Ultrastructural and Immunohistochemical Study of Double and Combined Intravitreal Administration of Antifungal Agents in the Retina of New Zealand Albino Rabbits: An Experimental Protocol
Abstract
1. Introduction
1.1. Exogenous Endophthalmitis
1.2. Endogenous Endophthalmitis
1.3. Etiology and Epidemiology
1.4. Therapeutic Approaches
1.4.1. Amphotericin B
1.4.2. Natamycin
1.4.3. Voriconazole
1.4.4. Micafungin
1.5. Immunohistochemical Markers
1.5.1. Interleukin 6—IL-6
1.5.2. Tumor Necrosis Factor-Alpha—TNF-a
1.6. Aim of Study
2. Methodology
2.1. Materials
2.2. Replacement, Reduction, Refinement (3Rs) Condition
2.3. Randomization
2.4. Medication and Dosages of Administration
2.5. Housing and Care of Animals
2.6. Description of Equipment
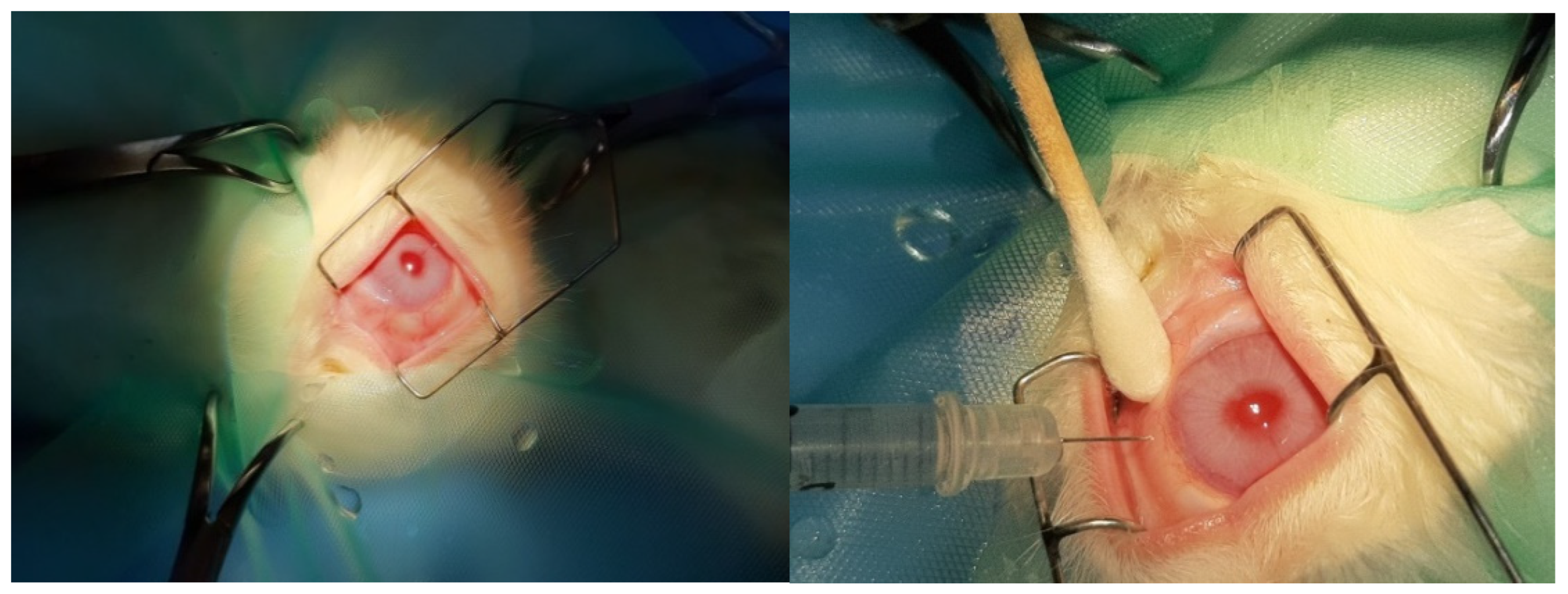
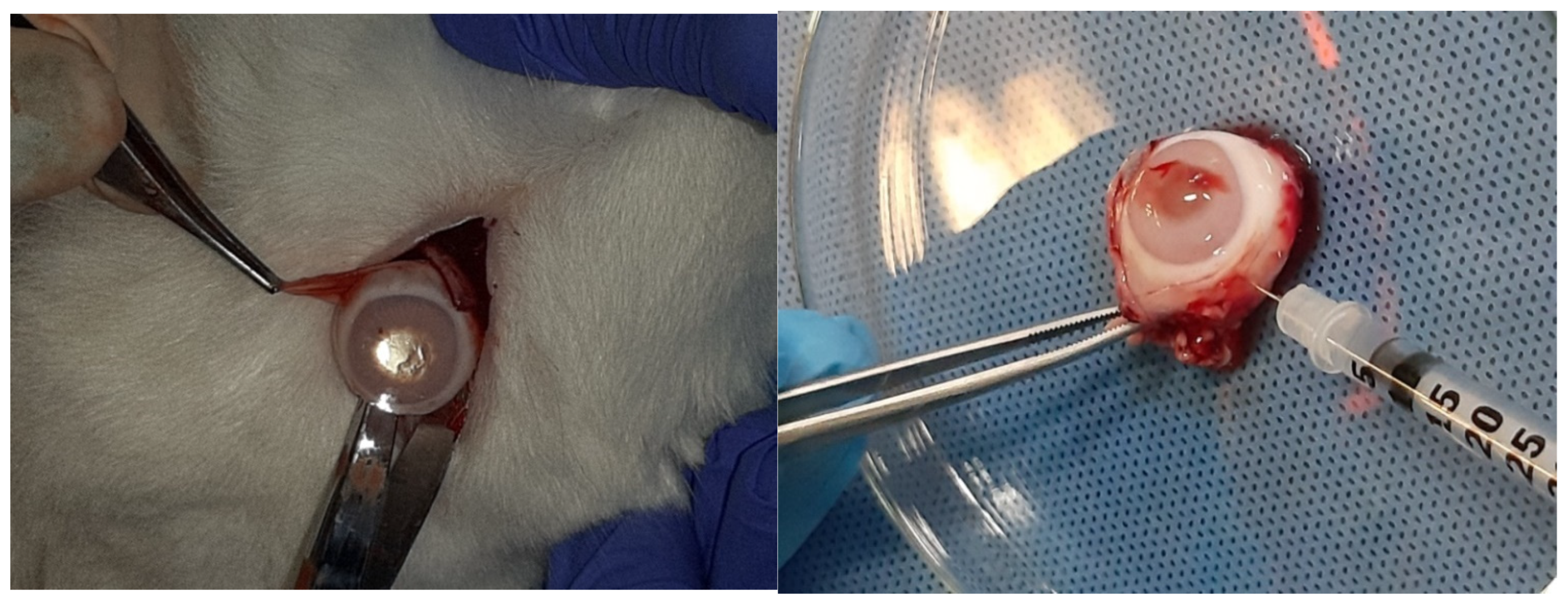
2.7. Description of the Procedure
2.8. Preparation for Observation Under the Optical Microscope
2.9. Double Blind Assessment
2.10. Preparation for Observation Under the Electron Microscope
2.11. Ethics Committee Approval
3. Results
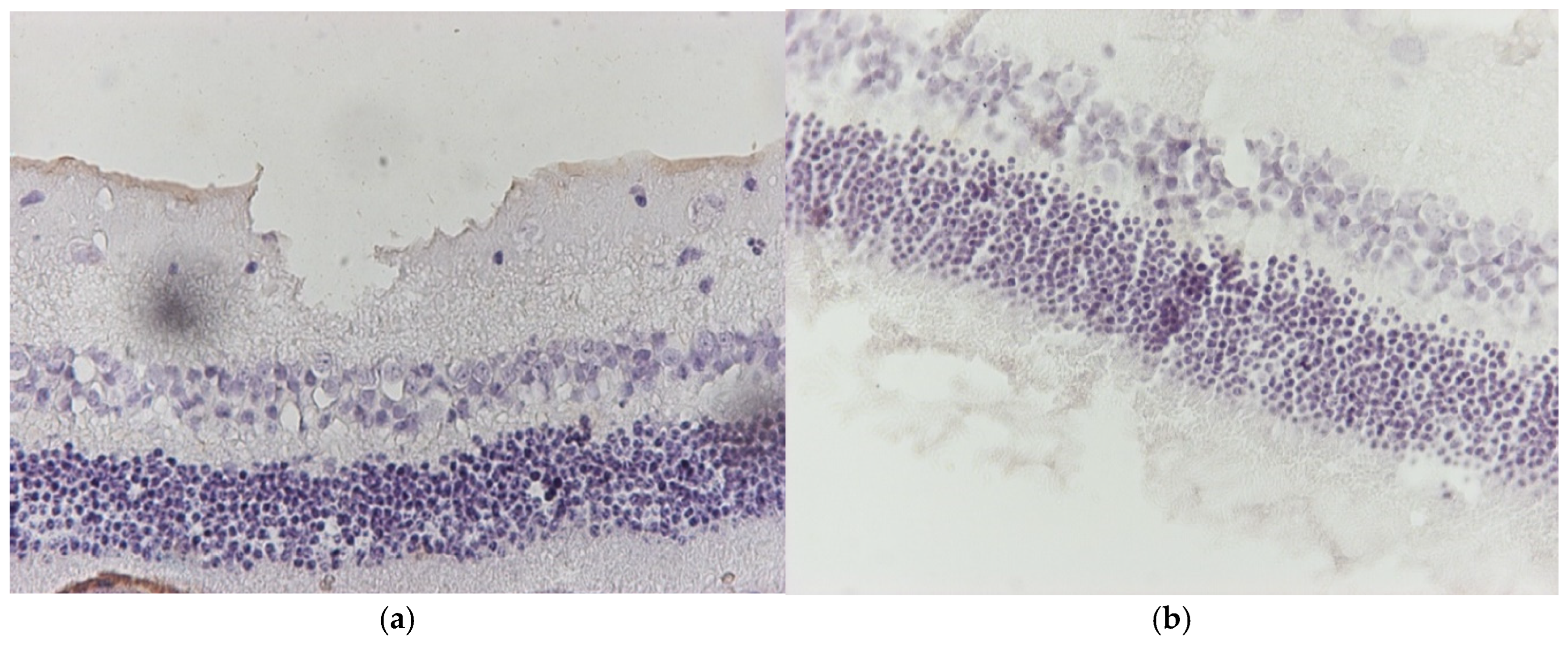
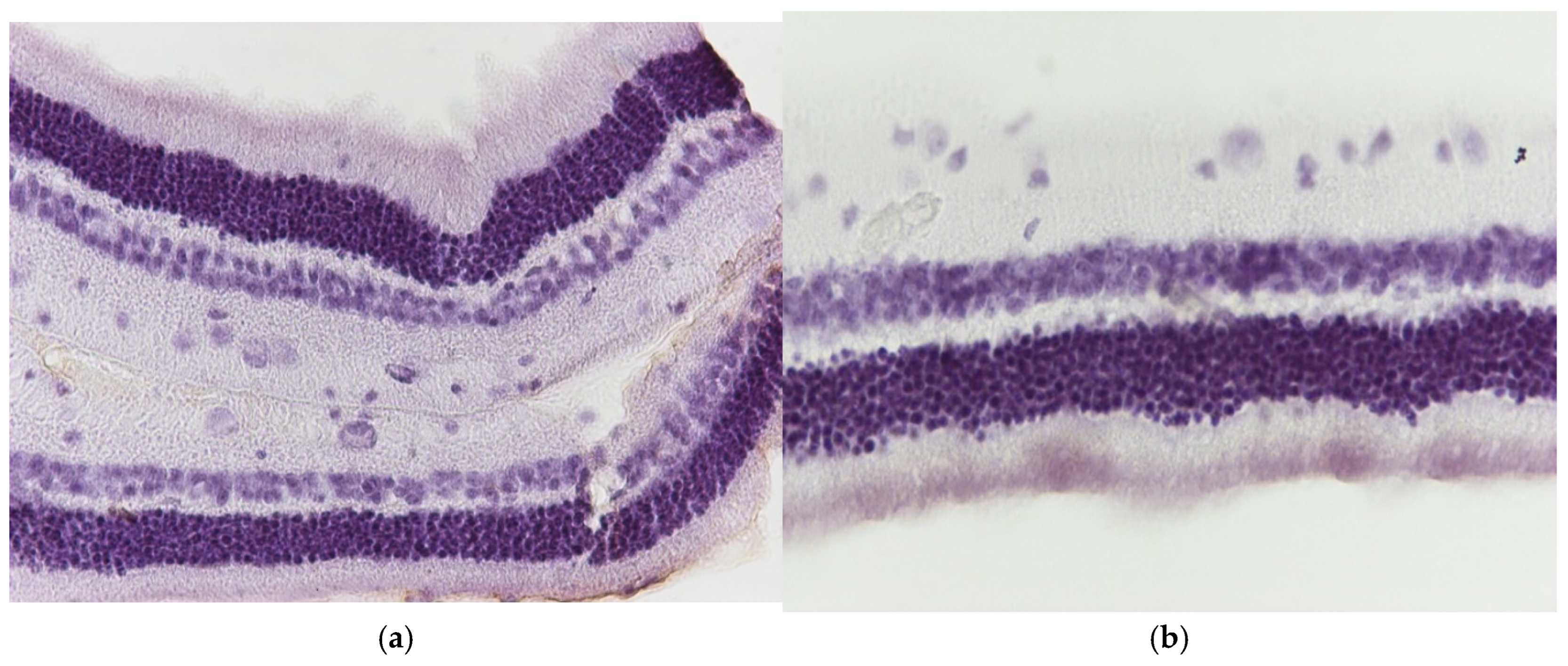
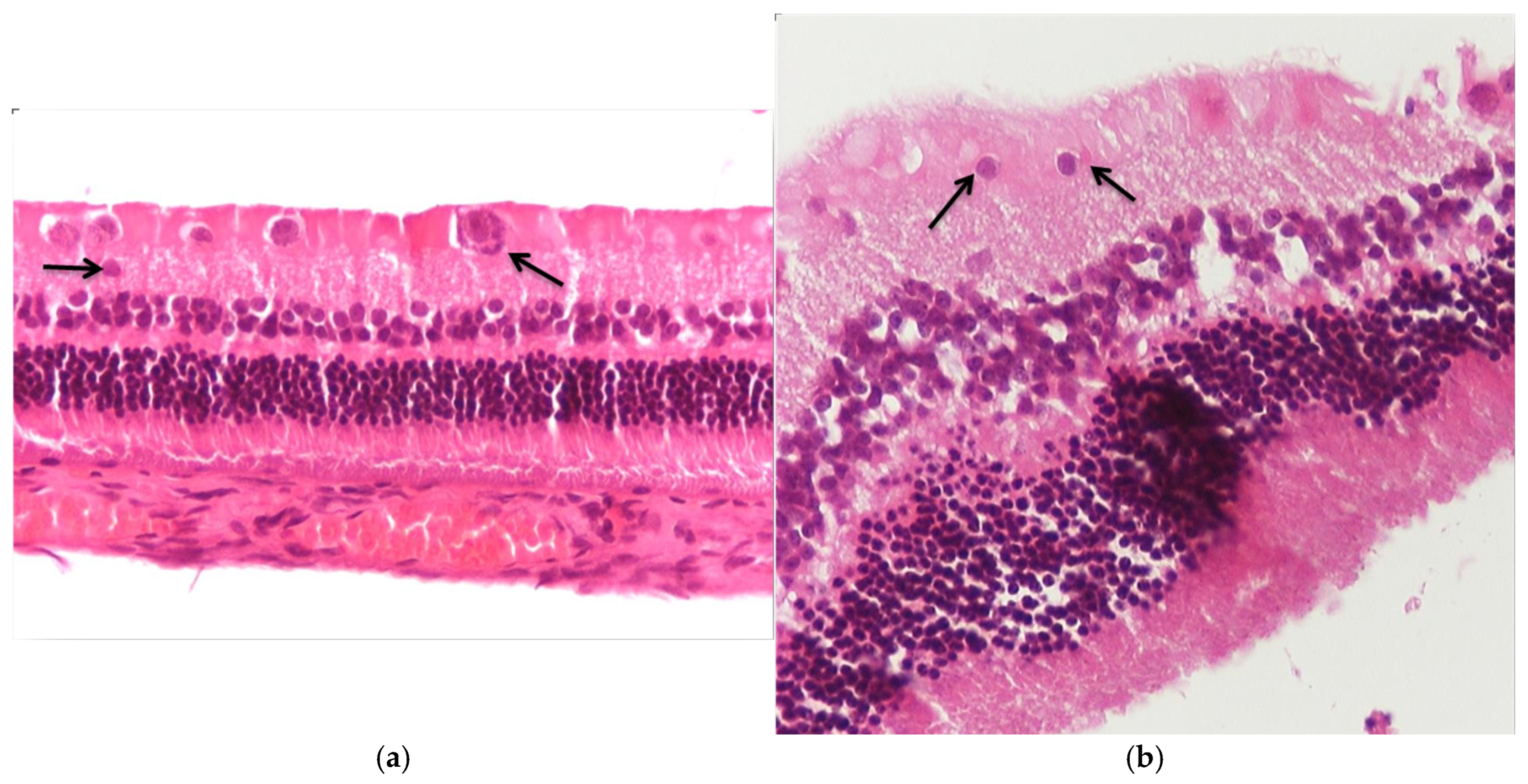
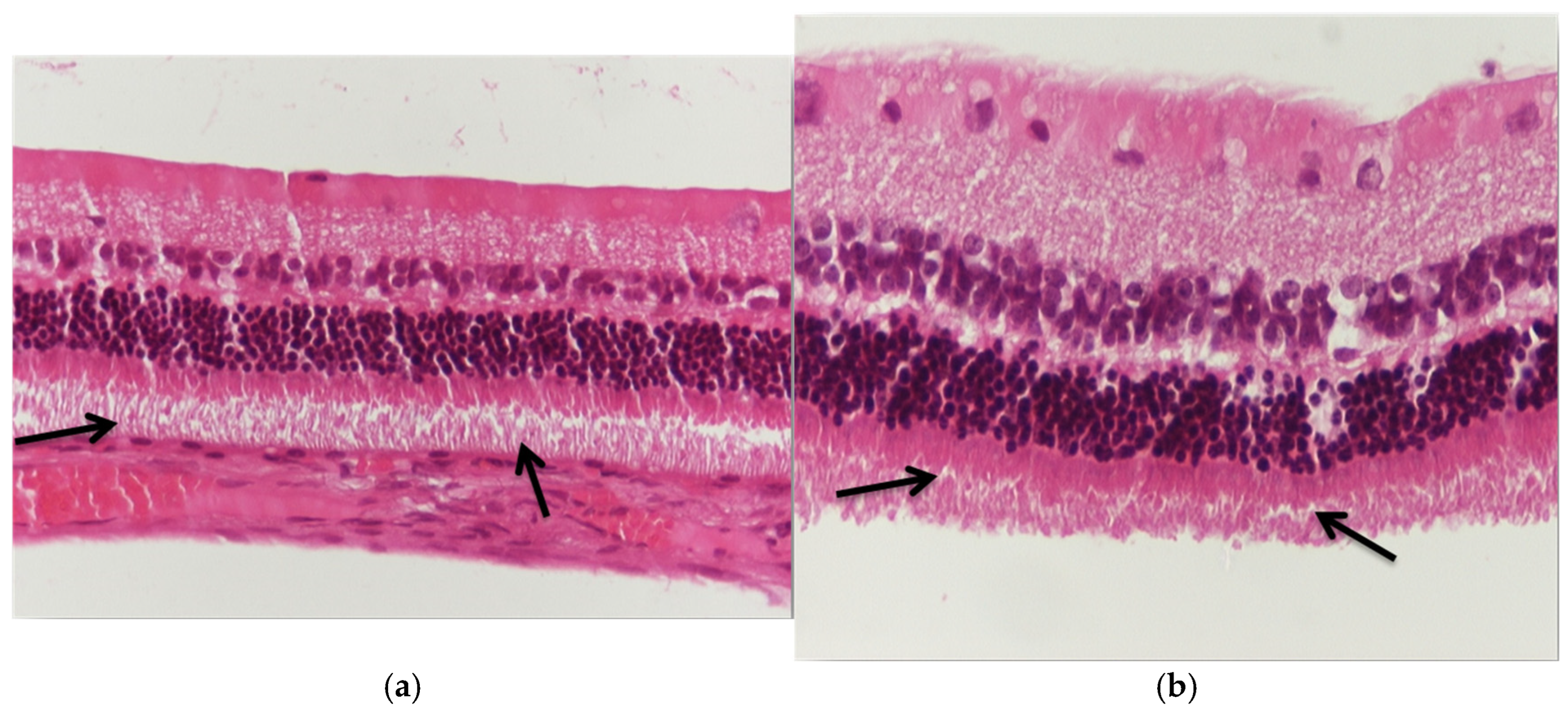
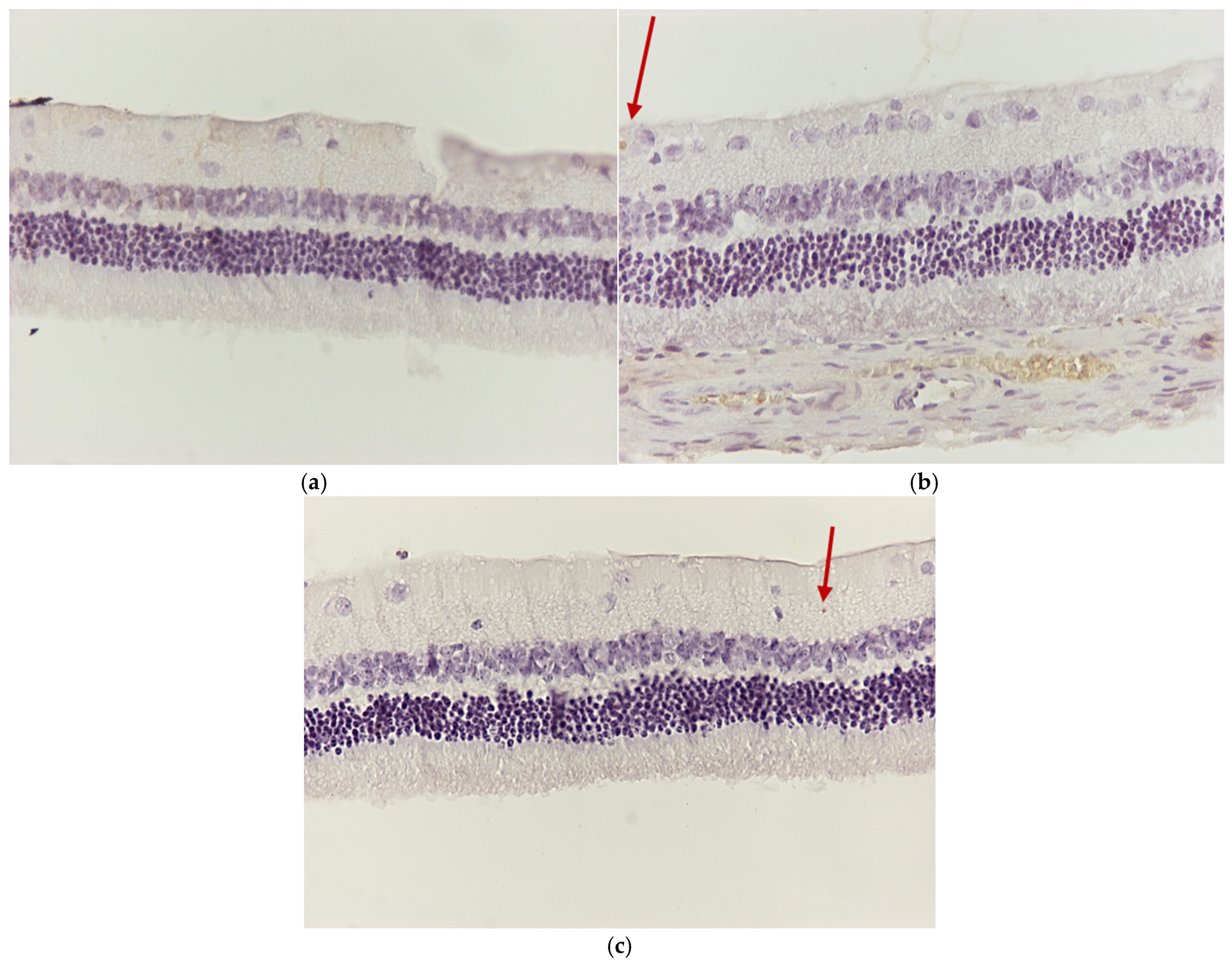
3.1. Results of Optical Microscope—Eosin–Hematoxylin Staining and Immunohistochemical Staining for IL-6 and TNF-a Markers
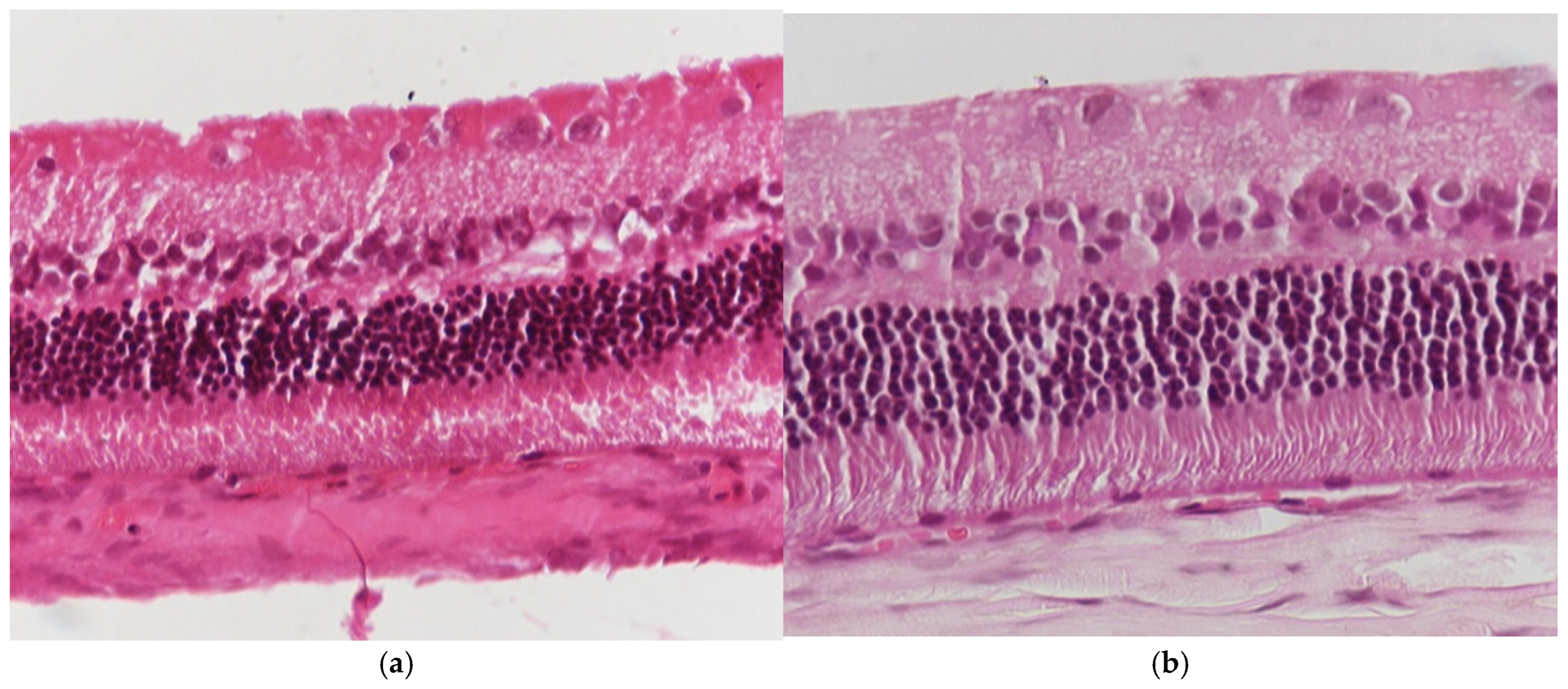
3.1.1. Group C2
3.1.2. Group V2
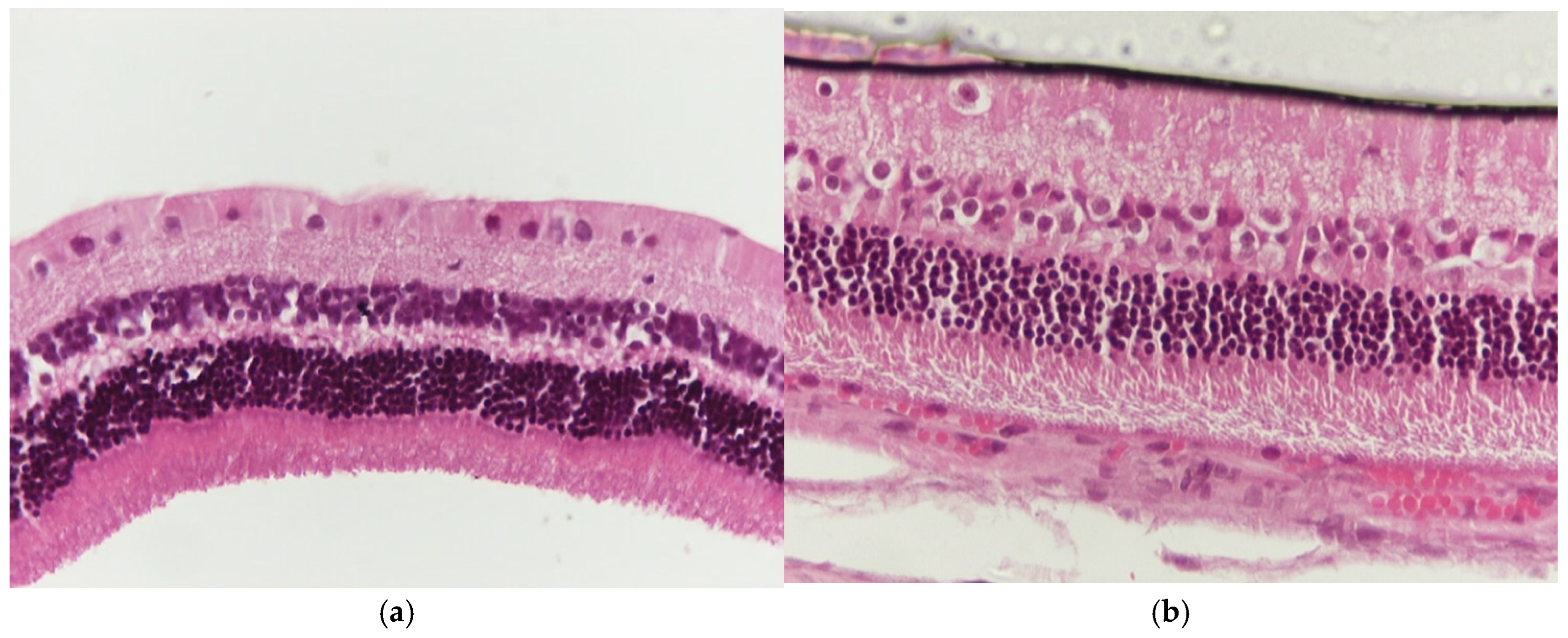
3.1.3. Group M2
3.1.4. Group VM
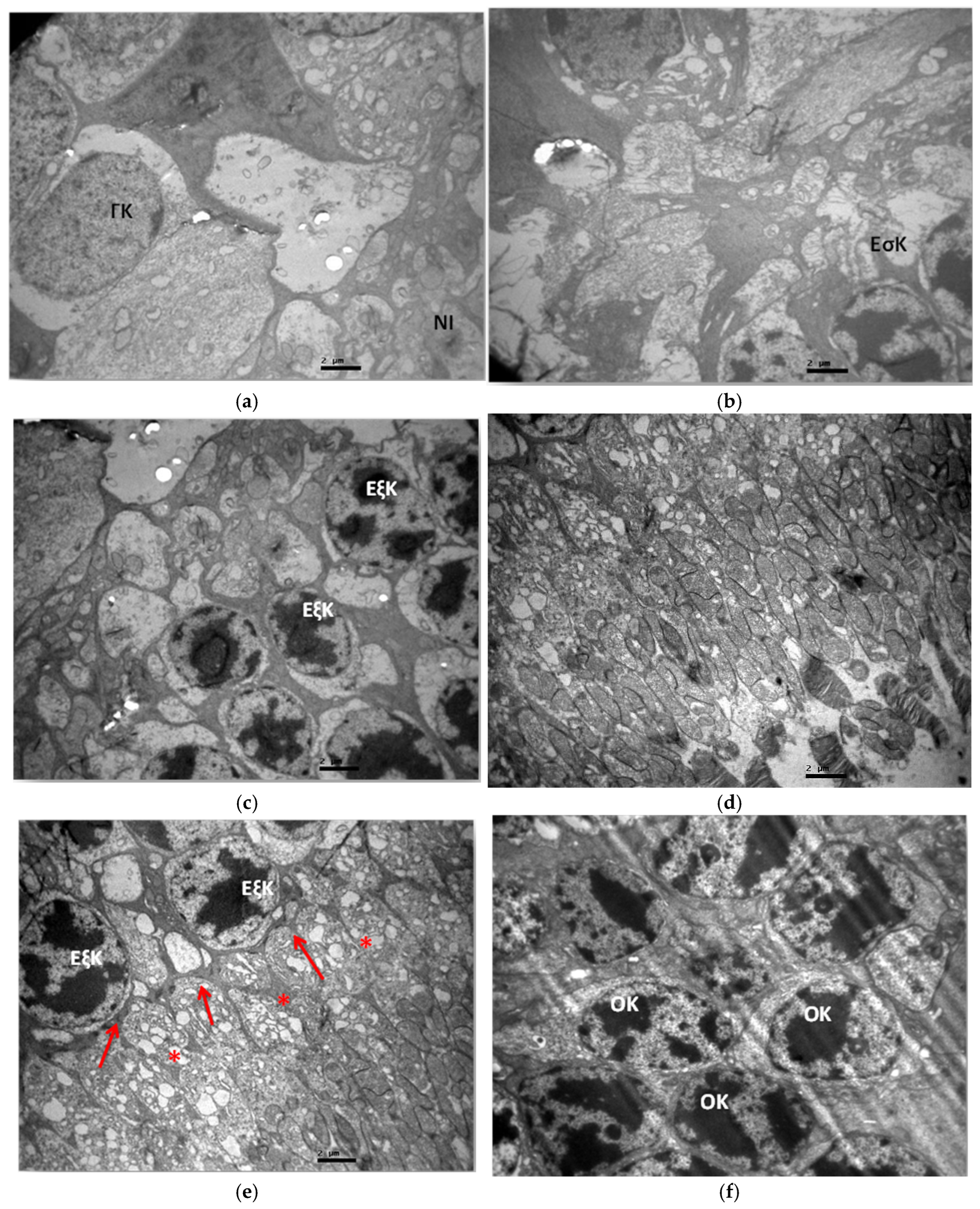
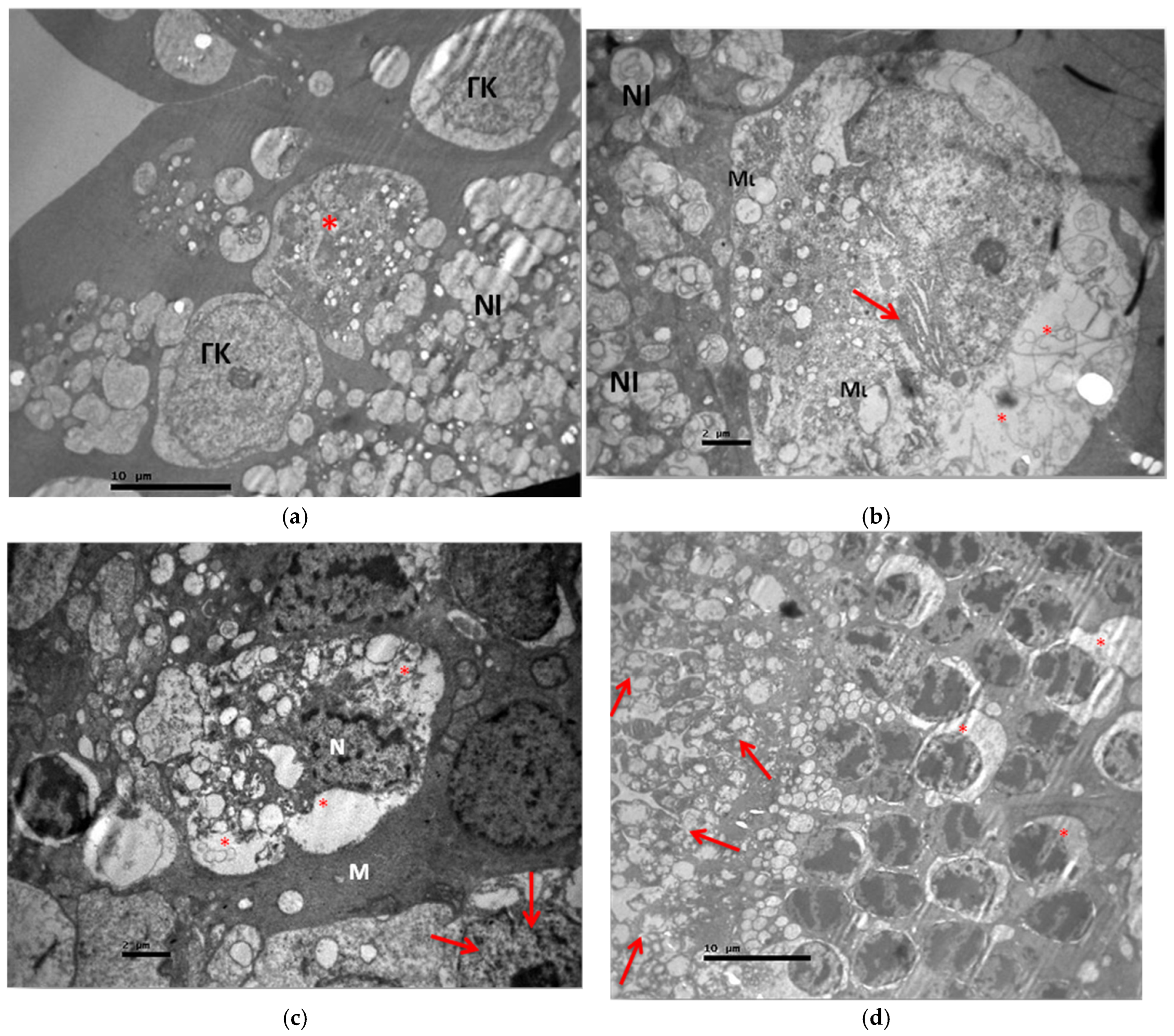
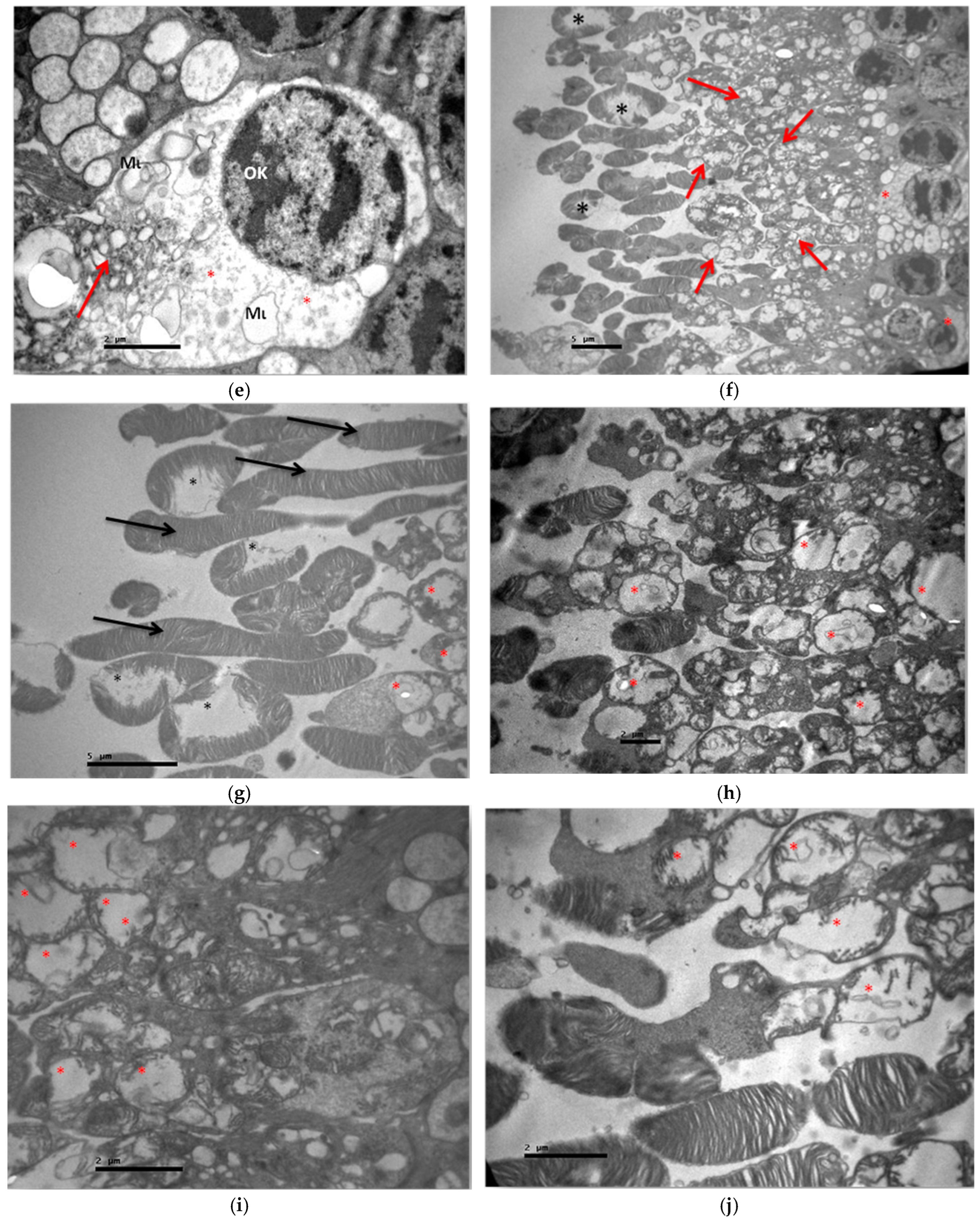
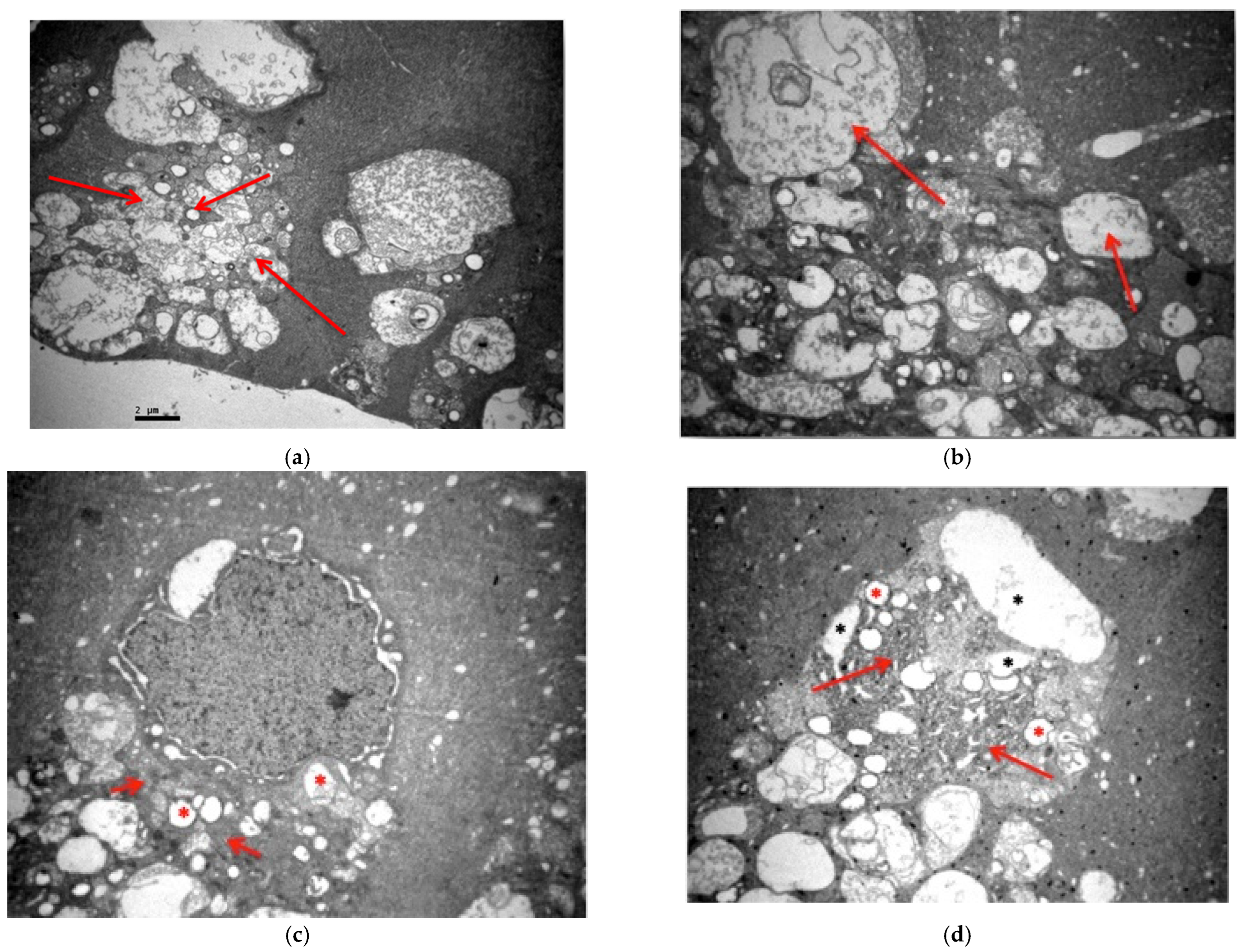
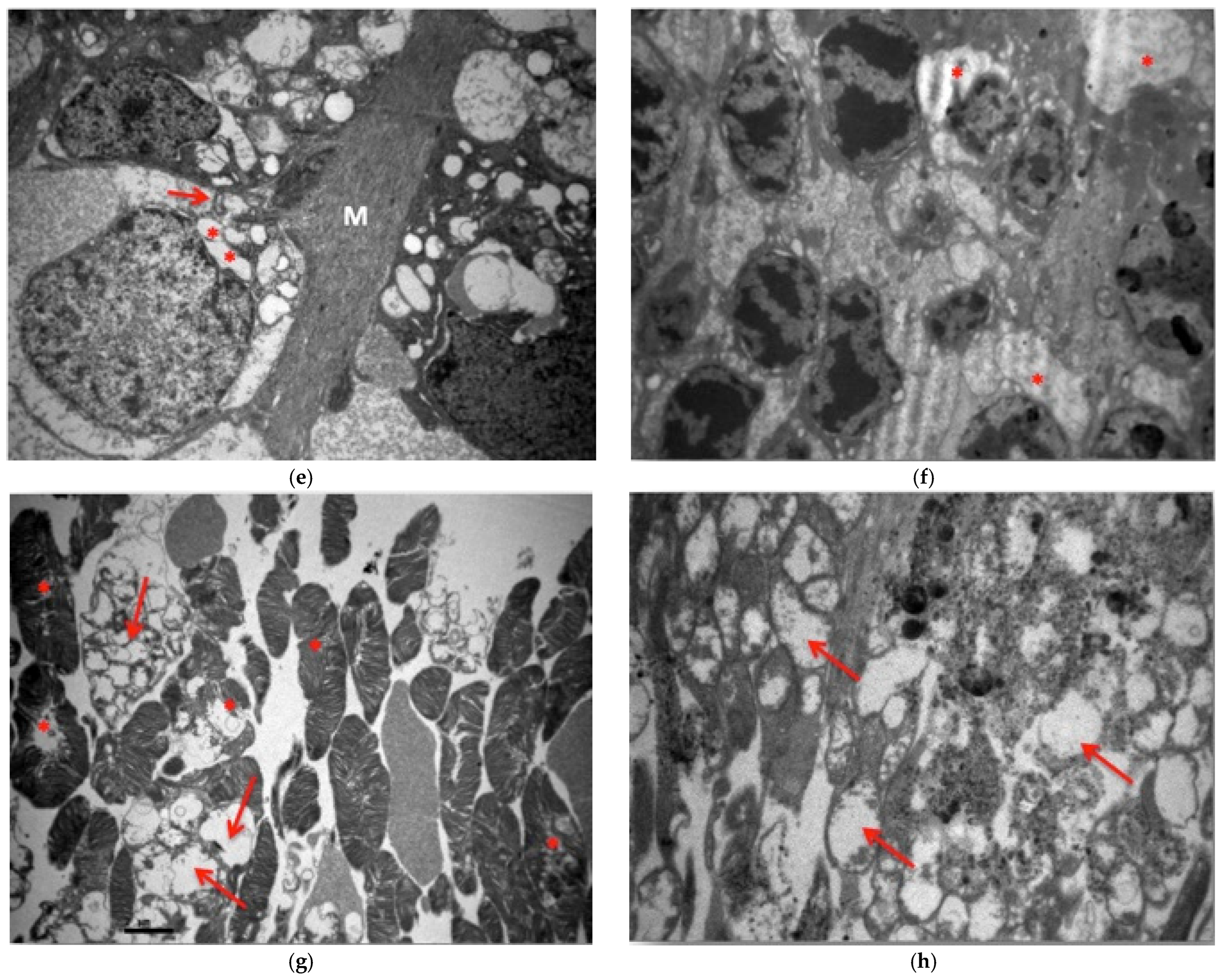
3.2. Electron Microscopy
3.2.1. Group C2
3.2.2. Group V2
3.2.3. Group M2
3.2.4. Group VM
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endophthalmitis—EyeWiki. Available online: https://eyewiki.org/Endophthalmitis (accessed on 27 October 2024).
- Ly, V.; Sallam, A. Fungal Endophthalmitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559257/ (accessed on 27 October 2024).
- Na, S.K.; Park, K.J.; Kim, H.J.; Lee, S.C. Hematogenous endophthalmitis in a patient with candidemia. Korean J. Intern. Med. 1997, 12, 242–244. [Google Scholar] [CrossRef]
- Fungal Eye Infections Basics|Fungal Eye Infections|CDC. Available online: https://www.cdc.gov/fungal-eye-infections/about/index.html?CDC_AAref_Val=https://www.cdc.gov/fungal/diseases/fungal-eye-infections/statistics.html (accessed on 27 October 2024).
- Sheu, S.-J. Endophthalmitis. Korean J. Ophthalmol. 2017, 31, 283. [Google Scholar] [CrossRef]
- Schwartz, S.G.; Flynn, H.W.; Davis, J.L. Exogenous Endophthalmitis. In Intraocular Inflammation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1437–1446. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Endogenous Endophthalmitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576391/ (accessed on 27 October 2024).
- Danielescu, C.; Anton, N.; Stanca, H.T.; Munteanu, M. Endogenous Endophthalmitis: A Review of Case Series Published between 2011 and 2020. J. Ophthalmol. 2020, 2020, 8869590. [Google Scholar] [CrossRef]
- Endogenous Endophthalmitis: Diagnosis and Treatment—American Academy of Ophthalmology. Available online: https://www.aao.org/eyenet/article/endogenous-endophthalmitis-diagnosis-treatment (accessed on 27 October 2024).
- Silva-Costa, C.; Melo-Cristino, J.; Ramirez, M. Streptococcus pneumoniae. In Molecular Medical Microbiology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 1479–1490. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus Infection. In Encyclopedia of Food Safety, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1–4, pp. V2-310–V2-318. [Google Scholar] [CrossRef]
- Ashurst, J.V.; Dawson, A. Klebsiella Pneumonia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519004/ (accessed on 27 October 2024).
- Schuster, J.E.; Fisher, B.T. Candidiasis. In Pediatric Transplant and Oncology Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 195–205.e3. [Google Scholar] [CrossRef]
- Das, T.; Agarwal, M.; Anand, A.R.; Behera, U.C.; Bhende, M.; Das, A.V.; Dasgupta, D.; Dave, V.P.; Gandhi, J.; Gunasekaran, R.; et al. Fungal Endophthalmitis: Analysis of 730 Consecutive Eyes from 7 Tertiary Eye Care Centers in India. Ophthalmol. Retin. 2022, 6, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Cheng, J.; Zhang, J.; Chen, N.; Gao, Y.; Xie, L.X. Risk Factors, Treatment Strategies, and Outcomes of Endophthalmitis Associated with Severe Fungal Keratitis. Retina 2019, 39, 1076–1082. [Google Scholar] [CrossRef]
- Patil, A.; Majumdar, S. Echinocandins in Ocular Therapeutics. J. Ocul. Pharmacol. Ther. 2017, 33, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.G.; Kara-José, N.; De Castro, R.S. Antifungals in eye infections: Drugs and routes of administration. Rev. Bras. Oftalmol. 2013, 72, 132–141. [Google Scholar] [CrossRef]
- Karachrysafi, S.; Sioga, A.; Komnenou, A.; Karamitsos, A.; Xioteli, M.; Dori, I.; Delis, G.; Kofidou, E.; Anastasiadou, P.; Sotiriou, S.; et al. Histological Effects of Intravitreal Injection of Antifungal Agents in New Zealand White Rabbits: An Electron Microscopic and Immunohistochemical Study. Pharmaceuticals 2020, 13, 267. [Google Scholar] [CrossRef]
- Danielescu, C.; Stanca, H.T.; Iorga, R.E.; Darabus, D.M.; Potop, V. The Diagnosis and Treatment of Fungal Endophthalmitis: An Update. Diagnostics 2022, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- IV, J.R.; McNeil, S.A.; Johnson, T.M.; Bradley, S.F.; Kazanjian, P.H.; Kauffman, C.A. Endogenous Aspergillus endophthalmitis: Report of 3 cases and review of the literature. Medicine 2002, 81, 311–320. [Google Scholar] [CrossRef]
- Grzybowski, A.; Turczynowska, M.; Schwartz, S.G.; Relhan, N.; Flynn, H.W. The Role of Systemic Antimicrobials in the Treatment of Endophthalmitis: A Review and an International Perspective. Ophthalmol. Ther. 2020, 9, 485. [Google Scholar] [CrossRef]
- Ito, J.; Hooshmand-Rad, R. Treatment of Candida Infections with Amphotericin B Lipid Complex. Clin. Infect. Dis. 2005, 40, S384–S391. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.; Genesoni, G. Treatment of severe fungal keratitis with subconjunctival amphotericin B. Cornea 2010, 30, 608–611. [Google Scholar] [CrossRef]
- Welscher, Y.M.T.; Napel, H.H.T.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Biju, R.; Sushil, D.; Georgy, N. Successful management of presumed Candida endogenous endophthalmitis with oral voriconazole. Indian J. Ophthalmol. 2009, 57, 306. [Google Scholar] [CrossRef]
- Varma, D.; Thaker, H.R.; Moss, P.J.; Wedgwood, K.; Innes, J.R. Use of voriconazole in Candida retinitis. Eye 2005, 19, 485–487. [Google Scholar] [CrossRef]
- Kong, D.; Al-Badriyeh, D.; Neoh, C.F.; Stewart, K. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin. Ophthalmol. 2010, 4, 391. [Google Scholar] [CrossRef]
- Klont, R.R.; Eggink, C.A.; Rijs, A.J.M.M.; Wesseling, P.; Verweij, P.E. Successful treatment of Fusarium keratitis with cornea transplantation and topical and systemic voriconazole. Clin. Infect. Dis. 2005, 40, e110–e112. [Google Scholar] [CrossRef] [PubMed]
- Cristiane, V.; Amaral, S.; Pereira, G.; Junior, N. Ketoconazole- and fluconazole-induced embryotoxicity and skeletal anomalies in wistar rats: A comparative study. Braz. Arch. Biol. Technol. 2008, 51, 1153–1161. [Google Scholar] [CrossRef]
- Bennett: Agentes Antimicrobianos, Agentes Antifúngicos—Μελετητής Google. Available online: https://scholar.google.com/scholar_lookup?title=Agentes+antimicrobianos,+agentes+antif%C3%BAngicos&author=Bennett,+J.&publication_year=2007&pages=1103%E2%80%931117 (accessed on 27 October 2024).
- Mycamine|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/mycamine (accessed on 27 October 2024).
- Pfaller, M.A.; Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N. Comparison of EUCAST and CLSI broth microdilution methods for the susceptibility testing of 10 systemically active antifungal agents when tested against Candida spp. Diagn. Microbiol. Infect. Dis. 2014, 79, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.C.; Mavrogiorgos, N.; Tillem, E.; Hector, R.; Walsh, T.J. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2001, 45, 3310–3321. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.A.; Slavin, M.A.; Sorrell, T.C. Echinocandin antifungal drugs in fungal infections: A comparison. Drugs 2011, 71, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Paris, G.R.; Trujillo, F.; Woodward, L.; Trigo, Y.; Ballentine, C.S.; Najvar, L.K.; Glickman, R.D.; Harrison, J.M.; Graybill, J.; Sponsel, W.E. Micafungin vs Amphotericin B in the Treatment of Experimental Aspergillosis Endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4014. [Google Scholar]
- Karagoz, E.; Ugan, R.A.; Duzgun, E.; Cadirci, E.; Keles, S.; Uyanik, M.H.; Yavan, I.; Turhan, V. A Comparative Study of the Effects of Intravitreal Anidulafungin, Voriconazole, and Amphotericin B in an Experimental Candida Endophthalmitis Model. Curr. Eye Res. 2017, 42, 225–232. [Google Scholar] [CrossRef]
- Kapur, R.; Kim, B.; Tu, E.Y.; Birnbaum, A.; Fiscella, R.; Navare, S.; Blair, M.P.; Edward, D.P.; Carroll, J.; Lim, J.I. The Safe and Non-Toxic Dose of Intravitreal Micafungin and Caspofungin in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3324. [Google Scholar]
- Pascual-Camps, I.; Hernández-Martínez, P.; Monje-Fernández, L.; Dolz-Marco, R.; Gallego-Pinazo, R.; Wu, L.; Arévalo, J.F.; Díaz-Llopis, M. Update on intravitreal anti-tumor necrosis factor alpha therapies for ocular disorders. J. Ophthalmic Inflamm. Infect. 2014, 4, 26. [Google Scholar] [CrossRef]
- Theodossiadis, P.G.; Markomichelakis, N.N.; Sfikakis, P.P. Tumor necrosis factor antagonists: Preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007, 27, 399–413. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF and the TNF Receptor Superfamily: Structure-Function Relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.I.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Dutz: Pathomechanisms of Cutaneous Lupus Erythematosus—Μελετητής Google. Available online: https://scholar.google.com/scholar_lookup?title=Pathomechanisms+of+Cutaneous+Lupus+Erythematosus&author=Wallace,+W.&author=Hahn,+B.&publication_year=2013&pages=310%E2%80%93318 (accessed on 27 October 2024).
- El Ayadi, A.; Herndon, D.N.; Finnerty, C.C. Biomarkers in burn patient care. In Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://www.sciencedirect.com/science/article/pii/B9780323476614000216 (accessed on 27 October 2024).
- Ravussin, E.; Smith, S.R. Role of the Adipocyte in Metabolism and Endocrine Function. In Endocrinology Adult and Pediatric: Diabetes Mellitus and Obesity E-Book; Saunders: Philadelphia, PA, USA, 2013; Available online: https://books.google.com/books?hl=el&lr=&id=c-yLXZJ3uYcC&oi=fnd&pg=SL5-PA157&ots=oXSYwoGv-e&sig=xl8HilngJ_ka-_CSPVG98Sznr-Q (accessed on 27 October 2024).
- Yao, M.; Brummer, G.; Acevedo, D.; Cheng, N. Cytokine Regulation of Metastasis and Tumorigenicity. Adv. Cancer Res. 2016, 132, 265–367. [Google Scholar] [CrossRef]
- Kinne, R.W.; Bräuer, R.; Stuhlmüller, B.; Palombo-Kinne, E.; Burmester, G.R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000, 2, 189–202. [Google Scholar] [CrossRef][Green Version]
- Harrison, J.M.; Glickman, R.D.; Ballentine, C.S.; Trigo, Y.; Pena, M.A.; Kurian, P.; Najvar, L.K.; Kumar, N.; Patel, A.H.; Sponsel, W.E.; et al. Retinal function assessed by ERG before and after induction of ocular aspergillosis and treatment by the anti-fungal, micafungin, in rabbits. Doc. Ophthalmol. 2005, 110, 37–55. [Google Scholar] [CrossRef]
- Gao, H.; Pennesi, M.; Shah, K.; Qiao, X.; Hariprasad, S.M.; Mieler, W.F.; Wu, S.M.; Holz, E.R. Safety of intravitreal voriconazole: Electroretinographic and histopathologic studies. Trans. Am. Ophthalmol. Soc. 2003, 101, 183–189. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1358987/ (accessed on 27 October 2024).
- Animal Randomization Tool—ACME Research Solutions. Available online: https://acmeresearchlabs.in/animal-randomization-tool/ (accessed on 27 October 2024).
- Shen, Y.C.; Wang, M.-Y.; Wang, C.-Y.; Tsai, T.-C.; Tsai, H.-Y.; Lee, Y.-F.; Wei, L.-C. Clearance of intravitreal voriconazole. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Pennesi, M.E.; Shah, K.; Qiao, X.; Hariprasad, S.M.; Mieler, W.F.; Wu, S.M.; Holz, E.R. Intravitreal voriconazole: An electroretinographic and histopathologic study. Arch. Ophthalmol. 2004, 122, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Missel, P.J. Simulating intravitreal injections in anatomically accurate models for rabbit, monkey, and human eyes. Pharm. Res. 2012, 29, 3251–3272. [Google Scholar] [CrossRef]
- Hariprasad, S.M.; Mieler, W.F. Antibiotics. Dev. Ophthalmol. 2016, 55, 344–356. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Kuhn, D.M. Voriconazole—Better chances for patients with invasive mycoses. Eur. J. Med. Res. 2002, 7, 242–256. Available online: https://europepmc.org/article/med/12069915 (accessed on 27 October 2024).
- Sabo, J.A.; Abdel-Rahman, S.M. Voriconazole: A new triazole antifungal. Ann. Pharmacother. 2000, 34, 1032–1043. [Google Scholar] [CrossRef]
- Hariprasad, S.M.; Mieler, W.F.; Lin, T.K.; Sponsel, W.E.; Graybill, J.R. Voriconazole in the treatment of fungal eye infections: A review of current literature. Br. J. Ophthalmol. 2008, 92, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Riddell, J., 4th; Comer, G.M.; Kauffman, C.A. Treatment of endogenous fungal endophthalmitis: Focus on new antifungal agents. Clin. Infect. Dis. 2011, 52, 648–653. [Google Scholar] [CrossRef]
- Weishaar, P.D.; Flynn, H.W.; Murray, T.G.; Davis, J.L.; Barr, C.C.; Gross, J.G.; Mein, C.E.; McLean, W.C.; Killian, J.H. Endogenous Aspergillus endophthalmitis. Clinical features and treatment outcomes. Ophthalmology 1998, 105, 57–65. [Google Scholar] [CrossRef]
- Chee, Y.E.; Eliott, D. The Role of Vitrectomy in the Management of Fungal Endophthalmitis. Semin. Ophthalmol. 2017, 32, 29–35. [Google Scholar] [CrossRef]
- Lingappan, A.; Wykoff, C.C.; Albini, T.A.; Miller, D.; Pathengay, A.; Davis, J.L.; Flynn, H.W. Endogenous fungal endophthalmitis: Causative organisms, management strategies, and visual acuity outcomes. Am. J. Ophthalmol. 2012, 153, 162–166.e1. [Google Scholar] [CrossRef]
- Pascual, A.; Csajka, C.; Buclin, T.; Bolay, S.; Bille, J.; Calandra, T.; Marchetti, O. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: Population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 2012, 55, 381–390. [Google Scholar] [CrossRef]
- Dolton, M.J.; McLachlan, A.J. Voriconazole pharmacokinetics and exposure-response relationships: Assessing the links between exposure, efficacy and toxicity. Int. J. Antimicrob. Agents 2014, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.M.; Hariprasad, S.M.; Mieler, W.F.; Shah, G.K.; Mills, M.D.; Grand, M.G. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am. J. Ophthalmol. 2005, 139, 135–140. [Google Scholar] [CrossRef]
- Lin, R.C.; Sanduja, N.; Hariprasad, S.M. Successful treatment of postoperative fungal endophthalmitis using intravitreal and intracameral voriconazole. J. Ocul. Pharmacol. Ther. 2008, 24, 245–248. [Google Scholar] [CrossRef] [PubMed]





| GROUPS | V2 | M2 | VM | C |
| NUMBER ASSIGNED | 7, 4, 3 | 1, 9, 2 | 6, 5, 8 | 1, 2, 3, 4, 5, 6, 7, 8, 9 |
| GENDER | XX, XX, XX | XY, XY, XX | XY, XY, XX | XY, XX, XX, XX, XY, XY, XX, XX, XY |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karachrysafi, S.; Kourti, M.; Tsokkou, S.; Ioannou, D.; Kofidou, E.; Delis, G.; Sotiriou, S.; Karamitsos, A.; Xioteli, M.; Dori, I.; et al. Ultrastructural and Immunohistochemical Study of Double and Combined Intravitreal Administration of Antifungal Agents in the Retina of New Zealand Albino Rabbits: An Experimental Protocol. J. Fungi 2025, 11, 564. https://doi.org/10.3390/jof11080564
Karachrysafi S, Kourti M, Tsokkou S, Ioannou D, Kofidou E, Delis G, Sotiriou S, Karamitsos A, Xioteli M, Dori I, et al. Ultrastructural and Immunohistochemical Study of Double and Combined Intravitreal Administration of Antifungal Agents in the Retina of New Zealand Albino Rabbits: An Experimental Protocol. Journal of Fungi. 2025; 11(8):564. https://doi.org/10.3390/jof11080564
Chicago/Turabian StyleKarachrysafi, Sofia, Maria Kourti, Sophia Tsokkou, Despoina Ioannou, Evangelia Kofidou, Georgios Delis, Sotiris Sotiriou, Athanasios Karamitsos, Maria Xioteli, Ioanna Dori, and et al. 2025. "Ultrastructural and Immunohistochemical Study of Double and Combined Intravitreal Administration of Antifungal Agents in the Retina of New Zealand Albino Rabbits: An Experimental Protocol" Journal of Fungi 11, no. 8: 564. https://doi.org/10.3390/jof11080564
APA StyleKarachrysafi, S., Kourti, M., Tsokkou, S., Ioannou, D., Kofidou, E., Delis, G., Sotiriou, S., Karamitsos, A., Xioteli, M., Dori, I., Anastasiadou, P., Konstantinidis, I., Kavvadas, D., Chatzinikolaou, F., Komnenou, A., Karampatakis, V., Sioga, A., & Papamitsou, T. (2025). Ultrastructural and Immunohistochemical Study of Double and Combined Intravitreal Administration of Antifungal Agents in the Retina of New Zealand Albino Rabbits: An Experimental Protocol. Journal of Fungi, 11(8), 564. https://doi.org/10.3390/jof11080564











