Establishment of Novel and Efficient Methods for Investigating Sexual Reproduction in Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Traditional Cross-Mating Method (TCM)
2.3. Conidia Mixed Mating Method (CMM)
2.4. Hyphal Segments Mixed Mating Method (HMM)
2.5. Observation of Sexual Structures
2.6. RNA Extraction and RT-qPCR
2.7. Condition Optimization for Sexual Reproduction
2.8. Phosphorylation Assays
3. Results
3.1. Mass Perithecia Generated via CMM and HMM Methods
3.2. Condition Optimization of CMM and HMM Methods
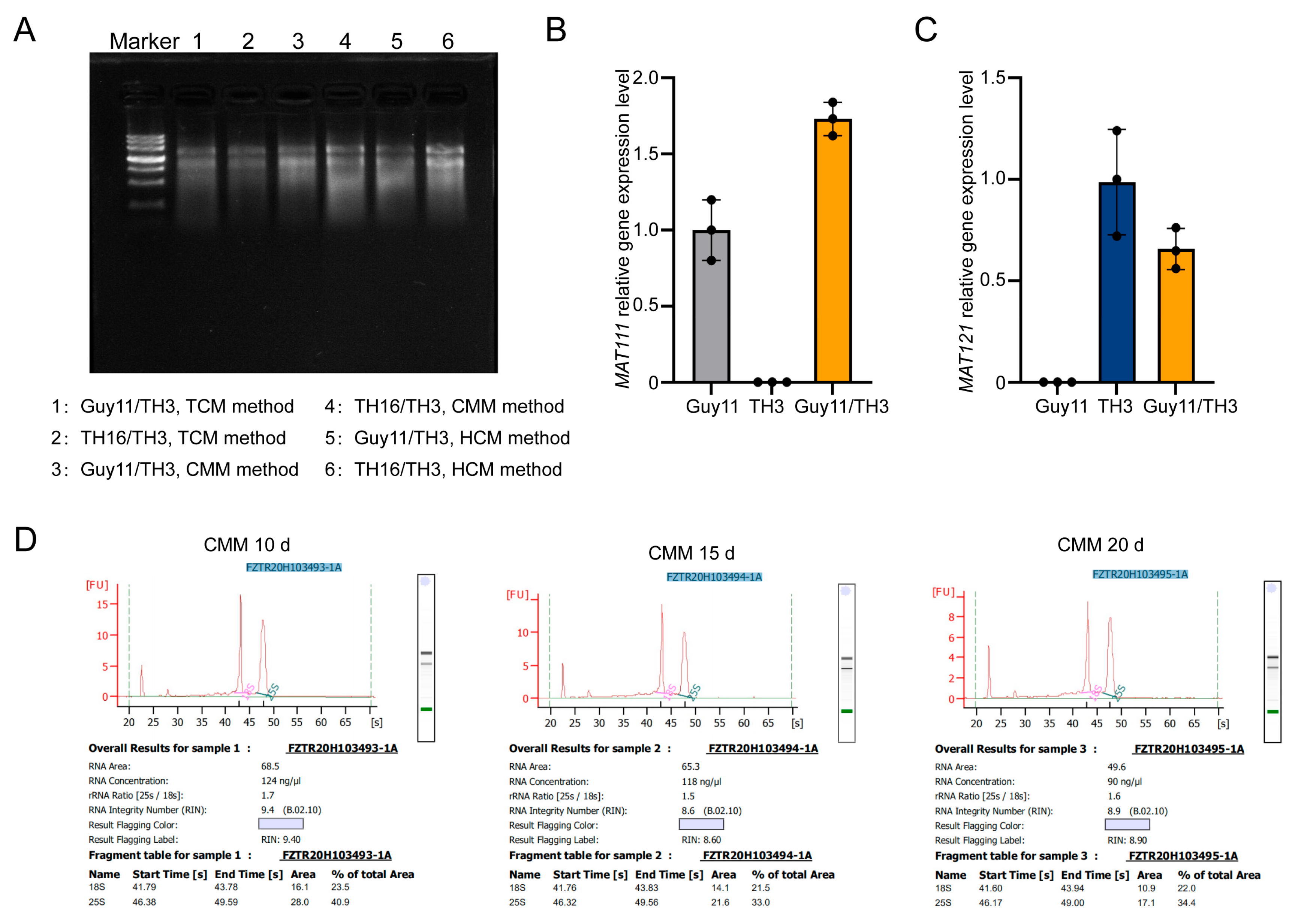
3.3. High-Purity RNA Extraction and Verification
3.4. The CMM and HMM Methods Generate More Perithecia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryder, L.S.; Dagdas, Y.F.; Kershaw, M.J.; Venkataraman, C.; Madzvamuse, A.; Yan, X.; Cruz-Mireles, N.; Soanes, D.M.; Oses-Ruiz, M.; Styles, V.; et al. A sensor kinase controls turgor-driven plant infection by the rice blast fungus. Nature 2019, 574, 423–427. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Wang, G.L.; Ning, Y.S.; Wang, R.Y. Insights into metabolite biosynthesis and regulation in rice immune signaling. Trends Microbiol. 2023, 31, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.J.; Shi, H.B.; Qiu, J.H.; Tao, Z.; Wang, W.M. Effectors and environment modulating rice blast disease: From understanding to effective control. Trends Microbiol. 2024, 32, 1007–1020. [Google Scholar] [CrossRef]
- Guo, N.H.; An, R.H.; Ren, Z.L.; Jiang, J.; Cai, B.N.; Hu, S.K.; Shao, G.N.; Jiao, G.A.; Xie, L.H.; Wang, L.; et al. Developing super rice varieties resistant to rice blast with enhanced yield and improved quality. Plant Biotechnol. J. 2025, 23, 232–234. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Wang, G.L.; Valent, B. Durable resistance to rice blast. Science 2017, 355, 906–907. [Google Scholar] [CrossRef]
- Saleh, D.; Milazzo, J.; Adreit, H.; Tharreau, D.; Fournier, E. Asexual reproduction induces a rapid and permanent loss of sexual reproduction capacity in the rice fungal pathogen Magnaporthe oryzae: Results of in vitro experimental evolution assays. BMC Evol. Biol. 2012, 12, 42. [Google Scholar] [CrossRef]
- Lassagne, A.; Brun, S.; Malagnac, F.; Adreit, H.; Milazzo, J.; Fournier, E.; Tharreau, D. Male fertility in Pyricularia oryzae: Microconidia are spermatia. Environ. Microbiol. 2022, 24, 6365–6375. [Google Scholar] [CrossRef] [PubMed]
- Kita, K.; Uchida, M.; Arie, T.; Teraoka, T.; Kaku, H.; Kanda, Y.; Mori, M.; Arazoe, T.; Kamakura, T. The MAT1 locus is required for microconidia-mediated sexual fertility in the rice blast fungus. FEMS Microbiol. Lett. 2024, 371, fnae004. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Wilken, P.M.; van der Nest, M.A.; Wingfield, M.J.; Wingfield, B.D. It’s All in the Genes: The Regulatory Pathways of Sexual Reproduction in Filamentous Ascomycetes. Genes 2019, 10, 330. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, S.Z.; Zhang, Z.; Hao, Z.N.; Shi, X.X.; Li, L.; Zhu, X.M.; Qiu, H.P.; Chai, R.Y.; Wang, Y.L.; et al. MAT Loci Play Crucial Roles in Sexual Development but Are Dispensable for Asexual Reproduction and Pathogenicity in Rice Blast Fungus Magnaporthe oryzae. J. Fungi 2021, 7, 858. [Google Scholar] [CrossRef]
- Zeyl, C. The role of sex in fungal evolution. Curr. Opin. Microbiol. 2009, 12, 592–598. [Google Scholar] [CrossRef]
- Fu, C.; Coelho, M.A.; David-Palma, M.; Priest, S.J.; Heitman, J. Genetic and genomic evolution of sexual reproduction: Echoes from LECA to the fungal kingdom. Curr. Opin. Genet. Dev. 2019, 58–59, 70–75. [Google Scholar] [CrossRef]
- Saleh, D.; Xu, P.; Shen, Y.; Li, C.; Adreit, H.; Milazzo, J.; Ravigne, V.; Bazin, E.; Notteghem, J.L.; Fournier, E.; et al. Sex at the origin: An Asian population of the rice blast fungus Magnaporthe oryzae reproduces sexually. Mol. Ecol. 2012, 21, 1330–1344. [Google Scholar] [CrossRef]
- Cruz-Mireles, N.; Osés-Ruiz, M.; Derbyshire, P.; Jégousse, C.; Ryder, L.S.; Bautista, M.J.A.; Eseola, A.; Sklenar, J.; Tang, B.Z.; Yan, X.; et al. The phosphorylation landscape of infection-related the rice blast fungus. Cell 2024, 187, 2557–2573. [Google Scholar] [CrossRef]
- Fernandez, J.; Lopez, V.; Kinch, L.; Pfeifer, M.A.; Gray, H.; Garcia, N.; Grishin, N.V.; Khang, C.H.; Orth, K. Role of Two Metacaspases in Development and Pathogenicity of the Rice Blast Fungus Magnaporthe oryzae. mBio 2021, 12, e03471-20. [Google Scholar] [CrossRef]
- Ryder, L.S.; Lopez, S.G.; Michels, L.; Eseola, A.B.; Sprakel, J.; Ma, W.; Talbot, N.J. A molecular mechanosensor for real-time visualization of appressorium membrane tension in Magnaporthe oryzae. Nat. Microbiol. 2023, 8, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, X.; Xiao, J.; Kang, X.; Hu, J.; Huang, Z.; Li, N.; Yang, C.; Pan, Y.; Zhang, S. A novel MAP kinase-interacting protein MoSmi1 regulates development and pathogenicity in Magnaporthe oryzae. Mol. Plant Pathol. 2024, 25, e13493. [Google Scholar] [CrossRef]
- Sakulkoo, W.; Oses-Ruiz, M.; Garcia, E.O.; Soanes, D.M.; Littlejohn, G.R.; Hacker, C.; Correia, A.; Valent, B.; Talbot, N.J. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 2018, 359, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Liang, M.L.; Naqvi, N.I.; Lin, C.X.; Qian, W.Q.; Zhang, L.H.; Deng, Y.Z. Phototrophy and starvation-based induction of autophagy upon removal of Gcn5-catalyzed acetylation of Atg7 in Magnaporthe oryzae. Autophagy 2017, 13, 1318–1330. [Google Scholar] [CrossRef]
- Kong, Y.; Guo, P.S.; Xu, J.Y.; Li, J.X.; Wu, M.; Zhang, Z.Q.; Wang, Y.F.; Liu, X.Y.; Yang, L.Y.; Liu, M.X.; et al. MoMkk1 and MoAtg1 dichotomously regulating autophagy and pathogenicity through MoAtg9 phosphorylation in Magnaporthe oryzae. mBio 2024, 15, e0334423. [Google Scholar] [CrossRef]
- Liu, M.; Wang, F.; He, B.; Hu, J.; Dai, Y.; Chen, W.; Yi, M.; Zhang, H.; Ye, Y.; Cui, Z.; et al. Targeting Magnaporthe oryzae effector MoErs1 and host papain-like protease OsRD21 interaction to combat rice blast. Nat. Plants 2024, 10, 618–632. [Google Scholar] [CrossRef]
- Chen, X.; Selvaraj, P.; Lin, L.L.; Fang, W.Q.; Wu, C.X.; Yang, P.; Zhang, J.; Abubakar, Y.S.; Yang, F.; Lu, G.D.; et al. Rab7/Retromer-based endolysosomal trafficking is essential for proper host invasion in rice blast. N. Phytol. 2023, 239, 1384–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Que, Y.W.; Liu, Y.T.; Yue, X.F.; Meng, X.L.; Zhang, Z.G.; Wang, Z.Y. The putative Gγ subunit gene is required for conidiation, appressorium formation, mating and pathogenicity in Magnaporthe oryzae. Curr. Genet. 2015, 61, 641–651. [Google Scholar] [CrossRef]
- Kato, H.; Yamaguchi, T. The perfect stage of Pyricularia oryzae Cav. from rice plants. In Proceedings of the International Conference on Plant Protection in the Tropics, Kuala Lumpur, Malaysia, 1–4 March 1982; pp. 161–164. [Google Scholar]
- Qiu, J.; Xie, J.; Chen, Y.; Shen, Z.; Shi, H.; Naqvi, N.I.; Qian, Q.; Liang, Y.; Kou, Y. Warm temperature compromises JA-regulated basal resistance to enhance Magnaporthe oryzae infection in rice. Mol. Plant 2022, 15, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Shen, X.T.; Liu, M.X.; Liu, X.Y.; Yin, Z.Y.; Li, X.; Feng, W.Z.; Hu, J.X.; Zhang, H.F.; Zheng, X.B.; et al. The rice blast fungus MoRgs1 functioning in cAMP signaling and pathogenicity is regulated by casein kinase MoCk2 phosphorylation and modulated by membrane protein MoEmc2. PLoS Pathog. 2021, 17, e1009657. [Google Scholar] [CrossRef]
- Qu, Y.M.; Cao, H.J.; Huang, P.Y.; Wang, J.; Liu, X.H.; Lu, J.P.; Lin, F.C. A kelch domain cell end protein, PoTea1, mediates cell polarization during appressorium morphogenesis in Pyricularia oryzae. Microbiol. Res. 2022, 259, 126999–127014. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Huang, L.; Liu, H. Fus3-regulated Tec1 degradation through SCFCdc4 determines MAPK signaling specificity during mating in yeast. Cell 2004, 119, 981–990. [Google Scholar] [CrossRef]
- Li, G.T.; Zhang, X.; Tian, H.; Choi, Y.E.; Tao, W.A.; Xu, J.R. MST50 is involved in multiple MAP kinase signaling pathways in Magnaporthe oryzae. Environ. Microbiol. 2017, 19, 1959–1974. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Wang, J.Y.; Wu, X.Y.; Liang, S.; Zhu, X.M.; Li, L.; Lu, J.P.; Liu, X.H.; Lin, F.C. MoOpy2 is essential for fungal development, pathogenicity, and autophagy in Magnaporthe oryzae. Environ. Microbiol. 2022, 24, 1653–1671. [Google Scholar]
- Schamber, A.; Leroch, M.; Diwo, J.; Mendgen, K.; Hahn, M. The role of mitogen-activated protein (MAP) kinase signalling components and the Ste12 transcription factor in germination and pathogenicity of Botrytis cinerea. Mol. Plant Pathol. 2010, 11, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Li, G.T.; Zhou, X.Y.; Xu, J.R. Genetic control of infection-related development in Magnaporthe oryzae. Curr. Opin. Microbiol. 2012, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Bayram, O.S.; Ahmed, Y.L.; Maruyama, J.; Valerius, O.; Rizzoli, S.O.; Ficner, R.; Irniger, S.; Braus, G.H. The Aspergillus nidulans MAPK module AnSte11-Ste50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet. 2012, 8, e1002816. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Y.; Liu, Y.; Zhang, C.; Ma, Z. The transmembrane protein FgSho1 regulates fungal development and pathogenicity via the MAPK module Ste50-Ste11-Ste7 in Fusarium graminearum. N. Phytol. 2015, 206, 315–328. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, L.; Deng, J.; Tan, C.; Geng, L.; Liao, Y.; Yuan, J.; Wang, S. A Cell Wall Integrity-Related MAP Kinase Kinase Kinase AflBck1 Is Required for Growth and Virulence in Fungus Aspergillus flavus. Mol. Plant-Microbe Interact. 2020, 33, 680–692. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Li, F.; Ma, W.; Guo, W.; Fang, X. Role of Pmk1, Mpk1, or Hog1 in the mitogen-activated protein kinase pathway of Aspergillus cristatus. Fungal Genet. Biol. 2024, 171, 103874–103883. [Google Scholar] [CrossRef]

| Temperature | Light Exposure | Conidium Density | |
|---|---|---|---|
| Optimal conditions | 20 °C | 24 h light exposure | 5 × 104 conidia/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Wang, J.; Noman, M.; Hao, Z.; Zhang, Z.; Qiu, H.; Chai, R.; Wang, Y.; Wang, J.; Lin, F. Establishment of Novel and Efficient Methods for Investigating Sexual Reproduction in Magnaporthe oryzae. J. Fungi 2025, 11, 604. https://doi.org/10.3390/jof11080604
Cai Y, Wang J, Noman M, Hao Z, Zhang Z, Qiu H, Chai R, Wang Y, Wang J, Lin F. Establishment of Novel and Efficient Methods for Investigating Sexual Reproduction in Magnaporthe oryzae. Journal of Fungi. 2025; 11(8):604. https://doi.org/10.3390/jof11080604
Chicago/Turabian StyleCai, Yingying, Jing Wang, Muhammad Noman, Zhongna Hao, Zhen Zhang, Haiping Qiu, Rongyao Chai, Yanli Wang, Jiaoyu Wang, and Fucheng Lin. 2025. "Establishment of Novel and Efficient Methods for Investigating Sexual Reproduction in Magnaporthe oryzae" Journal of Fungi 11, no. 8: 604. https://doi.org/10.3390/jof11080604
APA StyleCai, Y., Wang, J., Noman, M., Hao, Z., Zhang, Z., Qiu, H., Chai, R., Wang, Y., Wang, J., & Lin, F. (2025). Establishment of Novel and Efficient Methods for Investigating Sexual Reproduction in Magnaporthe oryzae. Journal of Fungi, 11(8), 604. https://doi.org/10.3390/jof11080604







