Appressorium: The Breakthrough in Dikarya
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Spores Harvesting and Inoculation
2.3. Microscopic Observations
3. Results
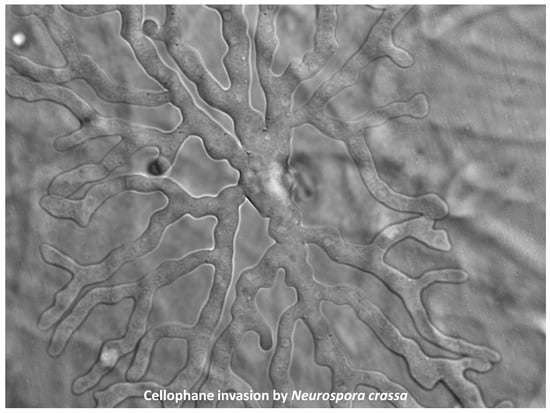
3.1. Cellophane for In Vitro Penetration Assays
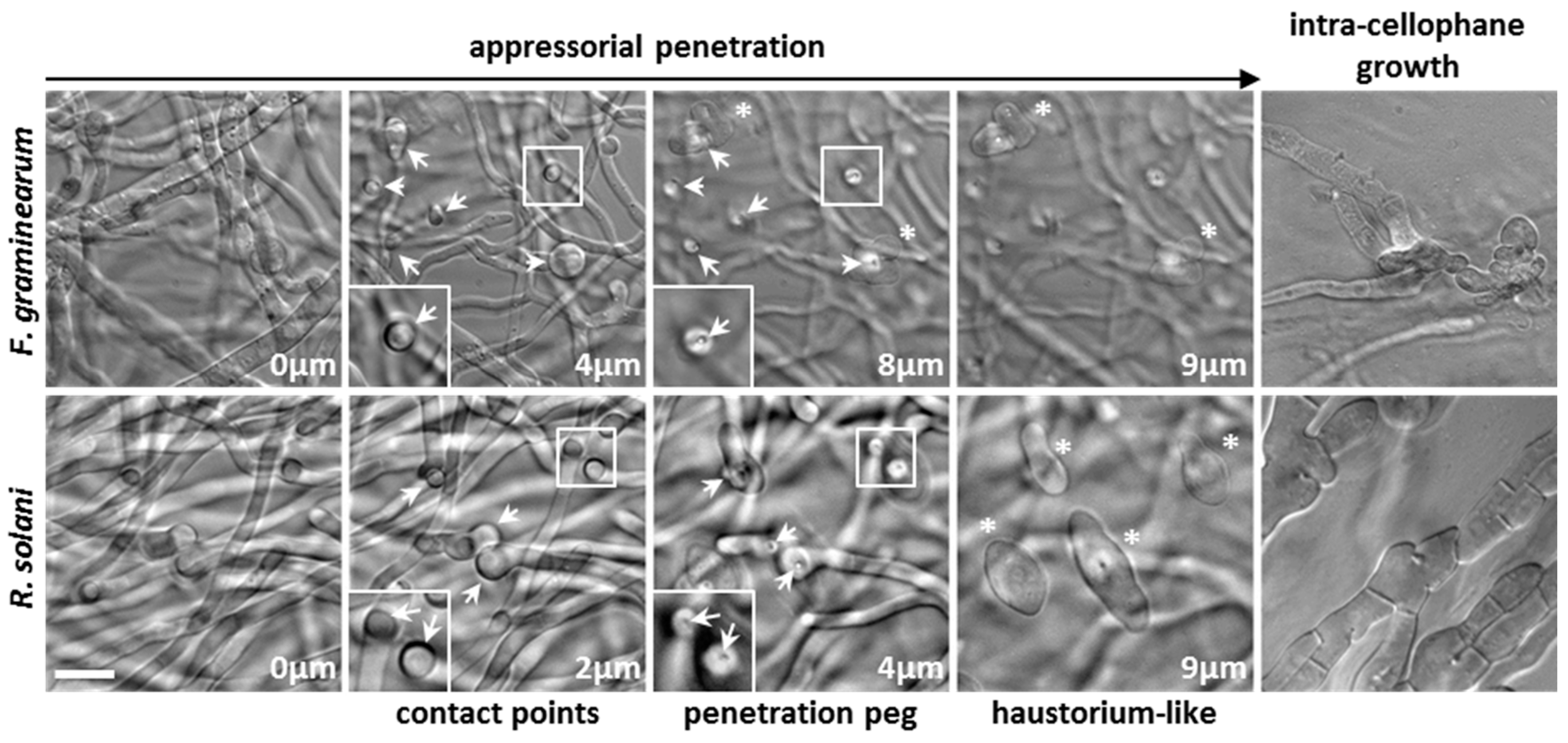
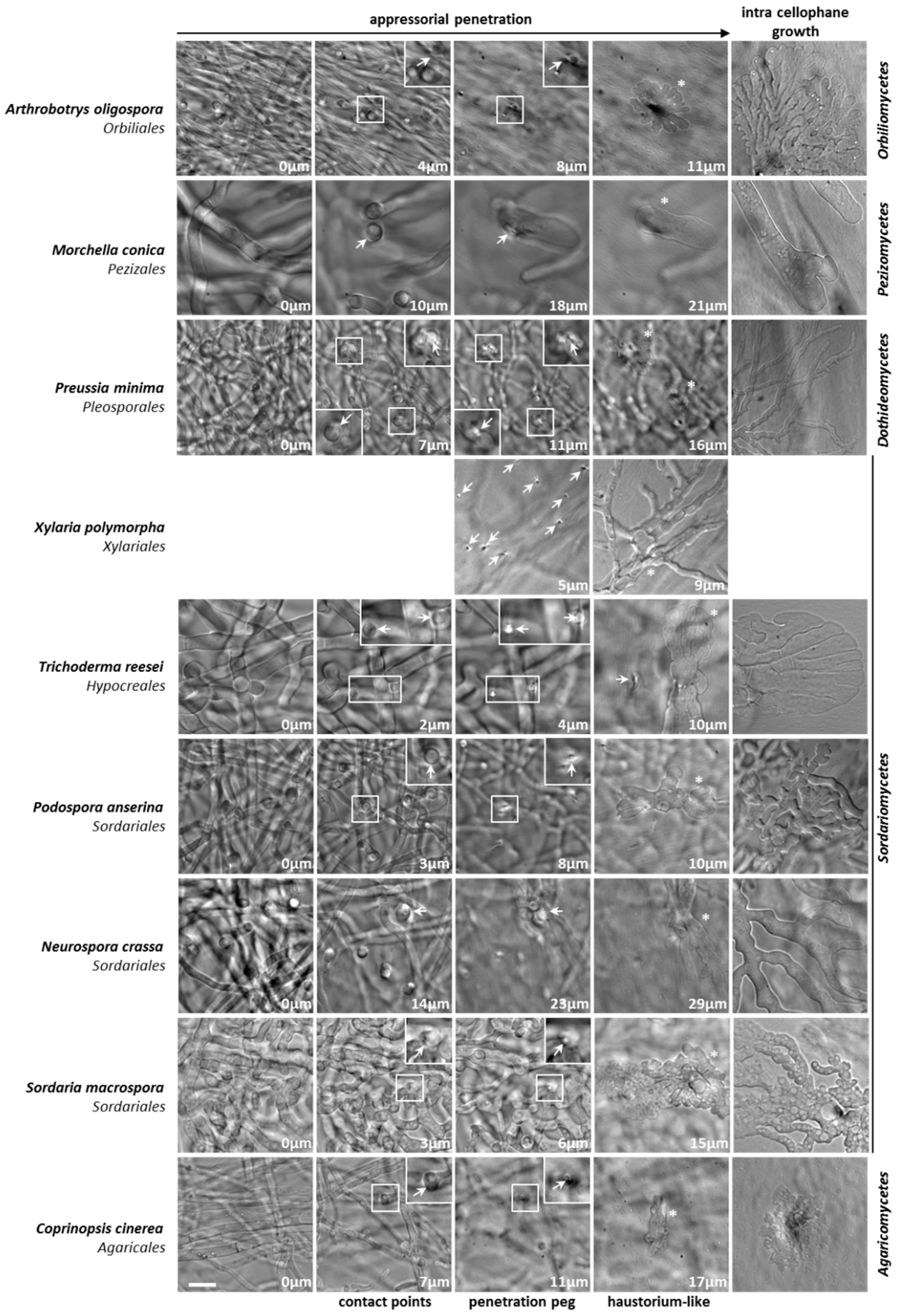
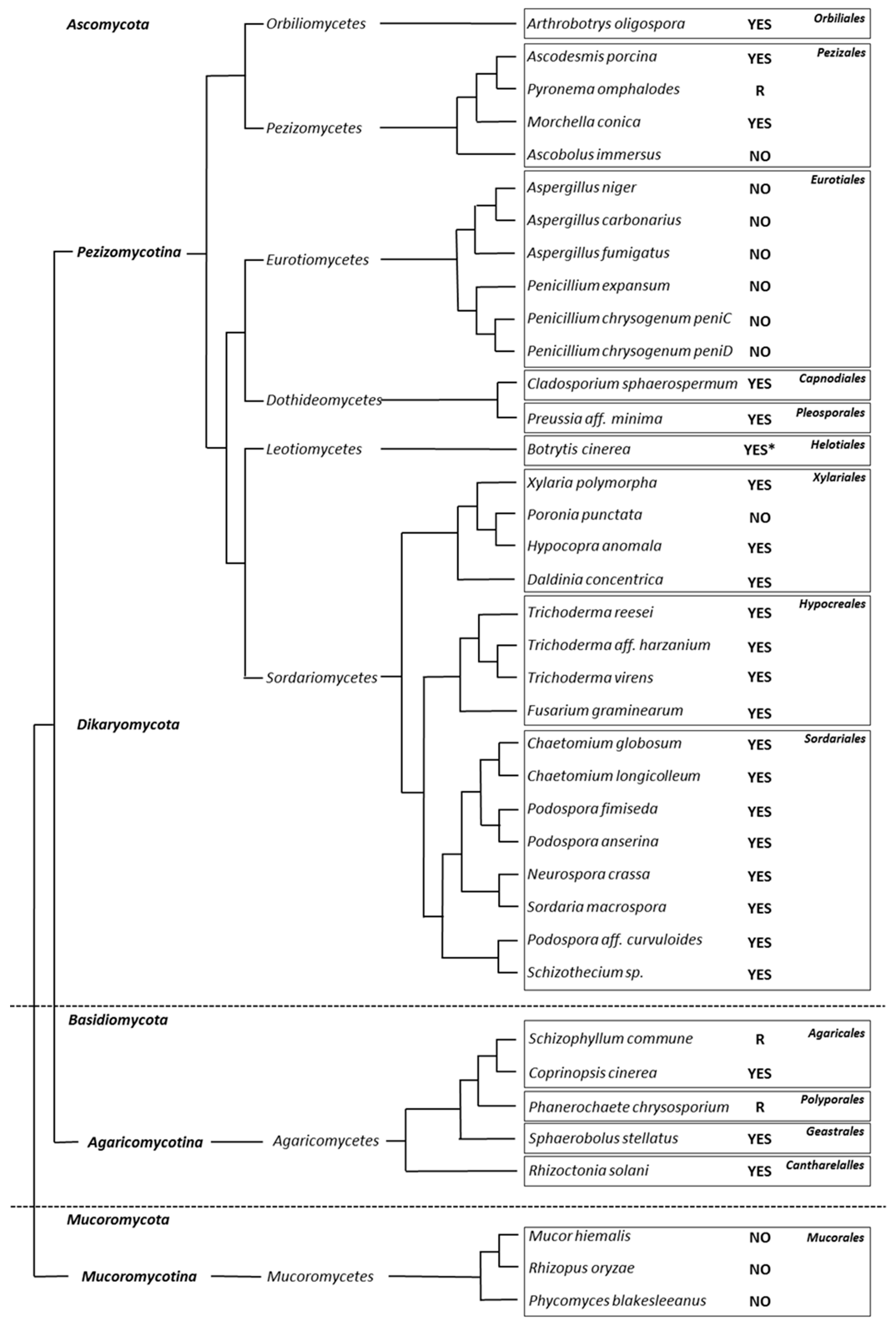
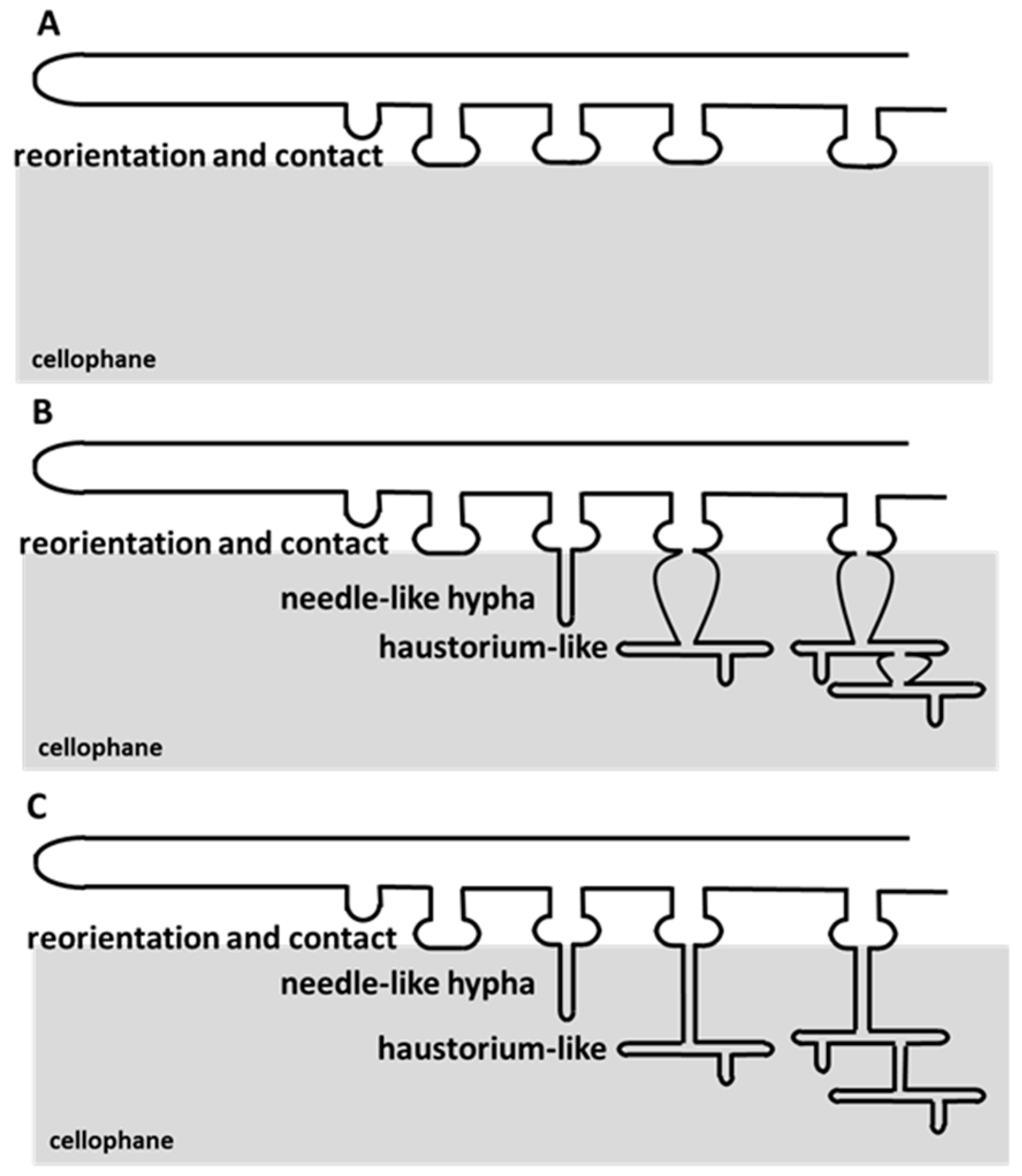
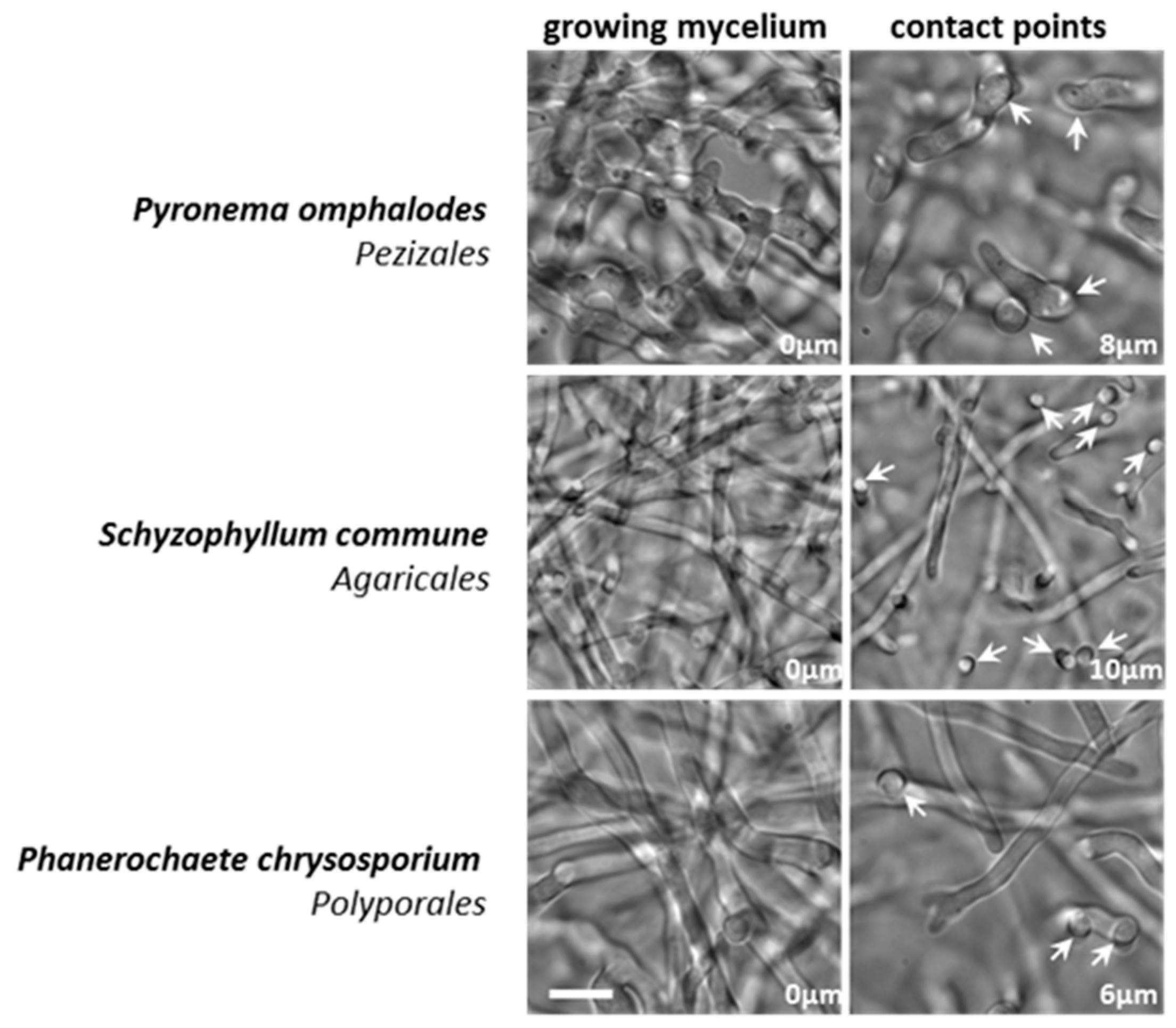
3.2. Appressorium Development is Widespread in Saprotrophic Dikarya
3.3. Mucorales and Eurotiales Do Not Penetrate Cellophane
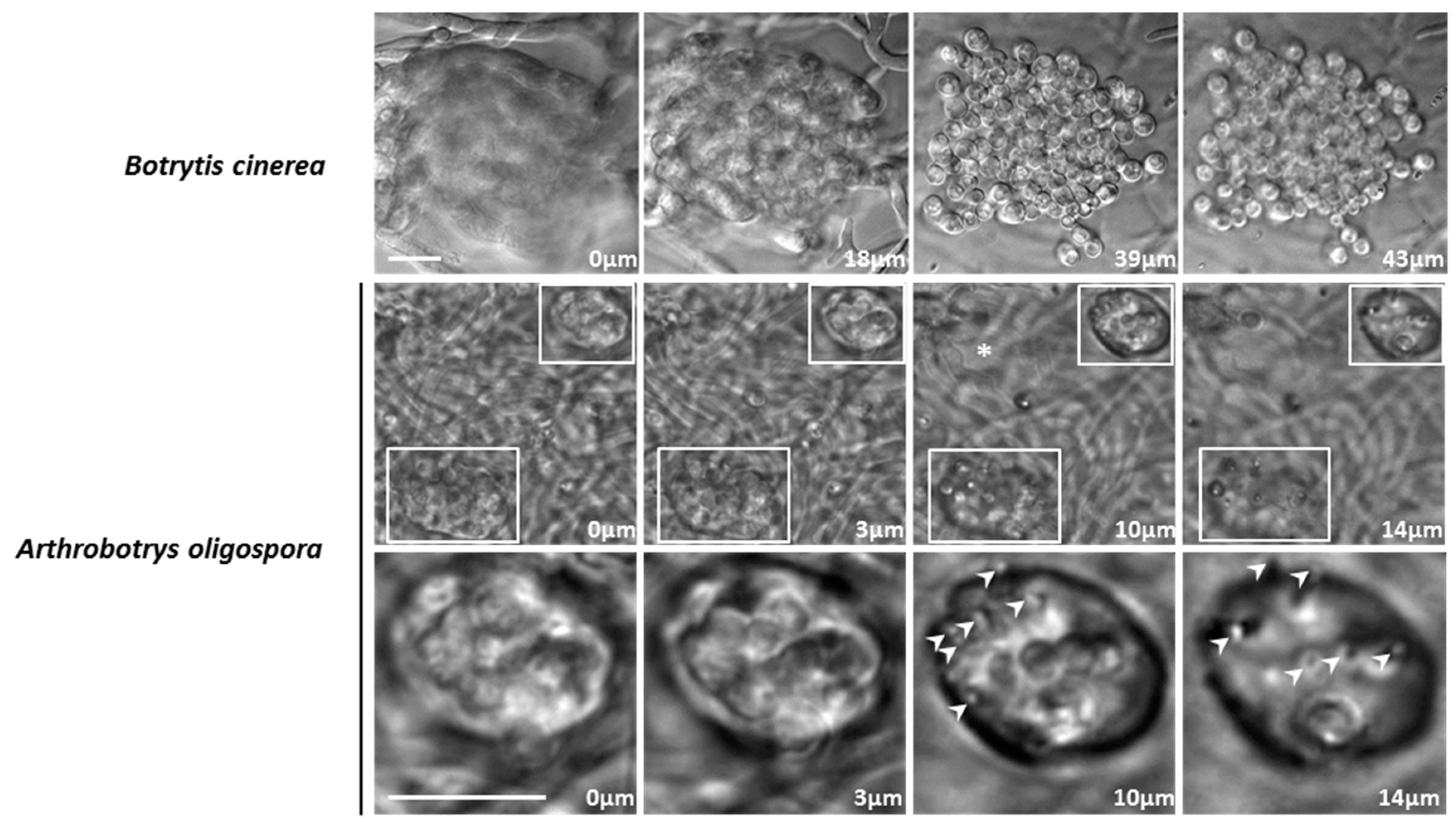
3.4. A. oligospora Differentiates Unicellular Appressoria and Pluricellular Infection Cushions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chundawat, S.P.S.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of Lignocellulosic Biomass to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Couturier, M.; Tangthirasunun, N.; Ning, X.; Brun, S.; Gautier, V.; Bennati-Granier, C.; Silar, P.; Berrin, J.-G. Plant biomass degrading ability of the coprophilic ascomycete fungus Podospora anserina. Biotechnol. Adv. 2016, 34, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Espagne, E.; Lespinet, O.; Malagnac, F.; Da Silva, C.; Jaillon, O.; Porcel, B.M.; Couloux, A.; Aury, J.-M.; Ségurens, B.; Poulain, J.; et al. The genome sequence of the model ascomycete fungus Podospora anserina. Genome Biol. 2008, 9, R77. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Chapeland-Leclerc, F.; Silar, P.; Ruprich-Robert, G. Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina: Wood degradation by laccases in Podospora anserina. Environ. Microbiol. 2014, 16, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Deising, H.B.; Werner, S.; Wernitz, M. The role of fungal appressoriain plant infection. Microbes Infect. 2000, 2, 1631–1641. [Google Scholar] [CrossRef]
- Frank, B. Ueber einige neue und weniger bekannte Pflanzenkrankheiten. Ber. Dtsch. Bot. Ges. 1883, 29–34. [Google Scholar]
- Emmett, R.W.; Parbery, D.G. Appressoria. Annu. Rev. Phytopathol. 1975, 13, 147–165. [Google Scholar] [CrossRef]
- Brun, S.; Malagnac, F.; Bidard, F.; Lalucque, H.; Silar, P. Functions and regulation of the Nox family in the filamentous fungus Podospora anserina: a new role in cellulose degradation. Mol. Microbiol. 2009, 74, 480–496. [Google Scholar] [CrossRef]
- Marschall, R.; Tudzynski, P. Reactive oxygen species in development and infection processes. Semin. Cell Dev. Biol. 2016, 57, 138–146. [Google Scholar] [CrossRef]
- Ryder, L.S.; Talbot, N.J. Regulation of appressorium development in pathogenic fungi. Curr. Opin. Plant Biol. 2015, 26, 8–13. [Google Scholar] [CrossRef]
- Hardham, A.R. The cell biology behind Phytophthora pathogenicity. Australas. Plant Pathol. 2001, 30, 91–98. [Google Scholar] [CrossRef]
- Harrison, M.J. MOLECULAR AND CELLULAR ASPECTS OF THE ARBUSCULAR MYCORRHIZAL SYMBIOSIS. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 361–389. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Backhouse, D.; Willetts, H.J. Development and structure of infection cushions of Botrytis cinerea. Trans. Br. Mycol. Soc. 1987, 89, 89–95. [Google Scholar] [CrossRef]
- Lambou, K.; Malagnac, F.; Barbisan, C.; Tharreau, D.; Lebrun, M.-H.; Silar, P. The crucial role of the Pls1 tetraspanin during ascospore germination in Podospora anserina provides an example of the convergent evolution of morphogenetic processes in fungal plant pathogens and saprobes. Eukaryot. Cell 2008, 7, 1809–1818. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bourett, T.M.; Howard, R.J. In vitro development of penetration structures in the rice blast fungus Magnaporthe grisea. Can. J. Bot. 1990, 68, 329–342. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Rittenour, W.R.; Harris, S.D. An in vitro method for the analysis of infection-related morphogenesis in Fusarium graminearum. Mol. Plant Pathol. 2010, 11, 361–369. [Google Scholar] [CrossRef]
- Fang, W.; Latgé, J.-P. Microbe Profile: Aspergillus fumigatus: a saprotrophic and opportunistic fungal pathogen. Microbiology 2018, 164, 1009–1011. [Google Scholar] [CrossRef]
- Niu, X.-M.; Zhang, K.-Q. Arthrobotrys oligospora: a model organism for understanding the interaction between fungi and nematodes. Mycology 2011, 2, 59–78. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef]
- Hardham, A.R. Cell Biology of Fungal Infection of Plants. In Biology of the Fungal Cell; The Mycota; Springer: Heidelberg, Germany, 2001; pp. 91–123. ISBN 978-3-662-06103-9. [Google Scholar]
- Van Den Heuvel, J.; Waterreus, L.P. Conidial concentration as an important factor determining the type of prepenetration structures formed by Botrytis cinerea on leaves of French bean (Phaseolus vulgaris). Plant Pathol. 1983, 32, 263–272. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and Mechanisms of Penetration by Plant Pathogenic Fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Boucher, C.; Nguyen, T.-S.; Silar, P. Species Delimitation in the Podospora anserina/ p. pauciseta/p. comata Species Complex (Sordariales). Cryptogam. Mycol. 2017, 38, 485–506. [Google Scholar] [CrossRef]
- Armentrout, V.N.; Downer, A.J. Infection cushion development by Rhizoctonia solani on cotton. Phytopathology 1987, 77, 619–623. [Google Scholar] [CrossRef]
- Galhano, R.; Illana, A.; Ryder, L.S.; Rodríguez-Romero, J.; Demuez, M.; Badaruddin, M.; Martinez-Rocha, A.L.; Soanes, D.M.; Studholme, D.J.; Talbot, N.J.; et al. Tpc1 is an important Zn(II)2Cys6 transcriptional regulator required for polarized growth and virulence in the rice blast fungus. PLOS Pathog. 2017, 13, e1006516. [Google Scholar] [CrossRef]
- Dagdas, Y.F.; Yoshino, K.; Dagdas, G.; Ryder, L.S.; Bielska, E.; Steinberg, G.; Talbot, N.J. Septin-Mediated Plant Cell Invasion by the Rice Blast Fungus, Magnaporthe oryzae. Science 2012, 336, 1590–1595. [Google Scholar] [CrossRef]
- Veneault-Fourrey, C.; Lambou, K.; Lebrun, M.-H. Fungal Pls1 tetraspanins as key factors of penetration into host plants: a role in re-establishing polarized growth in the appressorium? FEMS Microbiol. Lett. 2006, 256, 179–184. [Google Scholar] [CrossRef][Green Version]
- Lachance, M.-A.; Pang, W.-M. Predacious Yeasts. Yeast 1997, 13, 225–232. [Google Scholar] [CrossRef]
- Garriock, M.L.; Peterson, R.L.; Ackerley, C.A. Early stages in colonization of Allium porrwn (leek) roots by the vesicular—arbuscular mycorrhizal fungus, Glomus versiforme. New Phytol. 1989, 112, 85–92. [Google Scholar] [CrossRef]
- Hajek, A.E.; Filotas, M.J.; Ewing, D.C. Formation of appressoria by two species of lepidopteran-pathogenic Entomophthorales. Can. J. Bot. 2002, 80, 220–225. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demoor, A.; Silar, P.; Brun, S. Appressorium: The Breakthrough in Dikarya. J. Fungi 2019, 5, 72. https://doi.org/10.3390/jof5030072
Demoor A, Silar P, Brun S. Appressorium: The Breakthrough in Dikarya. Journal of Fungi. 2019; 5(3):72. https://doi.org/10.3390/jof5030072
Chicago/Turabian StyleDemoor, Alexander, Philippe Silar, and Sylvain Brun. 2019. "Appressorium: The Breakthrough in Dikarya" Journal of Fungi 5, no. 3: 72. https://doi.org/10.3390/jof5030072
APA StyleDemoor, A., Silar, P., & Brun, S. (2019). Appressorium: The Breakthrough in Dikarya. Journal of Fungi, 5(3), 72. https://doi.org/10.3390/jof5030072