Biological Characteristics and Domestication of Dichomitus squalens and the Antioxidant Activity of Its Cultivated Fruiting Bodies
Abstract
1. Introduction
2. Materials and Methods
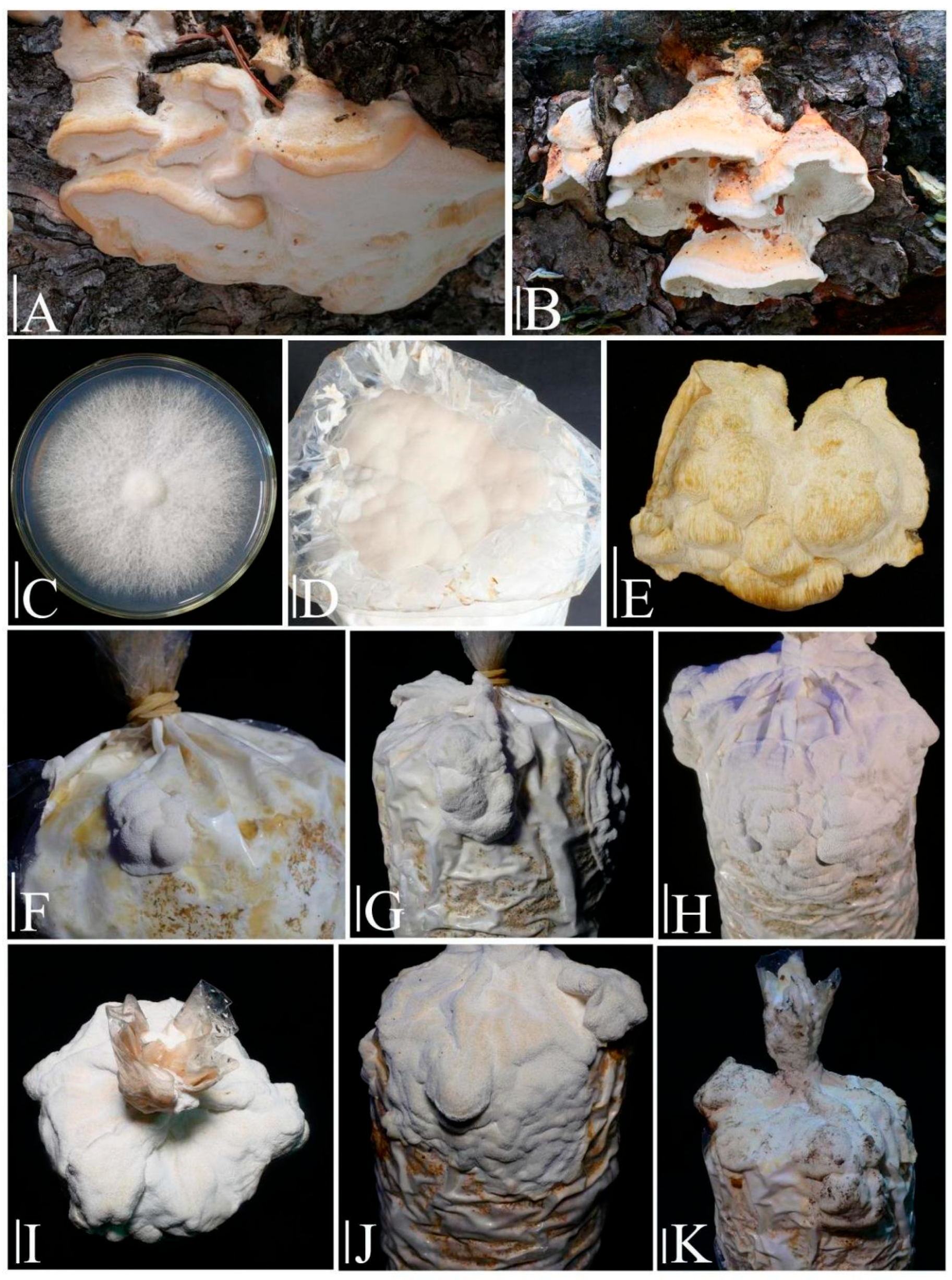
2.1. Experimental Strains and Specimens
2.2. Formulations of Experimental Media
2.3. Species Identification
2.4. Biological Characterization Studies
2.4.1. Single-Factor Experiment
- (1)
- Carbon source single-factor experimental design
- (2)
- Nitrogen source single-factor experimental design
- (3)
- pH gradient experimental design
- (4)
- Temperature gradient experimental design
2.4.2. Orthogonal Experiments
2.5. Domestication Cultivation Experiments
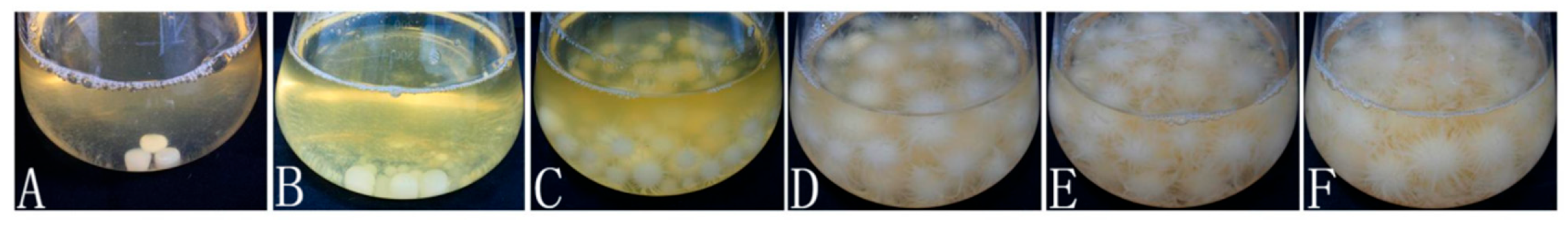
2.5.1. Liquid Strain Preparation
2.5.2. Production and Mycelial Colonization Management of Cultivation Spawn
2.5.3. Primordia Induction and Fruiting Body Development
2.6. Crude Polysaccharide Extraction
2.7. Total Polysaccharide Content Determination
2.8. Antioxidant Activity Determination
3. Results and Analysis
3.1. Species Identification
3.2. Biological Characterization Studies
3.2.1. Single-Factor Experiments
- (1)
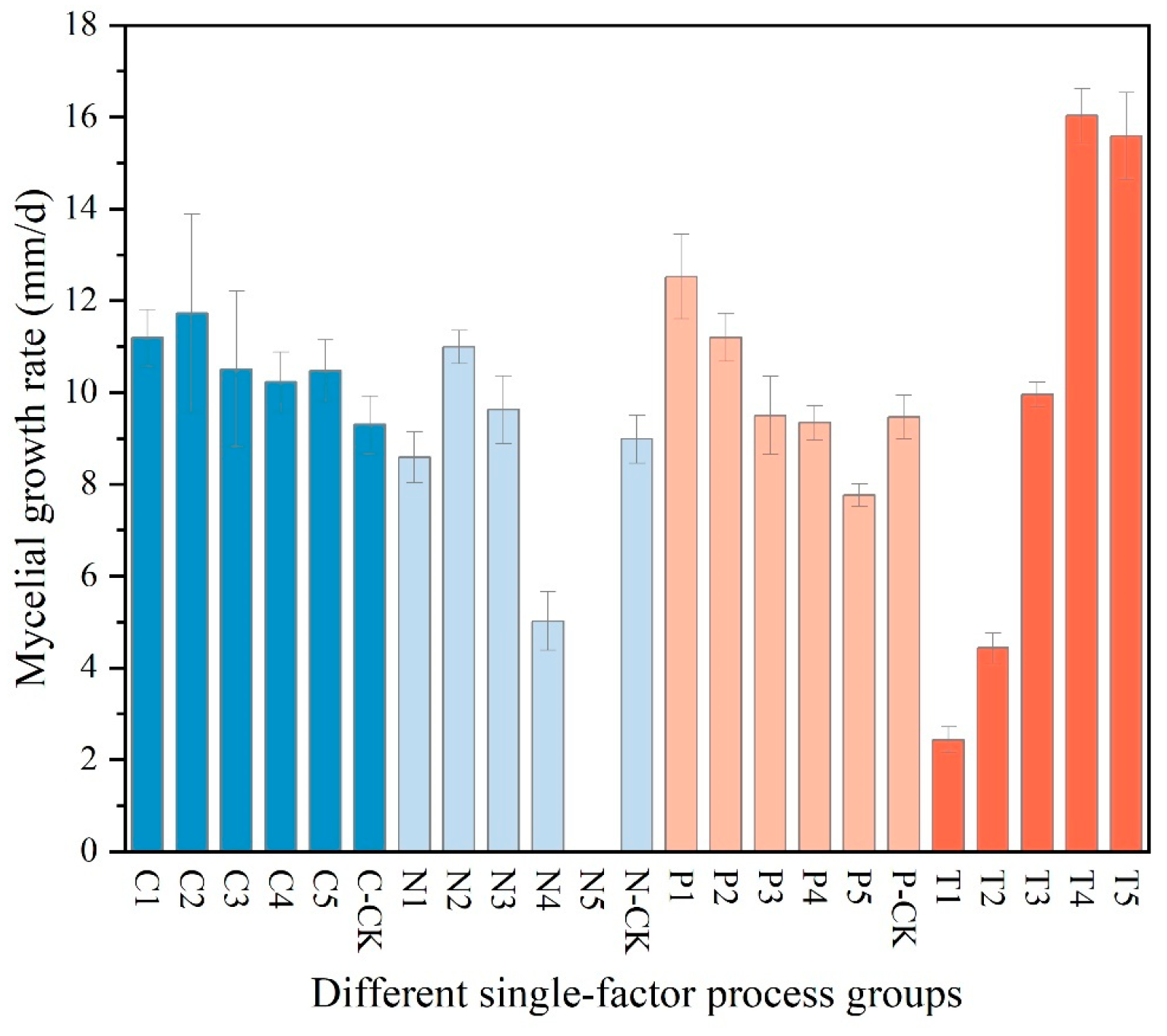
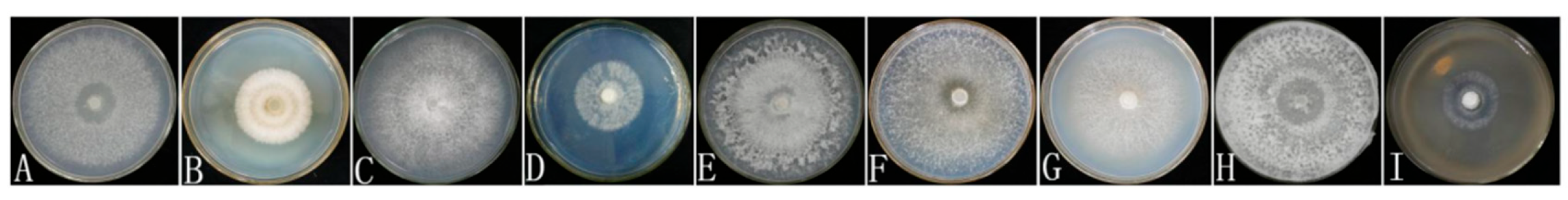
- The results of the single-factor experiment on carbon sources, as presented in Table 2, indicate that sucrose is the most effective carbon source for promoting the hyphal growth of Dichomitus squalens, achieving a growth rate of 11.73 ± 2.16 mm/d. Glucose was ranked second with a rate of 11.20 ± 0.61 mm/d, followed in descending order by soluble starch at 10.50 ± 1.70 mm/d, maltose at 10.48 ± 0.67 mm/d, and dextrin at 10.22 ± 0.66 mm/d. Under all experimental conditions utilizing a single carbon source, hyphal growth was relatively sparse (Figure 3 and Figure 4).
- (2)
- The results of the nitrogen source single-factor experiment, as presented in Table 2, demonstrate that yeast extract powder is the most effective nitrogen source for promoting the hyphal growth of Dichomitus squalens, achieving a growth rate of 10.99 ± 0.36 mm/d. Ammonium chloride exhibited the second highest rate of growth at 9.63 ± 0.74 mm/d, followed sequentially by peptone at 8.59 ± 0.56 mm/d and diammonium phosphate at 5.02 ± 0.64 mm/d. Notably, the urea treatment group completely inhibited hyphal growth, resulting in 0 mm/d, whereas the nitrogen-free control group sustained basal metabolic activity at 8.99 ± 0.52 mm/d (Figure 3 and Figure 4).
- (3)
- The results of the pH single-factor experiment, as presented in Table 2, indicate that pH exerts a significant influence on the hyphal growth of Dichomitus squalens. The optimal pH was 5.0, with a growth rate of 12.52 ± 0.92 mm/d, showing no significant difference from the pH 6.0 group (11.20 ± 0.52 mm/d). Under conditions ranging from neutral to alkaline pH levels (7.0–9.0), the growth rates demonstrated a linear decline (Figure 3 and Figure 4).
- (4)
- The results of the temperature single-factor experiment, as presented in Table 2, indicate a significant dependence of Dichomitus squalens hyphal growth on temperature. The optimal temperature for growth was identified as 30 °C, with a growth rate of 16.03 ± 0.59 mm/d, which did not differ significantly from the growth rate at 35 °C (15.59 ± 0.95 mm/d). However, the growth rate at 30 °C was significantly higher than those observed at 25 °C (9.96 ± 0.27 mm/d), 20 °C (4.43 ± 0.33 mm/d), and 15 °C (2.44 ± 0.28 mm/d). Hyphal growth was robust and dense across all tested temperatures, as illustrated in Figure 3. Furthermore, within the temperature range of 15–30 °C, there was a significantly positive correlation between growth rates and temperature, as depicted in Figure 4.
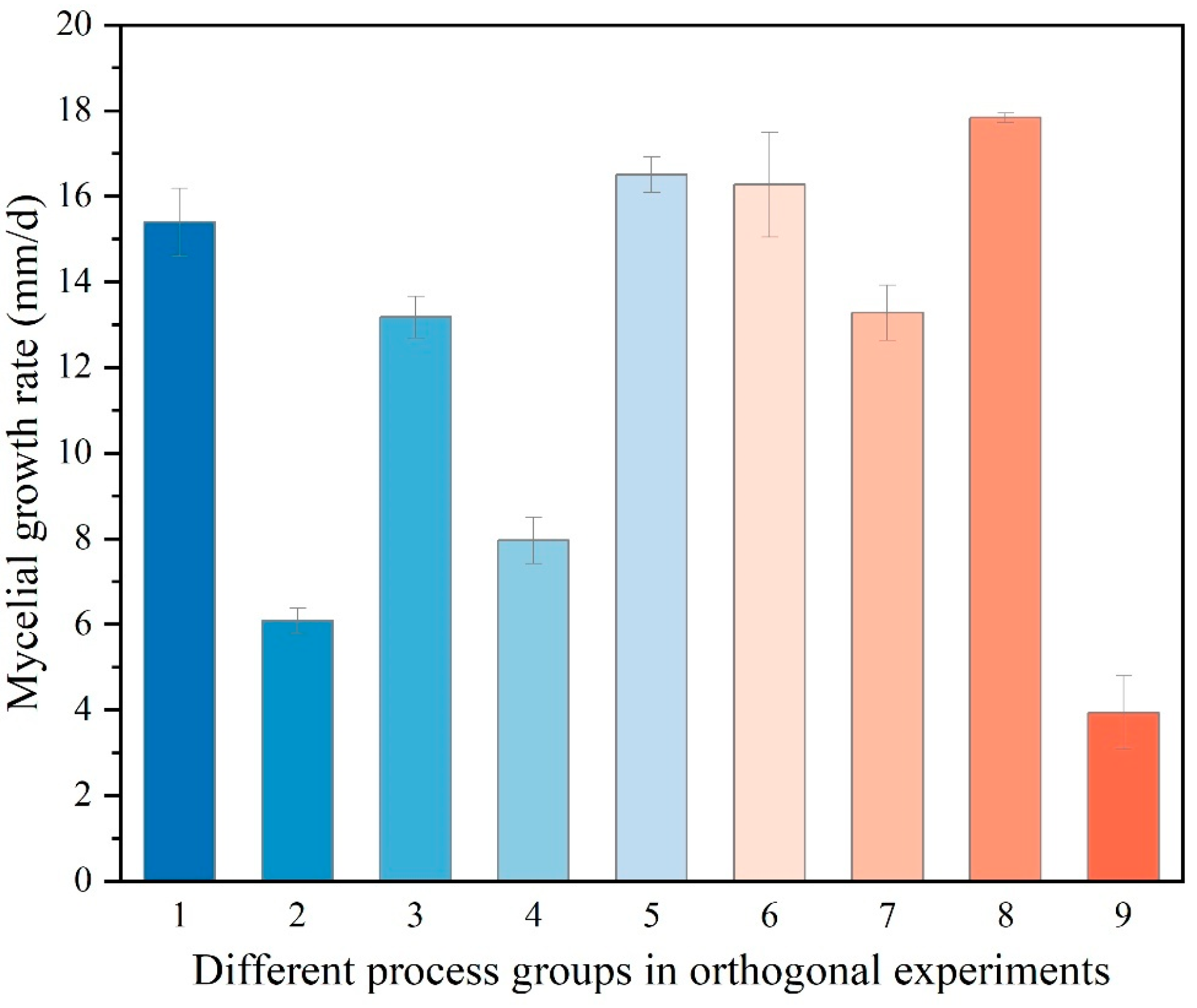
3.2.2. Orthogonal Experiments
3.3. Domestication Cultivation Trials
3.4. Crude Polysaccharide Extraction and Total Polysaccharide Content Determination
3.5. Antioxidant Activity Assays
3.5.1. Hydroxyl Radical Scavenging Capacity
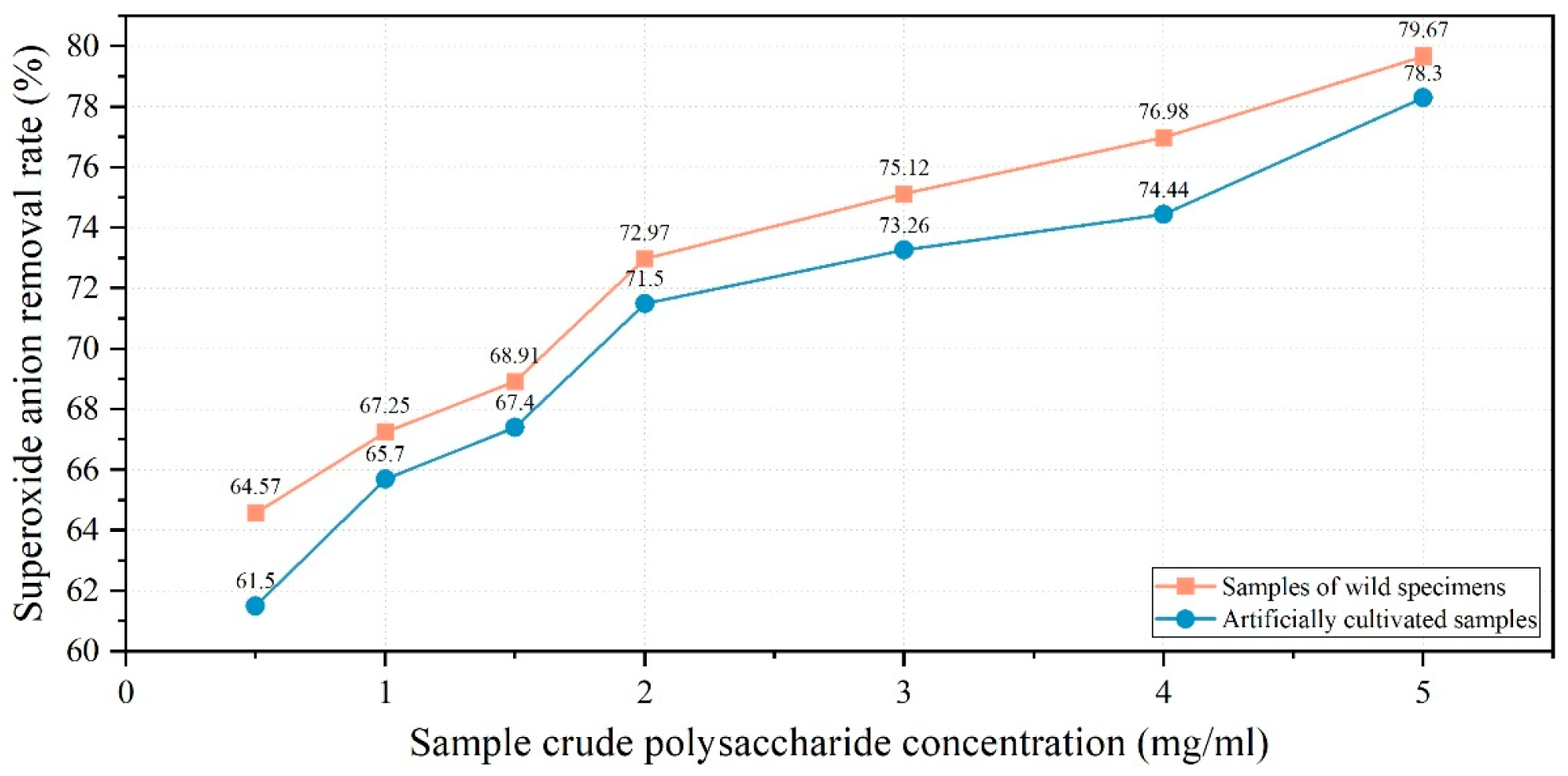
3.5.2. Superoxide Anion Radical Scavenging Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Li, T.H.; Yang, Z.L.; Bau, T.; Dai, Y.C. Atlas of Chinese Macrofungal Resources; Zhongyuan Farmer’s Publishing House: Zhengzhou, China, 2015; pp. 1–1351. (In Chinese) [Google Scholar]
- Rytioja, J.; Hilden, K.; Falco, M.D.; Zhou, M.; Aguilar-Pontes, M.V.; Sietio, O.M.; Tsang, A.; Vries, R.P.; Makela, M.R. The molecular response of the white-rot fungus Dichomitus squalens to wood and non-woody biomass as examined by transcriptome and exoproteome analyses. Environ. Microbiol. 2017, 19, 1237–1250. [Google Scholar] [CrossRef]
- Daly, P.; López, S.C.; Peng, M.; Lancefield, C.S.; Purvine, S.O.; Kim, Y.M.; Zink, E.M.; Dohnalkova, A.; Singan, V.R.; Lipzen, A.; et al. Dichomitus squalens partially tailors its molecular responses to the composition of solid wood. Environ. Microbiol. 2018, 20, 4141–4156. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, N.; He, B.; Igarashi, Y.; Luo, F. Transcriptome analysis of differential gene expression in Dichomitus squalens during interspecific mycelial interactions and the potential link with laccase induction. J. Microbiol. 2019, 57, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Šušla, M.; Novotný, C.; Svobodová, K. The implication of Dichomitus squalens laccase isoenzymes in dye decolorization by immobilized fungal cultures. Bioresour. Technol. 2007, 98, 2109–2115. [Google Scholar] [CrossRef]
- Daly, P.; Peng, M.; Falco, M.D.; Lipzen, A.; Wang, M.; Ng, V.; Grigoriev, I.V.; Tsang, A.; Makela, M.R.; Vries, R.P. Glucose-Mediated Repression of Plant Biomass Utilization in the White-Rot Fungus Dichomitus squalens. Appl. Environ. Microbiol. 2019, 85, e01828-19. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wiebenga, A.; Lipzen, A.; Ng, V.; Tejomurthula, S.; Zhang, Y.; Grigoriev, I.V.; Peng, M.; Vries, R.P. Comparative Genomics and Transcriptomics Analyses Reveal Divergent Plant Biomass-Degrading Strategies in Fungi. J. Fungi 2023, 9, 860. [Google Scholar] [CrossRef]
- Kowalczyk, J.E.; Saha, S.; Makela, M.R. Application of CRISPR/Cas9 Tools for Genome Editing in the White-Rot Fungus Dichomitus squalens. Biomolecules 2021, 11, 1526. [Google Scholar] [CrossRef]
- Daly, P.; Slaghek, G.G.; Lopez, S.C.; Wiebenga, A.; Hilden, K.S.; Vries, R.P.; Makela, M.R. Genetic transformation of the white-rot fungus Dichomitus squalens using a new commercial protoplasting cocktail. J. Microbiol. Methods 2017, 143, 38–43. [Google Scholar] [CrossRef]
- Venugopal, P.; Junninen, K.; Edman, M.; Kouki, J. Assemblage composition of fungal wood-decay species has a major influence on how climate and wood quality modify decomposition. FEMS Microbiol. Ecol. 2017, 93, fix002. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.F.; Liu, M.D.; Wang, Q.; Li, Y. Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. Int. J. Biol. Macromol. 2018, 112, 326–332. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 40, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J. Improvement of Tissue Isolation Method for Fruiting Bodies. Zhejiang Edible Fungi 1994, 6, 14. (In Chinese) [Google Scholar]
- Chen, G.F. Abrief Introduction to Common Methods for Observation of Mor-phology of Macrofungi. Hortic. Seed 2019, 39, 76–78. (In Chinese) [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Taylor, J., Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Dai, D.; Wang, Z.; Hu, J.; Dai, J.C.; Peng, X.H.; Sun, P.; Cheng, Z.T.; Wang, Z.W.; Wei, Y.H.; Li, Y. Biological characteristics and domestication of Piptoporellus soloniensis (Piptoporellaceae, Basidiomycota). Mycosystema 2024, 43, 79–89. (In Chinese) [Google Scholar]
- Guo, D.Z.; Ma, A.; Hu, J.J.; Li, D.; Zhang, B.; Li, Y. Solid incubation, domestic cultivation and cellulase activities of Elmerina hispida. Mycosystema 2019, 38, 1111–1119. (In Chinese) [Google Scholar]
- Wang, Y.; Li, S.; Zhang, T.; Guo, Y.F.; Li, D.; Zhang, B.; Li, Y. Biological Characteristics and Domestic Cultivation Analysis of Daedaleopsis confragosa. Edible Fungi China 2019, 38, 9–14, 18. (In Chinese) [Google Scholar]
- Zhang, F.; Zhang, T.; Li, D.; Zhang, B.; Li, Y. Biological characteristics, domestication cultivation and antioxidant activity of Trametes gibbosa. Mycosystema 2019, 38, 1480–1490. (In Chinese) [Google Scholar]
- Ma, A.; Hu, J.J.; Meng, L.S.; Xu, S.; Li, D.; Zhang, B.; Li, Y. Biological Characteristics and Artificial Domestication Cultivation of Trametes velutina. Mol. Plant Breed. 2019, 17, 7269–7276. (In Chinese) [Google Scholar]
- Liang, Y.; Dai, D.; Chang, W.Q.; Wang, Y.; Zhang, Z.H.; Li, D.; Zhang, B.; Li, Y. Biological Characteristics, Domesticated Cultivation Protocol, Antioxidant Activity, and Protective Effects against Cellular Oxidative Stress of an Underutilized Medicinal Mushroom: Fomitopsis palustris. J. Fungi 2024, 10, 380. [Google Scholar] [CrossRef]
- Qi, Z.X.; Rao, G.; Hu, J.J.; Tuo, Y.L.; Zhang, B.; Qian, K.Q. Biological characteristics and artificial domestication cultivation of Tyromyces chioneus. Mycosystema 2023, 42, 408–417. (In Chinese) [Google Scholar]
- Wei, H.; Zhang, D.; Lu, L.; Chen, F.C. Extraction, Purification and Antioxidant Activities of Several Polysaccharides from Edible and Medicinal Fungi. J. Nanjing Norm. Univ. (Nat. Sci. Ed.) 2017, 40, 72–75, 88. (In Chinese) [Google Scholar]
- Sun, G.Q.; Dong, J.Y.; Dong, J.S. Content Determination of Polysaccharides of Ganoderma By Phenol-Sulfuric Acid Method. Shanxi Chem. Ind. 2024, 44, 50–52, 72. (In Chinese) [Google Scholar]
- Zhao, J.D. Flora Fungorum Sinicorum VOL. 3 Polyporaceae; Science Press: Beijing, China, 1998; pp. 1–456. (In Chinese) [Google Scholar]
- Jancovicova, S. Dichomitus squalens (Polyporaceae, Basidiomycota)—in Slovakia again after many years. Acta Bot. Univ. Comen. 2021, 58, 61–72. [Google Scholar]
- Tomšovský, M.; Popelářová, P.; Baldrian, P. Production and regulation of lignocellulose-degrading enzymes of Poria-like wood-inhabiting basidiomycetes. Folia Microbiol. 2009, 54, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Cui, B.K. Taxonomy and phylogeny of the genus Megasporoporia and its related genera. Mycologia 2012, 105, 368–383. [Google Scholar] [CrossRef]
- Miettinen, O.; Rajchenberg, M. Obba and Sebipora.; new polypore genera related to Cinereomyces and Gelatoporia (Polyporales, Basidiomycota). Mycol Prog. 2012, 11, 131–147. [Google Scholar] [CrossRef]
- Floudas, D.; Hibbett, D.S. Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol. 2015, 119, 679–719. [Google Scholar] [CrossRef]
- Carlsson, F.; Edman, M.; Holm, S.; Eriksson, A.M.; Jonsson, B.G. Increased heat resistance in mycelia from wood fungi prevalent in forests characterized by fire: A possible adaptation to forest fire. Fungal Biol. 2012, 116, 1025–1031. [Google Scholar] [CrossRef]
- Wang, Y.R.; Dai, Y.C.; Liu, H.G.; Vlasák, J.; Buchanan, P.; Yuan, Y.; Wu, Y.D. A new contribution to Megasporoporia sensu lato: Six new species and three new combinations. Front. Microbiol. 2022, 13, 1046777. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Qi, Z.X. Diversity of macrofungi in Tili River Valley, Xinjiang Uygur Autonomous Region. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2023. (In Chinese). [Google Scholar]
- Wang, Q.; Ruan, X.; Li, Z.H.; Pan, C.D. Autotoxicity of Plants and Research of Coniferous Forest Autotoxicity. Sci. Silvae Sin. 2007, 43, 134–142. (In Chinese) [Google Scholar]
- Cheng, G.H.; An, X.Y.; Wang, X.; Zhang, B.; Li, Y. Cultural characteristics and domestic cultivation of Polyporus tuberaster. Mycosystema 2018, 37, 712–721. (In Chinese) [Google Scholar]
- Ma, L.; Bao, H.Y.; Han, Y.; Hu, R.W.; Bau, T.; Li, Y. Cultivation and biological characteristics of Trametes suaveolens. Mycosystema 2019, 38, 187–194. (In Chinese) [Google Scholar]
- Li, Y.Q. Studies on the Domestication Cultivation, Anti-Hyperglycemic and Antioxidative Effect of Inonotus hispidus. Master’s Thesis, Tarim University, Aral, China, 2010. (In Chinese). [Google Scholar]
- Qi, M.; Liu, C.Y.; Zhao, Q.; Zhang, Q.H.; Hu, K.H.; Fu, J.S. Polysaccharide antioxidant activities of Panus giganteus. Mycosystema 2018, 37, 1707–1716. (In Chinese) [Google Scholar]
- Wang, M.H.; Hao, Z.Q.; Chang, M.C.; Meng, J.L.; Liu, J.Y.; Feng, C.P. Characterization, antioxidant and immunity activities of Sparassis latifolia polysaccharides. Mycosystema 2019, 38, 707–716. (In Chinese) [Google Scholar]
- Nie, L.R.; Hao, L.M.; Wang, T.T.; Liu, Y.; Zhang, L.M.; Lu, J.K.; Kang, C.C.; Cui, Y.; Han, P.P.; Jia, S.R. Antioxidant Activity of Crude Polysaccharides from Fomitopsis pinicola from Different Geographical Origins. Food Sci. 2019, 40, 60–68. (In Chinese) [Google Scholar]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
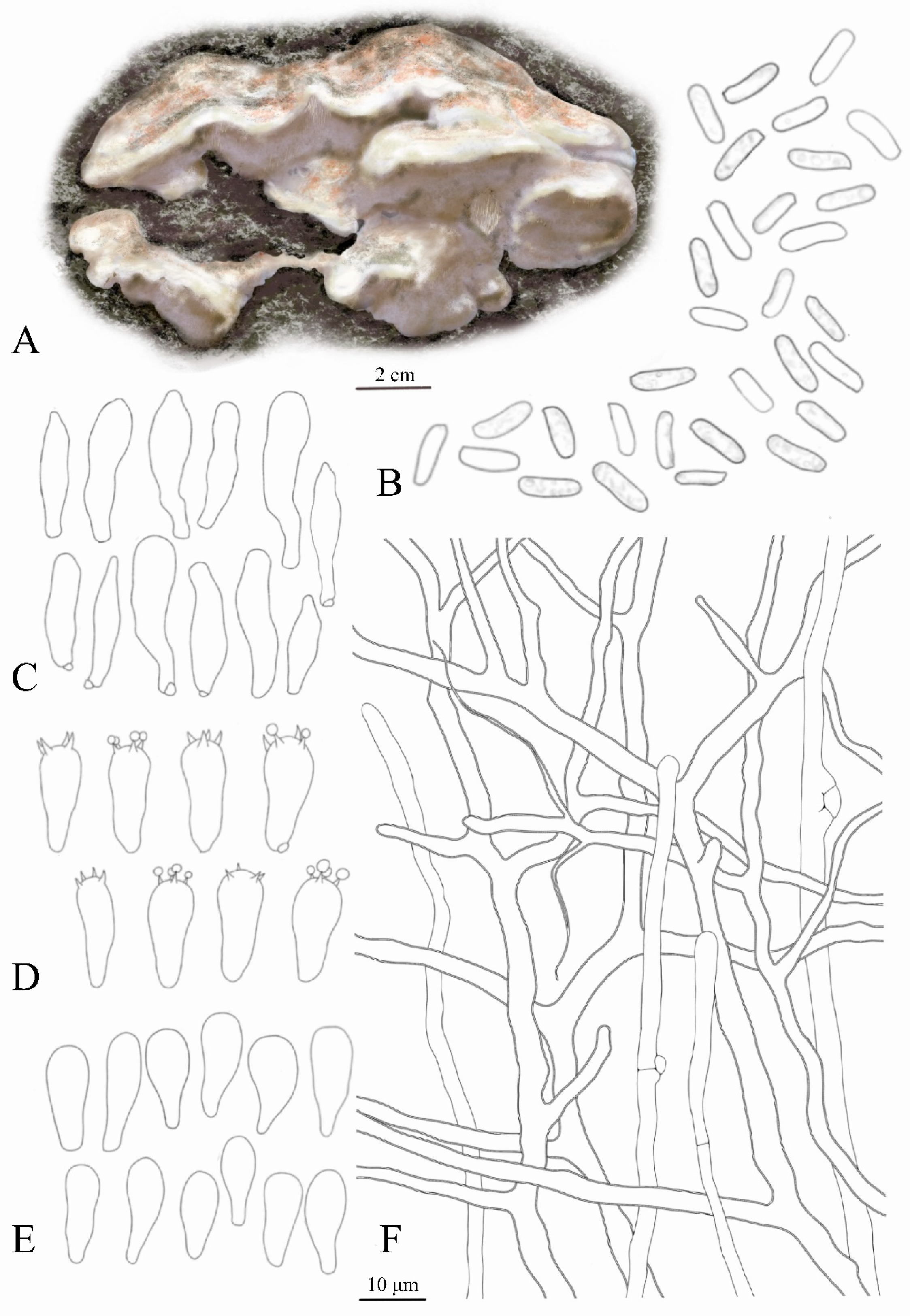
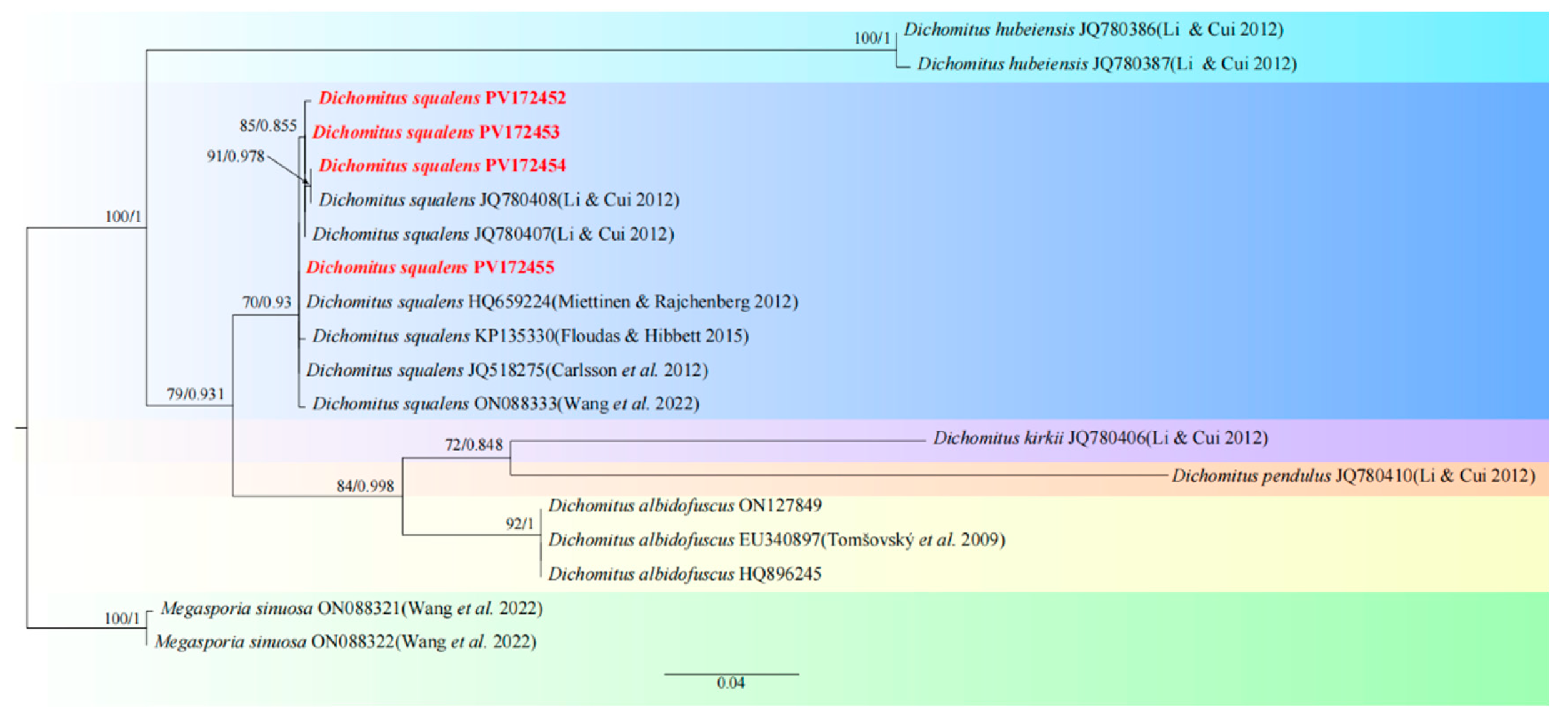


| Taxon | Collection | Country | GenBank No. | Reference |
|---|---|---|---|---|
| ITS | ||||
| Dichomitus albidofuscus | XSP-1 | China | ON127849 | |
| D. albidofuscus | MUAF843 | Czech Republic | EU340897 | Tomšovský et al. 2009 [27] |
| D. albidofuscus | FCL23 | Poland | HQ896245 | |
| D. hubeiensis | Wei2036 | China | JQ780386 | Li & Cui 2012 [28] |
| D. hubeiensis | Wei2045 | China | JQ780387 | Li & Cui 2012 [28] |
| D. kirkii | Yuan1237 | China | JQ780406 | Li & Cui 2012 [28] |
| D. pendulus | TL9948 | China | JQ780410 | Li & Cui 2012 [28] |
| D. squalens | FJAU66627 | China | PV172452 | This study |
| D. squalens | FJAU66628 | China | PV172453 | This study |
| D. squalens | FJAU66625 | China | PV172454 | This study |
| D. squalens | FJAU66626 | China | PV172455 | This study |
| D. squalens | Cui9639 | China | JQ780407 | Li & Cui 2012 [28] |
| D. squalens | Cui9725 | China | JQ780408 | Li & Cui 2012 [28] |
| D. squalens | s.n. (H) | Finland | HQ659224 | Miettinen & Rajchenberg 2012 [29] |
| D. squalens | LY-AD-421-SS1 | USA | KP135330 | Floudas & Hibbett 2015 [30] |
| D. squalens | Sweden | JQ518275 | Carlsson et al. 2012 [31] | |
| D. squalens | Dai15352 | China | ON088333 | Wang et al. 2022 [32] |
| Megasporia sinuosa | Dai22011 | China | ON088321 | Wang et al. 2022 [32] |
| M. sinuosa | Dai22210 | China | ON088322 | Wang et al. 2022 [32] |
| Text No. | Factor | Colony Characteristics | Mycelial Growth Rate (mm/d) | Significance | |
|---|---|---|---|---|---|
| 0.05 | 0.01 | ||||
| C1 | Glucose | Translucency relatively sparse | 11.2 ± 0.61 | ab | AB |
| C2 | Sucrose | White relatively sparse | 11.72 ± 2.16 | a | A |
| C3 | Soluble starch | White relatively sparse | 10.5 ± 1.70 | b | B |
| C4 | Dextrin | White relatively sparse | 10.22 ± 0.66 | bc | BC |
| C5 | Maltose | White relatively sparse | 10.48 ± 0.67 | b | B |
| C-CK | CK | Translucency relatively sparse | 9.30 ± 0.62 | c | C |
| N1 | Peptone | White dense | 8.59 ± 0.56 | c | C |
| N2 | Yeast extract powder | White dense | 10.99 ± 0.36 | a | A |
| N3 | NH4Cl | White dense | 9.63 ± 0.74 | b | B |
| N4 | Diammonium phosphate | White dense | 5.02 ± 0.64 | d | D |
| N5 | Carbamide | Not have | 0 | e | E |
| N-CK | CK | Transparent sparse | 8.99 ± 0.52 | c | C |
| P1 | pH 5 | White dense | 12.52 ± 0.92 | a | A |
| P2 | pH 6 | White dense | 11.20 ± 0.52 | b | B |
| P3 | pH 7 | White dense | 9.50 ± 0.85 | c | C |
| P4 | pH 8 | White dense | 9.34 ± 0.37 | c | C |
| P5 | pH 9 | White dense | 7.77 ± 0.24 | d | D |
| P-CK | Natural conditions | White dense | 9.47 ± 0.48 | c | C |
| T1 | 15 °C | White dense | 2.44 ± 0.28 | d | D |
| T2 | 20 °C | White dense | 4.43 ± 0.33 | c | C |
| T3 | 25 °C | White dense | 9.96 ± 0.27 | b | B |
| T4 | 30 °C | White dense | 16.03 ± 0.59 | a | A |
| T5 | 35 °C | White dense | 15.59 ± 0.95 | a | A |
| Text No. | Carbon Source | Nitrogen Source | Potential of Hydrogen | Temperature | Mycelial Growth Rate (mm/d) | Mycelial Characteristics |
|---|---|---|---|---|---|---|
| 1 | 1. Glucose | 1. Peptone | 1. pH5 | 1. 35 °C | 15.40 ± 0.79 | White thicker |
| 2 | 1. Glucose | 2. Yeas extract powder | 3. pH7 | 2. 25 °C | 6.09 ± 0.29 | White dense |
| 3 | 1. Glucose | 3. NH4Cl | 2. pH6 | 3. 30 °C | 13.17 ± 0.49 | White thicker |
| 4 | 2. Sucrose | 1. Peptone | 3. pH7 | 3. 30 °C | 7.96 ± 0.54 | White thicker |
| 5 | 2. Sucrose | 2. Yeast extract powder | 2. pH6 | 1. 35 °C | 16.50 ± 0.42 | White dense |
| 6 | 2. Sucrose | 3. NH4Cl | 1. pH5 | 2. 25 °C | 16.27 ± 1.23 | White thicker |
| 7 | 3. Dextrin | 1. Peptone | 2. pH6 | 2. 25 °C | 13.27 ± 0.64 | White thicker |
| 8 | 3. Dextrin | 2. Yeast extract powder | 1. pH5 | 3. 30 °C | 17.83 ± 0.11 | White dense |
| 9 | 3. Dextrin | 3. NH4Cl | 3. pH7 | 1. 35 °C | 3.94 ± 0.85 | Translucency sparse |
| K1 | 173.27 | 183.17 | 247.52 | 179.17 | ||
| K2 | 203.68 | 202.1 | 214.76 | 178.18 | ||
| K3 | 175.25 | 166.94 | 89.92 | 194.85 | ||
| K avg 1 | 11.55 | 12.21 | 16.5 | 11.94 | ||
| K avg 2 | 13.58 | 13.47 | 14.32 | 11.88 | ||
| K avg 3 | 11.68 | 11.13 | 5.99 | 12.99 | ||
| R | 2.03 | 2.34 | 10.51 | 1.11 |
| Source | Sum of Squares | Df | Mean Square | F | Significance |
|---|---|---|---|---|---|
| Carbon source | 38.612 | 2 | 19.306 | 42.64 | <0.001 |
| Nitrogen source | 41.296 | 2 | 20.648 | 45.604 | <0.001 |
| Pondus Hydrogenii | 922.163 | 2 | 461.082 | 1018.377 | <0.001 |
| Temperature | 11.662 | 2 | 5.831 | 12.878 | <0.001 |
| Error | 16.299 | 36 | 0.453 | ||
| Total | 7806.219 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-B.; Qi, Z.-X.; Zhang, T.; Qian, K.-Q.; Lv, H.-Y.; Zhang, B.; Li, Y. Biological Characteristics and Domestication of Dichomitus squalens and the Antioxidant Activity of Its Cultivated Fruiting Bodies. J. Fungi 2025, 11, 594. https://doi.org/10.3390/jof11080594
Wang L-B, Qi Z-X, Zhang T, Qian K-Q, Lv H-Y, Zhang B, Li Y. Biological Characteristics and Domestication of Dichomitus squalens and the Antioxidant Activity of Its Cultivated Fruiting Bodies. Journal of Fungi. 2025; 11(8):594. https://doi.org/10.3390/jof11080594
Chicago/Turabian StyleWang, Li-Bo, Zheng-Xiang Qi, Tao Zhang, Ke-Qing Qian, Hai-Yan Lv, Bo Zhang, and Yu Li. 2025. "Biological Characteristics and Domestication of Dichomitus squalens and the Antioxidant Activity of Its Cultivated Fruiting Bodies" Journal of Fungi 11, no. 8: 594. https://doi.org/10.3390/jof11080594
APA StyleWang, L.-B., Qi, Z.-X., Zhang, T., Qian, K.-Q., Lv, H.-Y., Zhang, B., & Li, Y. (2025). Biological Characteristics and Domestication of Dichomitus squalens and the Antioxidant Activity of Its Cultivated Fruiting Bodies. Journal of Fungi, 11(8), 594. https://doi.org/10.3390/jof11080594






