.; Yang, S.; Sun, J.; Liu, B.; Fu, R.; Wu, B.; Liu, X.; Cai, L.; Guo, L.; et al. Unveiling the Hidden Diversity of Rock-Inhabiting Fungi: Chaetothyriales from China. J. Fungi 2020, 6, 187. https://doi.org/10.3390/jof6040187
Sun W, Su L, Yang S, Sun J, Liu B, Fu R, Wu B, Liu X, Cai L, Guo L, et al. Unveiling the Hidden Diversity of Rock-Inhabiting Fungi: Chaetothyriales from China. Journal of Fungi. 2020; 6(4):187. https://doi.org/10.3390/jof6040187
Chicago/Turabian StyleSun, Wei, Lei Su, Shun Yang, Jingzu Sun, Bingjie Liu, Rong Fu, Bing Wu, Xingzhong Liu, Lei Cai, Liyun Guo, and et al. 2020. "Unveiling the Hidden Diversity of Rock-Inhabiting Fungi: Chaetothyriales from China" Journal of Fungi 6, no. 4: 187. https://doi.org/10.3390/jof6040187
APA StyleSun, W., Su, L., Yang, S., Sun, J., Liu, B., Fu, R., Wu, B., Liu, X., Cai, L., Guo, L., & Xiang, M. (2020). Unveiling the Hidden Diversity of Rock-Inhabiting Fungi: Chaetothyriales from China. Journal of Fungi, 6(4), 187. https://doi.org/10.3390/jof6040187







