Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature
Abstract
:1. Introduction
2. Methods
3. Results
4. Literature Review
4.1. N-Acetylcysteine—The Archetypal Classical Mucolytic
4.2. Pharmacokinetics of N-Acetylcysteine
4.3. N-Acetylcysteine Products on the Market
4.4. Nebulized versus Oral N-Acetylcysteine
4.5. Asthma with Hypersecretion of Mucus—Use of N-Acetylcysteine Down A Bronchoscope
4.6. Intravenous N-Acetylcysteine
4.7. Mucoid Impaction—What Is It and Does N-Acetylcysteine Help?
4.8. Allergic Bronchopulmonary Aspergillosis and Hyperattenuated Allergic Mucin
5. Conclusions
Funding
Conflicts of Interest
References
- Majima, Y.; Sakakura, Y.; Matsubara, T.; Hattori, T. Possible mechanisms of reduction of nasal mucociliary clearance in chronic sinusitis. Clin. Otolaryngol. 1986, 11, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.K. Aerosol medications for treatment of mucus clearance disorders. Respir. Care 2015, 60, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.K. Mucolytics, expectorants, and mucokinetic medications. Respir. Care 2007, 52, 859–865. [Google Scholar] [PubMed]
- Elkins, M.R.; Robinson, M.; Rose, B.R.; Robinson, M.; Rose, B.R.; Harbour, C.; Moriarty, C.P.; Marks, G.B.; Belousova, E.G.; Xuan, W.; et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 2006, 354, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Regnis, J.A.; Bailey, D.L.; King, M.; Bautovich, G.J.; Bye, P.T. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 153, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L.; Auci, R.M.; Shields, D.E.; Christiansen, D.H.; Lucas, M.K.; Fuchs, H.J.; Rosenstein, B.J. Effect of rhDNase on airflow obstruction and mucociliary clearance in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 153, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Willis, P.J.; Hall, R.L.; Chan, W.; Cole, P.J. Sodium chloride increases ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus depleted bovine trachea. J. Clin. Investig. 1997, 99, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kellett, F.; Niven, M.R. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir. Med. 2011, 105, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Bennett, W.D.; Zeman, K.L.; Knowles, M.R.; Tarran, R.; Boucher, R.C. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med. 2006, 354, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Pinamonti, S.; Venturoli, L.; Leis, M.; Chicca, M.; Barbieri, A.; Sostero, S.; Ravenna, F.; Daffonchio, L.; Novellini, R.; Ciaccia, A. Antioxidant activity of carbocysteine lysine salt monohydrate. Panminerva Med. 2001, 43, 215–220. [Google Scholar] [PubMed]
- Braga, P.C.; Allegra, L.; Rampoldi, C.; Ornaghi, A.B.; Beghi, G. Long-lasting effects on rheology and clearance of bronchial mucus after short term administration of high doses of carbocysteine lysine to patients with chronic bronchitis. Respiration 1990, 57, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.F.; Steel, A.E.; Scott, J.K.; Jordan, J.W. S-carboxymethyl-cysteine in the humidification of sputum and treatment of chronic airways disease. Chest 1976, 70, 505–513. [Google Scholar] [CrossRef]
- Fahy, J.V.; Kim, K.W.; Liu, J.; Boushey, H.A. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J. Allergy Clin. Immunol. 1995, 95, 843–852. [Google Scholar] [CrossRef]
- Flume, P.A.; O’Sullivan, B.P.; Robinson, K.A.; Goss, C.H.; Mogayzel, P.J.; Willey-Courand, D.B.; Bujan, J.; Finder, J.; Lester, M.; Quittell, L.; et al. Cystic fibrosis pulmonary guidelines: Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 2007, 176, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Broughton-Head, V.J.; Shur, J.; Carroll, M.P.; Smith, J.R.; Shute, J.K. Unfractionated heparin reduces the elasticity of sputum from patients with cystic fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L1240–L1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, M.; Moënnoz, D.; Montigon, F.; Mettraux, C.; Mercier, S.; Schiffrin, E.J.; Obled, C.; Breuillé, D.; Boza, J. Mucin production and composition is altered in dextran sulfate sodium-induced colitis in rats. Dig. Dis. Sci. 2003, 48, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Garrett, H.; Speert, D.P.; King, M. Improved clearability of cystic fibrosis sputum with dextran treatment in vitro. Am. J. Respir. Crit. Care Med. 1998, 157, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Whyte, I.M.; Francis, B.; Dawson, A.H. Safety and efficacy of intravenous N-acetylcysteine for acetaminophen overdose: Analysis of the Hunter Area Toxicology Service (HATS) database. Curr. Med. Res. Opin. 2007, 10, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.J.; Heard, K.; Sloan, E.P.; Jagoda, A.S. Clinical policy: Critical issues in the management of patients presenting to the emergency department with acetaminophen overdose. Ann. Emerg. Med. 2007, 50, 292–313. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. High attenuation mucoid impaction in allergic bronchopulmonary aspergillosis. World J. Radiol. 2010, 2, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W.; Fotter, R.; Steiner, H.; Beaufort, F.; Stammberger, H. Aspergillosis of the paranasal sinuses. Radiology 1985, 156, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Zinreich, S.J.; Kennedy, D.W.; Malat, J.; Curtin, H.D.; Epstein, J.I.; Huff, L.C.; Kumar, A.J.; Johns, M.E.; Rosenbaum, A.E. Fungal sinusitis: Diagnosis with CT and MR imaging. Radiology 1988, 169, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Gupta, D.; Aggarwal, A.N.; Saxena, A.K.; Chakrabarti, A.; Jindal, S.K. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: An analysis of 155 patients. Chest 2007, 132, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Logan, P.M.; Muller, N.L. High-attenuation mucous plugging in allergic bronchopulmonary aspergillosis. Can. Assoc. Radiol. J. 1996, 47, 374–377. [Google Scholar] [PubMed]
- Morozov, A.; Applegate, K.E.; Brown, S.; Howenstine, M. High-attenuation mucus plugs on MDCT in a child with cystic fibrosis: Potential cause and differential diagnosis. Pediatr. Radiol. 2007, 37, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Bulcke, J.A.; Termote, J.L.; Palmers, Y.; Crolla, D. Computed tomography of the human skeletal muscular system. Neuroradiology 1979, 17, 127–136. [Google Scholar] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cai, W.; Lei, S.; Zhang, Z. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD 2014, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fowdar, K.; Chen, H.; He, Z.; Zhang, J.; Zhong, X.; Zhang, J.; Li, M.; Bai, J. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: A meta-analysis and systematic review. Heart Lung 2017, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Nash, E.F.; Ratjen, F.; Tullis, E.; Stephenson, E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst. Rev. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, E.M.; Berthet, P.; Ruffmann, R.; Leuenberger, P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: A meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000, 22, 209–221. [Google Scholar] [CrossRef]
- Holdiness, M.R. Clinical pharmacokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and bioavailability of reduced and oxidized N.-acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Borgström, L.; Kågedal, B.; Paulsen, O. Pharmacokinetics of N-acetylcysteine in man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar]
- Cotgreave, I.A.; Eklund, A.; Larsson, K.; Moldeus, P.W. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur. J. Respir. Dis. 1987, 70, 73–77. [Google Scholar] [PubMed]
- Millar, A.B.; Pavia, D.; Agnew, J.E.; Lopez-Vidriero, M.T.; Lauque, D.; Clarke, S.W. Effect of oral N-acetylcysteine on mucus clearance. Br. J. Dis Chest 1985, 79, 262–266. [Google Scholar] [CrossRef]
- Bonanomi, L.; Gazzaniga, A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. 1980, 111, 217–222. [Google Scholar]
- Braga, P.C.; Allegra, L. Drugs in Bronchial Mucology; Raven Press: New York, NY, USA, 1989; pp. 77–102. [Google Scholar]
- Papiris, S.; Kotanidou, A.; Malagari, K.; Roussos, C. Clinical review: Severe asthma. Crit. Care 2002, 6, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Oair, H.A.; Elgishy, A.F.; Alzeeret, A.H. Bronchoscopy as a rescue therapy in patients with status asthmaticus: Two case reports and review of literature. Saudi J. Anaesth. 2013, 7, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Shridharani, M.; Maxson, T.R. Pulmonary lavage in a patient in status asthmaticus receiving mechanical ventilation: A case report. Ann. Allergy 1982, 49, 156–158. [Google Scholar] [PubMed]
- Millman, M.; Goodman, A.H.; Goldstein, I.M.; Millman, F.M.; van Campen, S.S. Status Asthmaticus: Use of acetylcysteine during bronchoscopy and lavage to remove mucous plugs. Ann. Allergy 1983, 50, 85–93. [Google Scholar] [PubMed]
- Konrad, F.; Schoenberg, M.H.; Wiedmann, H.; Kilian, J.; Georgieff, M. The application of n-acetylcysteine as an antioxidant and mucolytic in mechanical ventilation in intensive care patients. A prospective, randomized, placebo-controlled, double-blind study. Anaesthesist 1995, 44, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.R. Mucoid impaction of the bronchi. J. Thorac. Surg. 1951, 22, 149. [Google Scholar] [PubMed]
- Shah, R.J.; Kotloff, R.M. Beware of Gloved Fingers. Ann. Am. Thorac. Soc. 2013, 10, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Heyneman, L.E.; McAdams, P.; Rossi, S.E.; Restrepo, C.S.; Eraso, A. Mucoid impactions: Finger-in-glove sign and other CT and radiographic Features. Radiographics 2008, 28, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Urschel, H.C.; Paulson, D.L. Mucoid impaction of the bronchi. J. Soc. Thorac. Surg. Assoc. 1966, 2, 1–16. [Google Scholar] [CrossRef]
- Agarwal, R.; Khan, A.; Gupta, D.; Aggarwal, A.N.; Saxena, A.K.; Chakrabarti, A. An alternate method of classifying allergic bronchopulmonary aspergillosis based on high- attenuation mucus. PLoS ONE 2007, 5, e15346. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, N.; Baraket, M.; Lim, S.; Ridley, L. Case quiz. Thoracic CT illustrating hyperdense bronchial mucous plugging: Allergic bronchopulmonary aspergillosis. Australas. Radiol. 2003, 47, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Shah, A. Allergic bronchopulmonary and sinus aspergillosis: The roentgenologic spectrum. Front. Biosci. 2003, 8, e138–e146. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Gupta, D.; Aggarwal, A.N.; Saxena, A.K.; Saikia, B.; Chakrabarti, A.; Jindal, S.K. Clinical significance of decline in serum IgE Levels in allergic bronchopulmonary aspergillosis. Respir. Med. 2010, 104, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Gupta, D.; Aggarwal, A.N.; Behera, D.; Jindal, S.K. Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in north India. Chest 2006, 130, 442–448. [Google Scholar] [CrossRef] [PubMed]
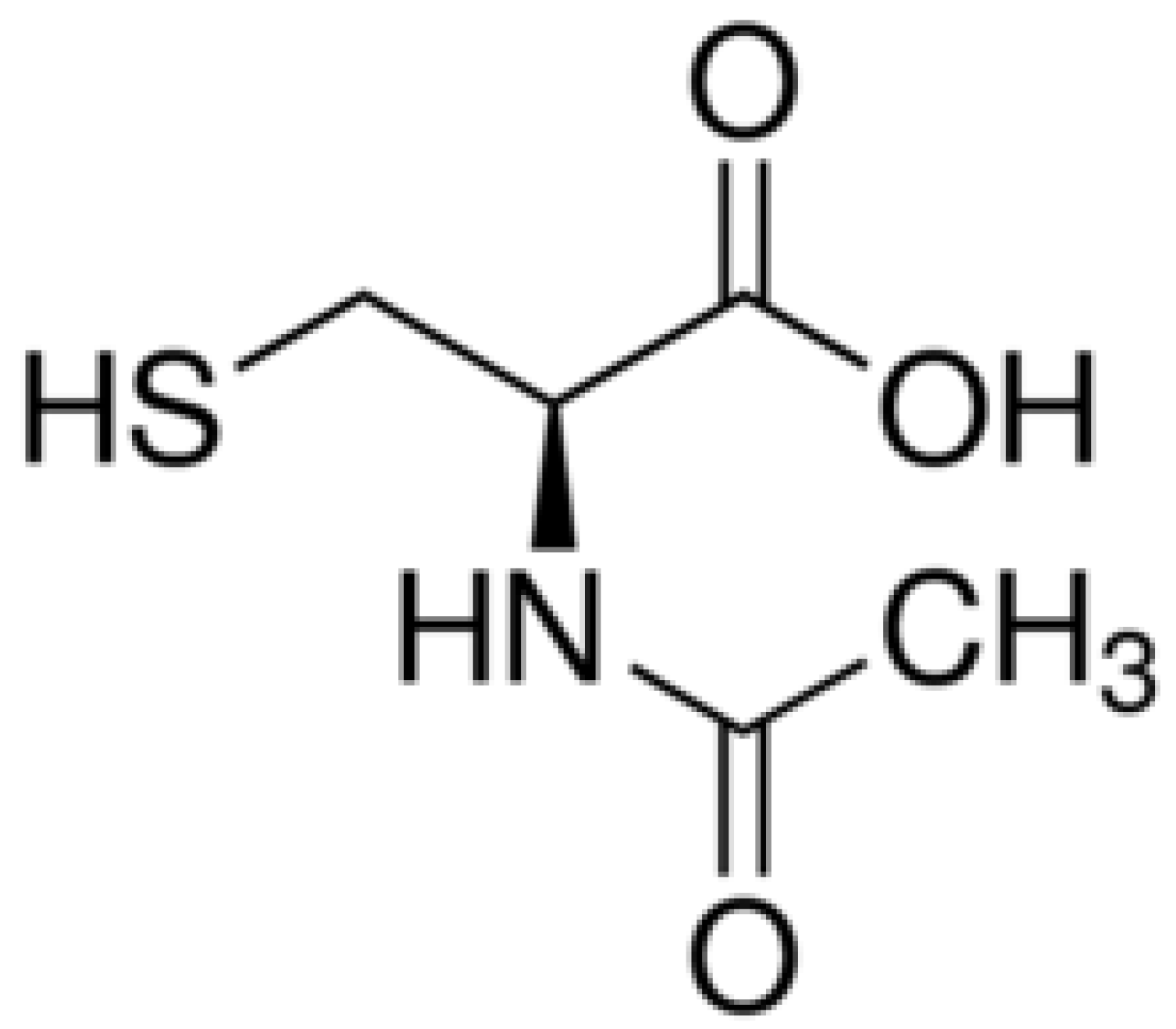

| Sex | Age | Diagnosis | Co-Morbidities | Duration of NAC Use | Outcome | FEV1 Pre/Post (Liters) | Any Other Relevant Clinical Information? |
|---|---|---|---|---|---|---|---|
| Female | 50 | CPA, ABPA | Asthma, bronchiectasis, adrenal insufficiency, and previous left upper lobectomy for bronchiectasis | 14 days | Discontinued. No change in symptoms | 1.67/1.53 | Trialed in order to try and avoid bronchoscopy. She was already receiving daily physiotherapy after 8 mL of 7% saline via nebulizer. The challenge with NAC was followed by intermittent positive pressure breathing therapy, 8 mL 7% saline and active cycle of the breathing technique |
| Male | 63 | ABPA | Asthma, coeliac disease, sinusitis, hypertension vitamin D deficiency, liver cysts, and hyperaldosteronism | 1 | Failed initial challenge due to increased breathlessness. However, he was able to expectorate freely and provided a sputum sample which he hadn’t managed to do previously | 2.25/1.96 | Had not taken his usual dose of Fostair/salbutamol that morning. He was noted to be wheezy pre-challenge. Post NAC challenge, he was given 2 puffs of salbutamol via a metered dose inhaler. |
| Female | 59 | ABPA | Asthma, vocal cord dysfunction, bronchiectasis, and tracheal stenosis | Long term since 2/10/2014 | Discontinued. No change in symptoms | Not performed-tracheostomy patient. No change on auscultation/pulse oximetry (98%)/heart rate | Prior to challenge that had already self-suctioned 6 times that morning. Already nebulizing budesonide, salbutamol, 7% saline and colistin |
| Male | 47 | ABPA | Right upper lobectomy | 1 week | Discontinued. No change in symptoms | 4.15/4.10 | Never tried 7% saline via a nebulizer |
| Female | 57 | Aspergillus terreus and Trichoderma bronchitis | Hypothyroidism, migraine, and bronchiectasis | 8 months. A clinical decision was made to stop NAC as she felt much better post bronchoscopy | Well tolerated, increased expectoration of phlegm | 1.99/1.89 | Already on 7% saline via nebulizer. Underwent bronchoscopy with lavage 8 months after commencing NAC |
| Female | 83 | ABPA Recurrent left lower lobe collapse | Hypertension, osteoarthritis, glaucoma, and nasal disease of uncertain aetiology | 7 months | Tolerated NAC. Eventually discontinued as she derived greater benefit from 7% saline nebs | NAC appeared to aggravate her cough. No further lobar collapse recorded |
| Drug | Device | Indication | Proposed Mechanism of Action | Notes |
|---|---|---|---|---|
| Expectorants | ||||
| Hypertonic saline 7% | Nebulizer | Cystic fibrosis, and bronchiectasis | Increases the amount of sodium and chloride in airway surface liquid, thereby increasing the osmotic gradient and rehydrating the mucus layer [5,7] | Improves lung function and quality of life in bronchiectasis [8]. Should not be given via a vibrating mesh nebulizer. Improves mucus clearance, airflow, and reduces rates of exacerbation among patients with cystic fibrosis [4,9]. |
| Classical mucolytics | ||||
| NAC (Mucomyst®) | Nebulizer | ABPA | Severs disulfide bonds that link mucin monomers to polymers, and solubilizes sputum antioxidant and anti-inflammatory | No evidence for use in any lung disease. |
| S-carboxymethylcysteine (carbocysteine) | Oral | COPD, and cystic fibrosis | Increases concentrations of sialomucins and reduces that of fucomucins, acts as a free radical scavenger [10], and has antioxidant and anti-inflammatory properties | Reduces measured sputum viscosity [11,12]. |
| Dry powder mannitol (Bronchitol®) | Dry powder inhaler | Cystic fibrosis, bronchiectasis, and COPD | Increases mucus secretion | Nonabsorbable. Associated with bronchoconstriction and cough when used in children with cystic fibrosis. |
| Peptide mucolytics | ||||
| Dornase alfa (Pulmozyme®) | Nebulizer | Cystic fibrosis | Hydrolyzes DNA polymer and reduces DNA length | Hydrolyzes DNA, improves lung function, and decreases the frequency of exacerbation [13,14]. |
| Nondestructive mucolytics | ||||
| Unfractionated heparin (UFH) | Nebulizer | COPD, and cystic fibrosis | Modifies ionic interactions and the intermolecular hydrogen bonds between mucin molecules, and untangles the charged oligosaccharide side chains of mucin | UFH reduces the elasticity and yield stress in the samples from cystic fibrosis patients [15]. |
| Low molecular weight dextran (DCF 987) | Nebulizer | COPD | Disrupts the polyionic oligosaccharide mucin network and increases secretion hydration | Proven lung safety in animal studies [16,17]. |
| Brand | Preparation | Administration | Side Effects |
|---|---|---|---|
| Mucomyst® by Bristol-Myers Squibb | Sterile unpreserved solutions (not for injection) of 20% (Mucomyst-20) or 10% (Mucomyst-10) acetylcysteine, with edetate disodium in purified water. Sodium hydroxide is added to adjust pH to 7. | Nebulization using face mask, mouth piece, tracheostomy, tent or croupette, direct introduction into a segment of the bronchopulmonary tree via a plastic catheter, and can also be given via a percutaneous intratracheal catheter and during bronchoscopy. | Stomatitis, nausea, vomiting, fever, rhinorrhea, drowsiness, clamminess, and bronchoconstriction. Acquired sensitization to NAC may rarely occur. |
| Fluimucil by Zambon | Capsules 200 mg, effervescent tablets 600 mg, paediatric sachet 100 mg, granules 200 mg, dry syrup 100 mg/5 mL, and injection suspension (ampoule) 300 mg/3 mL | Oral and aerosol administration | Capsule/granule/dry syrup: heartburn, nausea, vomiting, diarrhoea, stomatitis, dizziness, tinnitus, allergic and reduced blood pressure. IV: hypersensitivity reactions, rhinorrhea, stomatitis, nausea and vomiting. |
| Acetadote® injection by Cumberland Pharmaceuticals Inc. Nashville, TN 37203 | 20% solution in 30 mL (200 mg/mL) single-dose glass vials, preservative-free for IV administration. | For IV administration | Anaphylactoid reaction due to pyrogens, flushing, oedema, urticaria, pruritus, nausea, pharyngitis, rhinorrhea |
| Cetylev® tablets by Alpex Pharma SA | Effervescent tablets 500 mg or 2.5 grams of NAC. | Oral administration | Nausea, vomiting, other gastrointestinal symptoms, and rash with or without fever. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otu, A.; Langridge, P.; Denning, D.W. Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature. J. Fungi 2018, 4, 117. https://doi.org/10.3390/jof4040117
Otu A, Langridge P, Denning DW. Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature. Journal of Fungi. 2018; 4(4):117. https://doi.org/10.3390/jof4040117
Chicago/Turabian StyleOtu, Akaninyene, Philip Langridge, and David W. Denning. 2018. "Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature" Journal of Fungi 4, no. 4: 117. https://doi.org/10.3390/jof4040117
APA StyleOtu, A., Langridge, P., & Denning, D. W. (2018). Nebulised N-Acetylcysteine for Unresponsive Bronchial Obstruction in Allergic Brochopulmonary Aspergillosis: A Case Series and Review of the Literature. Journal of Fungi, 4(4), 117. https://doi.org/10.3390/jof4040117





