Review of the Novel Investigational Antifungal Olorofim
Abstract
1. Introduction
2. Mechanism of Action
3. In Vitro Spectrum of Activity
4. In Vivo Effectiveness
5. Pharmacokinetics
6. Adverse Effects and Drug Interactions
7. Clinical Outcomes
8. Conclusions
Funding
Conflicts of Interest
References
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant? Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J. Faculty Opinions recommendation of Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and Outcome of Invasive Fungal Infection in Adult Hematopoietic Stem Cell Transplant Recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Denning, D. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2010, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Chronic forms of pulmonary aspergillosis. Clin. Microbiol. Infect. 2001, 7, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Mellado, E.; Melchers, W.J.G. Multiple-Triazole–Resistant Aspergillosis. New Engl. J. Med. 2007, 356, 1481–1483. [Google Scholar] [CrossRef]
- Bueid, A.; Howard, S.J.; Moore, C.B.; Richardson, M.D.; Harrison, E.; Bowyer, P.; Denning, D.W. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J. Antimicrob. Chemother. 2010, 65, 2116–2118. [Google Scholar] [CrossRef]
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Laverdière, M.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and Evolution of Azole Resistance in Aspergillus fumigatus Associated with Treatment Failure1. Emerg. Infect. Dis. 2009, 15, 1068–1076. [Google Scholar] [CrossRef]
- Meis, J.F.G.M.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. B Boil. Sci. 2016, 371, 20150460. [Google Scholar] [CrossRef]
- Sharpe, A.R.; Lagrou, K.; Meis, J.F.G.M.; Chowdhary, A.; Lockhart, S.R.; Verweij, P.E.; on behalf of the ISHAM/ECMM Aspergillus Resistance Surveillance working group. Triazole resistance surveillance in Aspergillus fumigatus. Med. Mycol. 2018, 56, S83–S92. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, B.; Mahto, K.K.; Meis, J.F.G.M.; Chowdhary, A. High-Frequency Direct Detection of Triazole Resistance in Aspergillus fumigatus from Patients with Chronic Pulmonary Fungal Diseases in India. J. Fungi 2020, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Kano, R.; Baddley, J.W.; Moser, S.A.; Marr, K.A.; Alexander, B.D.; Andes, D.; Kontoyiannis, D.P.; Perrone, G.; Peterson, S.; et al. Molecular Identification of Aspergillus Species Collected for the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 2009, 47, 3138–3141. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Mellado, E.; Pelaez, T.; Peman, J.; Zapico, S.; Alvarez, M.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M. Population-Based Survey of Filamentous Fungi and Antifungal Resistance in Spain (FILPOP Study). Antimicrob. Agents Chemother. 2013, 57, 4604. Available online: https://www.google.com/search?tbm=bks&q=13.+13.+Alastruey-Izquierdo+A%2C+Mellado+E%2C+Pelaez+T%2C+Peman+J%2C+Zapico+S%2C+Alvarez+M%2C+et+al.+Population-Based+Survey+of+Filamentous+Fungi+and+Antifungal+Resistance+in+Spain+%28FILPOP+Study%29.+Antimicrob+Agents+Chemother.+2013%3B57%289%29%3A4604 (accessed on 29 July 2020). [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; De Hoog, G.S.; Meis, J.F. Multiresistant Fusarium Pathogens on Plants and Humans: Solutions in (from) the Antifungal Pipeline? Infect. Drug Resist. 2019, 12, 3727–3737. [Google Scholar] [CrossRef]
- Lackner, M.; Hagen, F.; Meis, J.F.; Gerrits van den Ende, A.H.; Vu, D.; Robert, V.; Fritz, J.; Moussa, T.A.A.; De Hoog, G.S. Susceptibility and Diversity in the Therapy-Refractory Genus Scedosporium. Antimicrob. Agents Chemother. 2014, 58, 5877–5885. [Google Scholar] [CrossRef]
- Lewis, R.E.; Wiederhold, N.P.; Klepser, M.E. In Vitro Pharmacodynamics of Amphotericin B, Itraconazole, and Voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob. Agents Chemother. 2005, 49, 945–951. [Google Scholar] [CrossRef]
- Johnson, L.; Shields, R.; Clancy, C.J. Epidemiology, clinical manifestations, and outcomes of Scedosporium infections among solid organ transplant recipients. Transpl. Infect. Dis. 2014, 16, 578–587. [Google Scholar] [CrossRef]
- Husain, S.; Muñoz, P.; Forrest, G.; Alexander, B.D.; Somani, J.; Brennan, K.; Wagener, M.M.; Singh, N. Infections Due to Scedosporium apiospermum and Scedosporium prolificans in Transplant Recipients: Clinical Characteristics and Impact of Antifungal Agent Therapy on Outcome. Clin. Infect. Dis. 2005, 40, 89–99. [Google Scholar] [CrossRef]
- Lamaris, G.A.; Chamilos, G.; Lewis, R.E.; Safdar, A.; Raad, I.I.; Kontoyiannis, D.P. Scedosporium Infection in a Tertiary Care Cancer Center: A Review of 25 Cases from 1989–2006. Clin. Infect. Dis. 2006, 43, 1580–1584. [Google Scholar] [CrossRef]
- Nucci, M. Emerging moulds: Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr. Opin. Infect. Dis. 2003, 16, 607–612. [Google Scholar] [CrossRef]
- Doligalski, C.T.; Benedict, K.; Cleveland, A.A.; Park, B.; Derado, G.; Pappas, P.G.; Baddley, J.W.; Zaas, D.W.; Harris, M.T.; Alexander, B.D. Epidemiology of invasive mold infections in lung transplant recipients. Am. J. Transplant. 2014, 14, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Prevention. Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 217–221. [Google Scholar] [CrossRef]
- Cooksey, G.S.; Nguyen, A.; Knutson, K.; Tabnak, F.; Benedict, K.; McCotter, O.; Jain, S.; Vugia, D. Notes from the Field: Increase in Coccidioidomycosis—California, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.G.R.; Barker, B.M.; Wiederhold, N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017, 61, e02634-16. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Sibley, G.E.M.; Beckmann, N.; Dobb, K.S.; Slater, M.J.; McEntee, L.; Du Pré, S.; Livermore, J.; Bromley, M.; Wiederhold, N.P.; et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc. Natl. Acad. Sci. USA 2016, 113, 12809–12814. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5. [Google Scholar]
- Du Pré, S.; Beckmann, N.; Almeida, M.C.; Sibley, G.E.M.; Law, D.; Brand, A.; Birch, M.; Read, N.D.; Oliver, J.D. Effect of the Novel Antifungal Drug F901318 (Olorofim) on Growth and Viability of Aspergillus fumigatus. Antimicrob. Agents Chemother. 2018, 62, AAC.00231-18. [Google Scholar] [CrossRef]
- Du Du Pré, S.; Birch, M.; Law, D.; Beckmann, N.; Sibley, G.E.M.; Bromley, M.; Read, N.D.; Oliver, J.D. The Dynamic Influence of Olorofim (F901318) on the Cell Morphology and Organization of Living Cells of Aspergillus fumigatus. J. Fungi 2020, 6, 47. [Google Scholar] [CrossRef]
- Walker, L.A.; Lee, K.K.; Munro, C.A.; Gow, N.A.R. Caspofungin Treatment of Aspergillus fumigatus Results in ChsG-Dependent Upregulation of Chitin Synthesis and the Formation of Chitin-Rich Microcolonies. Antimicrob. Agents Chemother. 2015, 59, 5932–5941. [Google Scholar] [CrossRef]
- Cota, J.M.; Grabinski, J.L.; Talbert, R.L.; Burgess, D.S.; Rogers, P.D.; Edlind, T.D.; Wiederhold, N.P. Increases in SLT2 Expression and Chitin Content Are Associated with Incomplete Killing of Candida glabrata by Caspofungin. Antimicrob. Agents Chemother. 2007, 52, 1144–1146. [Google Scholar] [CrossRef]
- Veses, V.; Richards, A.; Gow, N.A.R. Vacuoles and fungal biology. Curr. Opin. Microbiol. 2008, 11, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Law, D.; Birch, M. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob. Chemother. 2017, 72, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Najvar, L.K.; Jaramillo, R.; Olivo, M.; Birch, M.; Law, D.; Rex, J.H.; Catano, G.; Patterson, T.F. The Orotomide Olorofim Is Efficacious in an Experimental Model of Central Nervous System Coccidioidomycosis. Antimicrob. Agents Chemother. 2018, 62, AAC.00999-18. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Law, D.; Birch, M.; Halliday, C.; Sorrell, T.C.; Rex, J.; Slavin, M.; Chen, S.C.-A. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med. Mycol. 2018, 56. [Google Scholar] [CrossRef]
- Jørgensen, K.M.; Astvad, K.M.T.; Hare, R.K.; Arendrup, M.C. EUCAST Determination of Olorofim (F901318) Susceptibility of Mold Species, Method Validation, and MICs. Antimicrob. Agents Chemother. 2018, 62, AAC.00487-18. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2019, 74, 1586–1590. [Google Scholar] [CrossRef]
- Fothergill, A.W.; Wiederhold, N.P.; Sibley, G.; Kennedy, A.; Oliver, J.; Birch, M.; Law, D. Spectrum of activity of F901318, the first agent from the newly discovered orotomide class of antifungals. ICAAC/ICC 2015, 2015. [Google Scholar]
- Buil, J.B.; Rijs, A.J.M.M.; Meis, J.F.M.; Birch, M.; Law, D.; Melchers, W.J.G.; Verweij, P.E. Activity of F901318 against azole-resistant and difficult-to-treat Aspergillus species (abstr. P1605). In Proceedings of the 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 9–12 April 2016. [Google Scholar]
- Seyedmousavi, S.; Chang, Y.C.; Law, D.; Birch, M.; Rex, J.H.; Kwon-Chung, K.J. Efficacy of Olorofim (F901318) against Aspergillus fumigatus, A. nidulans, and A. tanneri in Murine Models of Profound Neutropenia and Chronic Granulomatous Disease. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Buil, J.B.; Rijs, A.J.M.M.; Meis, J.F.; Birch, M.; Law, D.; Melchers, W.J.G.; Verweij, P.E. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J. Antimicrob. Chemother. 2017, 72, 2548–2552. [Google Scholar] [CrossRef]
- Lackner, M.; Birch, M.; Naschberger, V.; Grässle, D.; Beckmann, N.; Warn, P.; Gould, J.; Law, D.; Lass-Flörl, C.; Binder, U. Dihydroorotate dehydrogenase inhibitor olorofim exhibits promising activity against all clinically relevant species within Aspergillus section Terrei. J. Antimicrob. Chemother. 2018, 73, 3068–3073. [Google Scholar] [CrossRef]
- Lim, W.; Eadie, K.; Konings, M.; Rijnders, B.; Fahal, A.H.; Oliver, J.D.; Birch, M.; Verbon, A.; Van De Sande, W. Madurella mycetomatis, the main causative agent of eumycetoma, is highly susceptible to olorofim. J. Antimicrob. Chemother. 2020, 75, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Patterson, H.P.; Birch, M.; Law, D. (Eds.) Evaluation of the in vitro activity of olorofim against Fusarium species. In Proceedings of the 9th Trends in Medical Mycology, Nice, France, 11–19 October 2019. [Google Scholar]
- Hope, W.W.; McEntee, L.; Livermore, J.; Whalley, S.; Johnson, A.; Farrington, N.; Kolamunnage-Dona, R.; Schwartz, J.; Kennedy, A.; Law, D.; et al. Pharmacodynamics of the Orotomides against Aspergillus fumigatus: New Opportunities for Treatment of Multidrug-Resistant Fungal Disease. mBio 2017, 8, e01157-17. [Google Scholar] [CrossRef] [PubMed]
- Negri, C.; Johnson, A.; McEntee, L.; Box, H.; Whalley, S.; Schwartz, J.; Ramos-Martín, V.; Livermore, J.; Kolamunnage-Dona, R.; Colombo, A.L.; et al. Pharmacodynamics of the Novel Antifungal Agent F901318 for Acute Sinopulmonary Aspergillosis Caused by Aspergillus flavus. J. Infect. Dis. 2017, 217, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Allen, G.; Steiner, J.; Oliver, J.; Birch, M.; Sibley, G. Pharmacokinetics of F901318 in man from an intravenous single ascending dose study (abstr. F-751). In Proceedings of the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 17–22 September 2015. [Google Scholar]
- Kennedy, T.; Allen, G.; Steiner, J.; Heep, M.; Birch, M. (Eds.) Assessment of the duration of infusion on the tolerability and repeat dose pharmacokinetics of F901318 in healthy volunteers (abstr. P1711). In Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Kennedy, T.; Allen, G.; Steiner, J.; Heep, M.; Oliver, J.; Sibley, G.; Law, D. Multiple dose pharmacokinetics of an immediate-release tablet formulation of F901318 in healthy male and female subjects. In Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Law, D.; Birch, M.; Oliver, J.; Sibley, G.; Goodwin, J.; Livermore, J.; Whalley, S.; Hope, W. Pharmacokinetics of the novel antifungal agents F901318 in mice, rats, and cynomolgus monkeys (abstr. F-757). In Proceedings of the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 17–22 September 2015. [Google Scholar]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef]
- Kennedy, T.; Allen, G.; Steiner, J.; Oliver, J.; Birch, M.; Sibley, G.; Law, D. An open-label study in healthy volunteers to evaluate the potential for cytochrome P450 3A4 inhibition by F901318 using oral midazolam as a probe. In Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Tio, S.H.; Thursky, K.; Ng, G.; Rex, J.H.; Carney, D.; Slavin, M. Olorofim for a case of severe disseminated Lomentospora prolificans infections. In Proceedings of the 30th European Congress of Clinical Microbiology and Infectious Diseases, Paris, France, 18–21 April 2020. [Google Scholar]
- Chen, S.; Rai, N.J.; Cunneen, S.; Cornelissen, K.; Rex, J.H.; Heath, C.H.; Harvey, E. A case of Lomentospora prolificans treated with the novel antifungal olorofim. In Proceedings of the 30th European Congress of Clinical Microbiology and Infectious Diseases, Paris, France, 18–21 April 2020. abstr. 2585. [Google Scholar]
- Harvey, E.; Fitton, L.; Rex, J.H.; Thompson, G. Successful use of the novel antifungal olorofim in the treatment of disseminated coccidioidomycosis. In Proceedings of the 30th European Congress of Clinical Microbiology and Infectious Diseases, Paris, France, 18–21 April 2020. [Google Scholar]
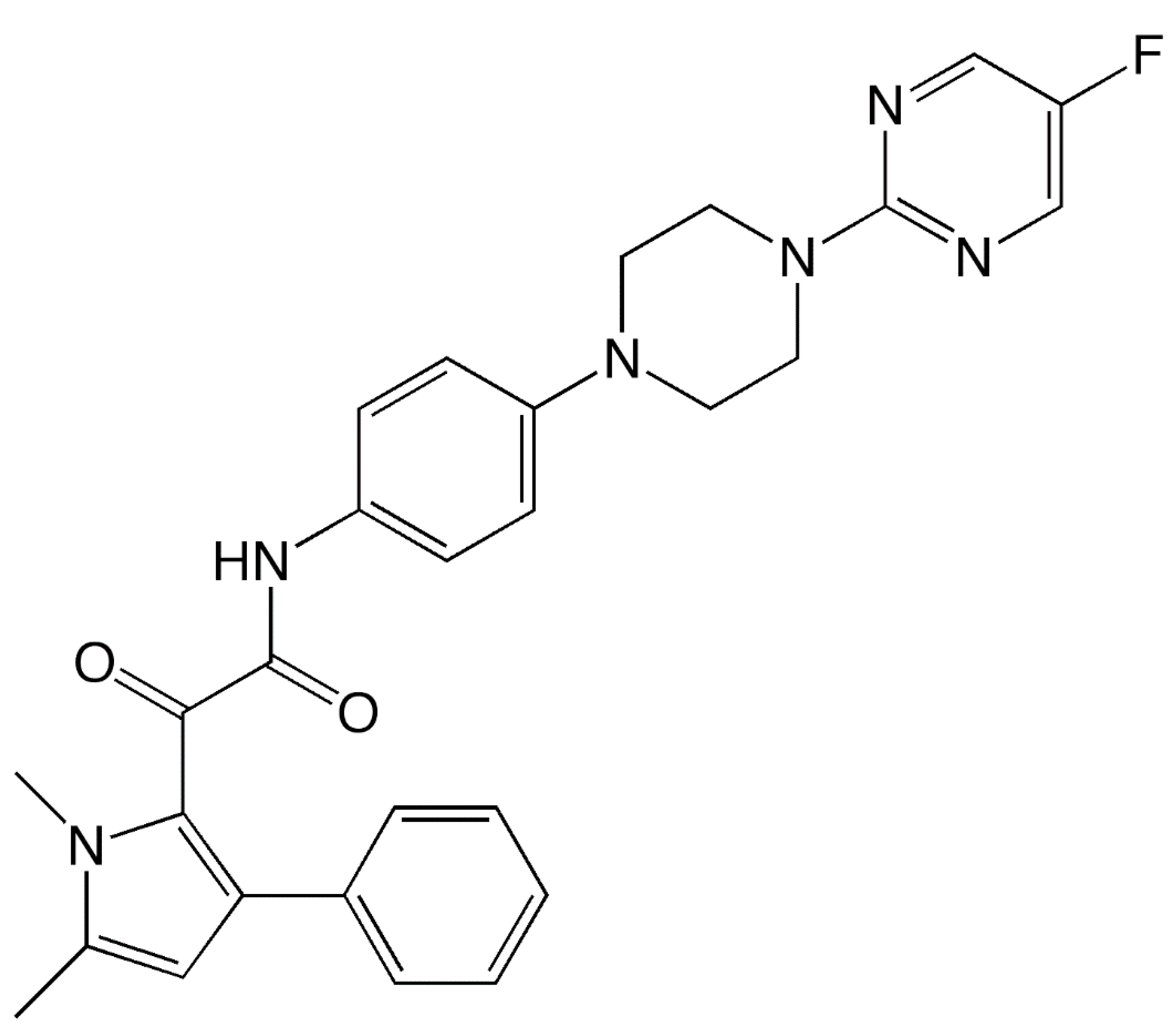
| Feature | Description |
|---|---|
| Mechanism of action | Targets pyrimidine biosynthesis through inhibition of dihydroorotate dehydrogenase |
| Spectrum of in vitro activity | Aspergillus spp., including azole resistant isolates Scedosporium spp., and Lomentospora prolificans Variable and species-specific vs. Fusarium Endemic fungi (Blastomyces, Coccidioides, Histoplasma) Microascus/Scoulariopsis spp. Penicillium, Paecilomyces, Purpureocillium, Talaromyces Madurella No activity against yeasts or the Mucorales |
| Activity in in vivo models | Improvements in survival and histopathology, reductions in galactomannan in a neutropenic murine model of pulmonary aspergillosis due to A. fumigatus (wild-type and isolates harboring mutations in cyp51A (TR34/L98H and G138C)) Improvements in survival and histopathology, and reductions in galactomannan in murine sinopulmonary model of invasive aspergillosis secondary to A. flavus Improvements in survival and histopathology, and reductions in galactomannan and tissue fungal burden in a CGD murine model of invasive aspergillosis secondary to A. fumigatus, A. nidulans, and A. tanneri Improvements in survival and reductions in brain tissue fungal burden in murine model of CNS coccidioidomycosis |
| Clinical Characteristic | Description |
|---|---|
| Pharmacokinetic parameters | Large volume of distribution (~3 L/kg); some CNS distribution High plasma protein binding (>99%) Half-life approximately 20–30 h Bioavailability >45% Pharmacokinetic/pharmacodynamic parameter Cmin/MIC |
| Clinical status | Currently in Phase IIb clinical study Case reports describe success against disseminated lomentosporiosis and coccidioidomycosis |
| Adverse effects/drug–drug interactions | Infusion site pain and phlebitis with intravenous infusion Dizziness Weak inhibitor of CYP3A4 Metabolized by multiple CYP450 isoenzymes |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiederhold, N.P. Review of the Novel Investigational Antifungal Olorofim. J. Fungi 2020, 6, 122. https://doi.org/10.3390/jof6030122
Wiederhold NP. Review of the Novel Investigational Antifungal Olorofim. Journal of Fungi. 2020; 6(3):122. https://doi.org/10.3390/jof6030122
Chicago/Turabian StyleWiederhold, Nathan P. 2020. "Review of the Novel Investigational Antifungal Olorofim" Journal of Fungi 6, no. 3: 122. https://doi.org/10.3390/jof6030122
APA StyleWiederhold, N. P. (2020). Review of the Novel Investigational Antifungal Olorofim. Journal of Fungi, 6(3), 122. https://doi.org/10.3390/jof6030122





