Trends in Antifungal Resistance Among Candida Species: An Eight-Year Retrospective Study in the Galveston–Houston Gulf Coast Region
Abstract
1. Introduction
2. Materials and Methods
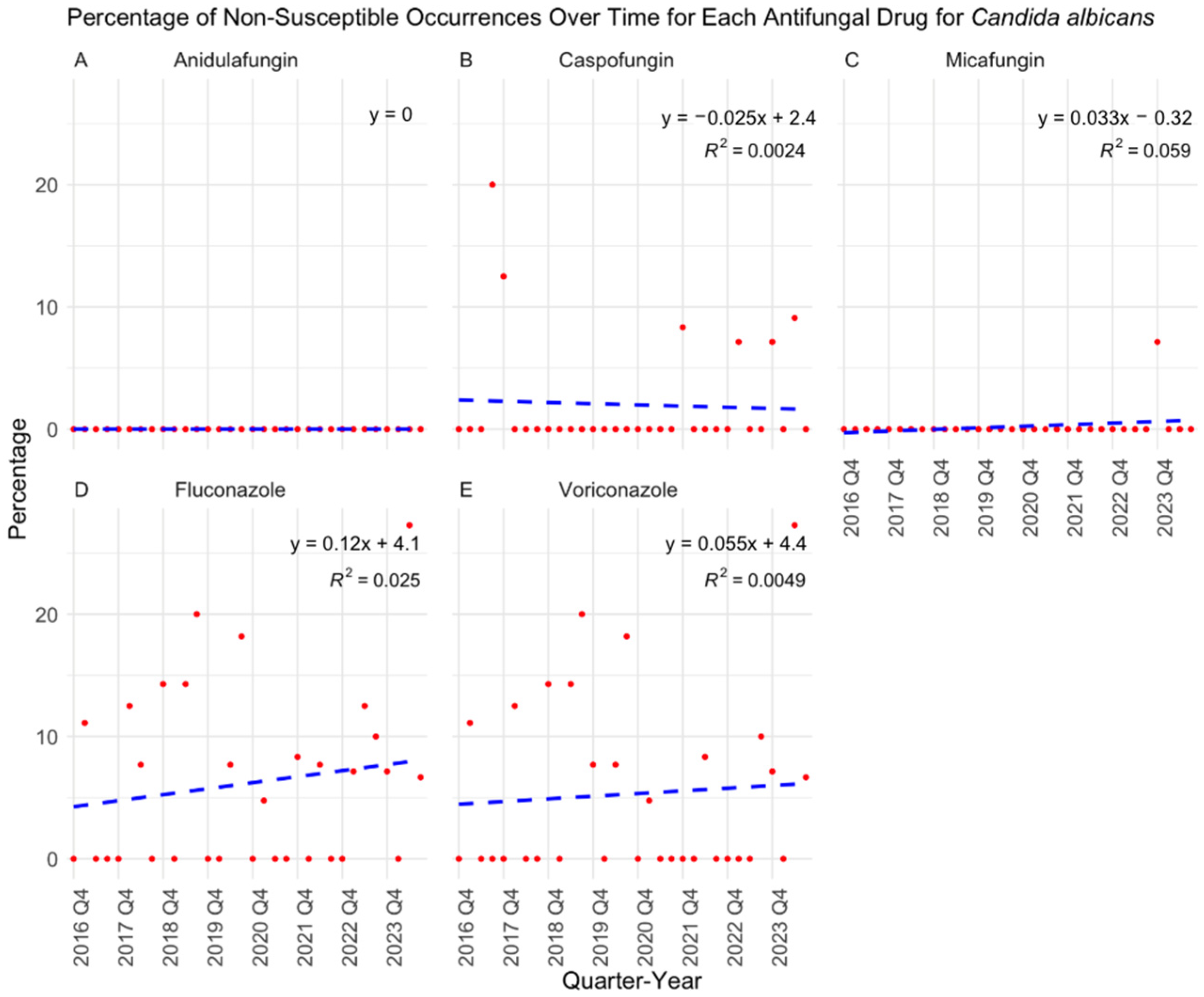
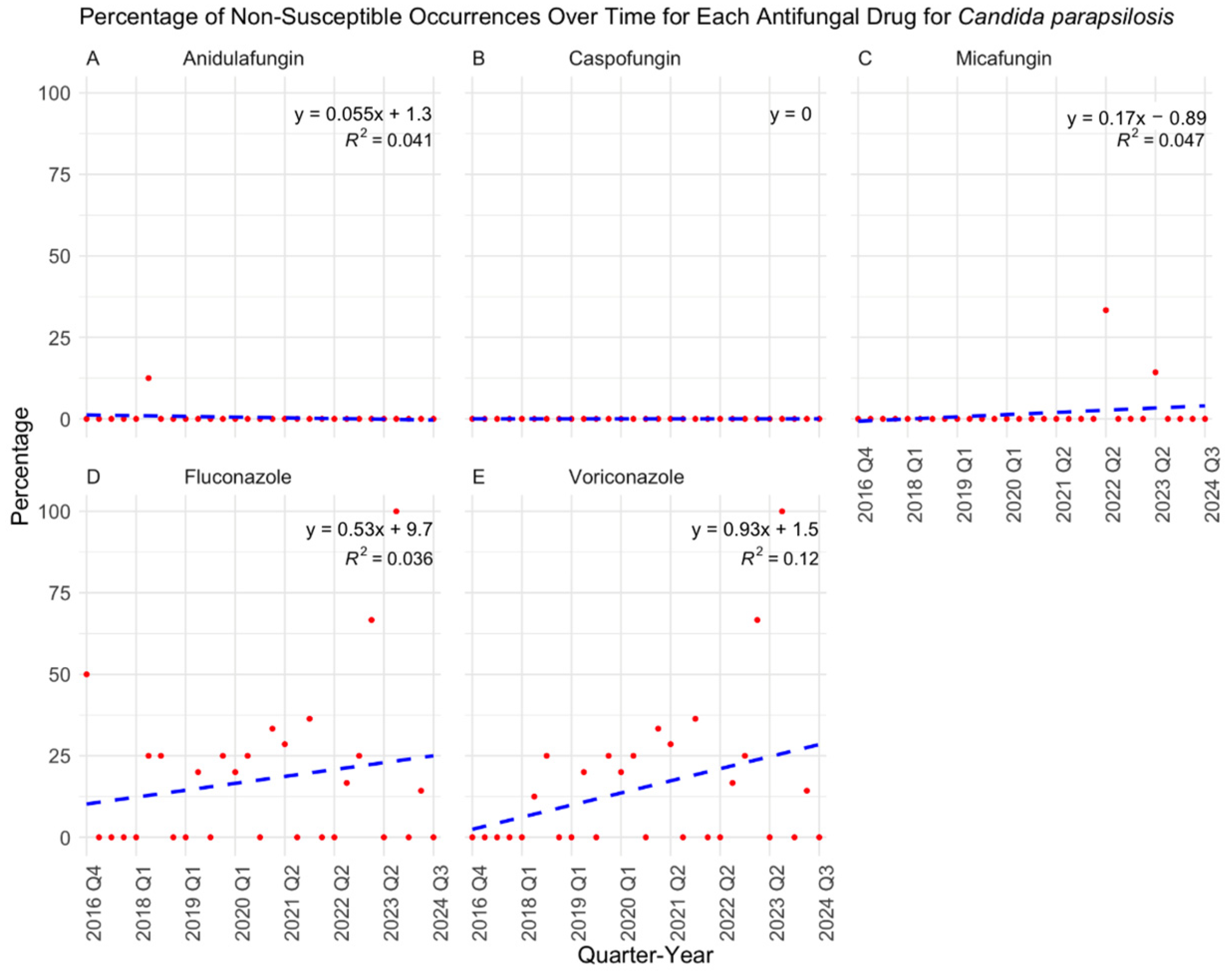
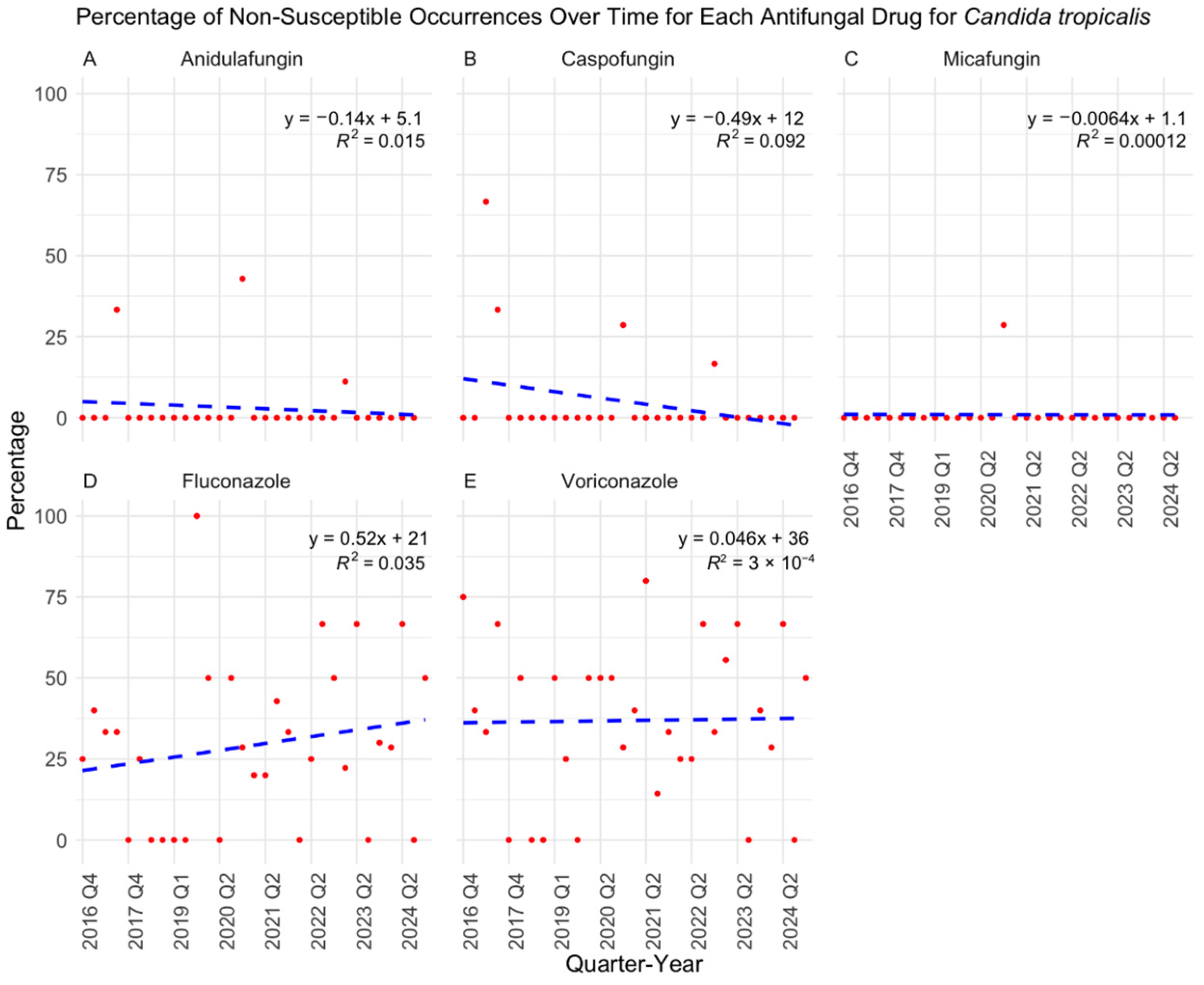
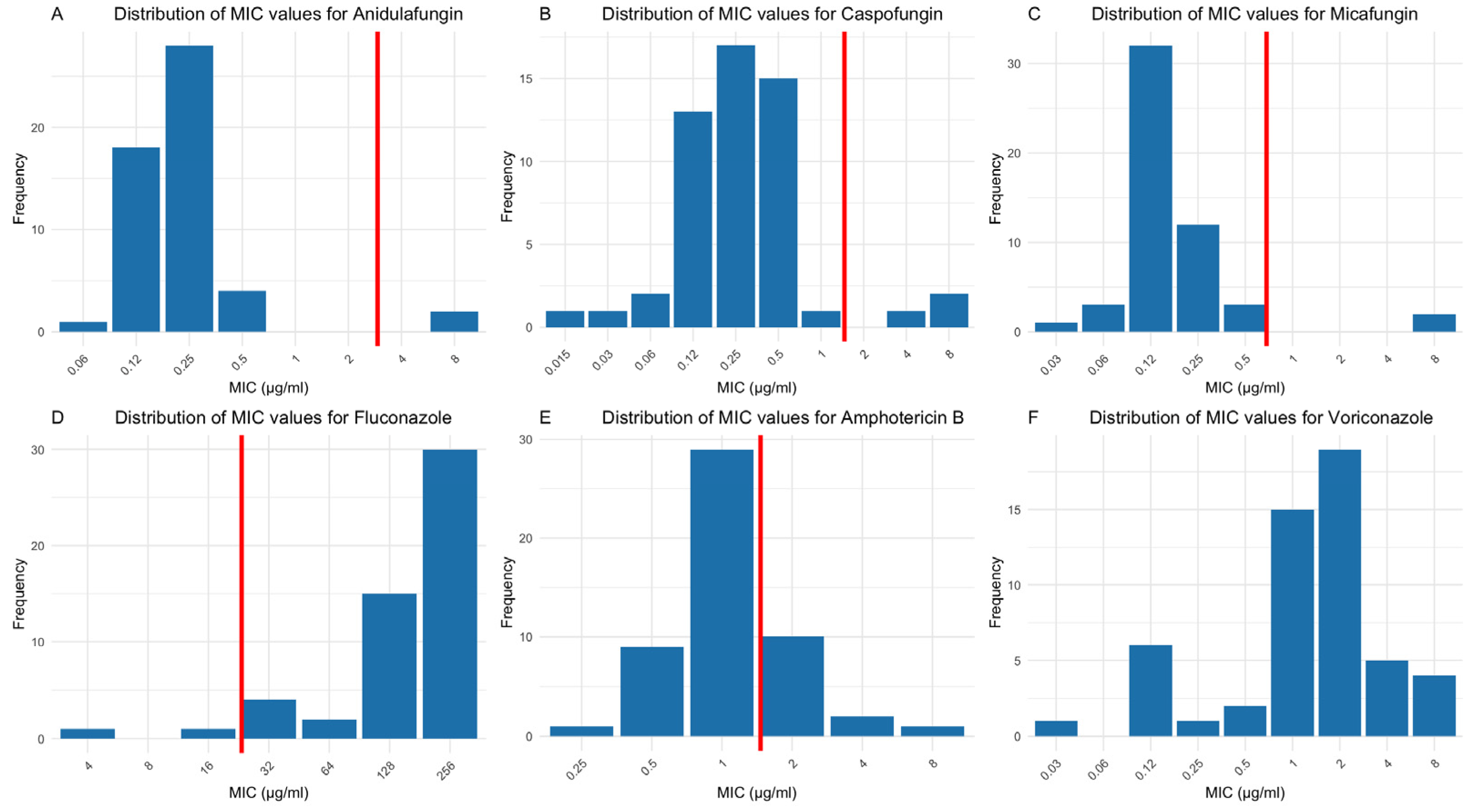
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, C.-Y.; Rotstein, C. Emerging fungal infections in immunocompromised patients. F1000 Med. Rep. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Mora Carpio, A.L.; Climaco, A. Candidemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jamil, Y.; Akinleye, A.; Mirzaei, M.; Lempel, M.; Farhat, K.; Pan, S. Candida endocarditis: Update on management considerations. World J. Cardiol. 2023, 15, 469–478. [Google Scholar] [CrossRef]
- Lockhart, S.R. Current epidemiology of candida infection. Clin. Microbiol. Newsl. 2014, 36, 131–136. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Non-albicans Candida Infection: An Emerging Threat. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 615958. [Google Scholar] [CrossRef]
- Sikora, A.; Hashmi, M.F.; Zahra, F. Candida auris. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hayes, J.F. Candida auris: Epidemiology Update and a Review of Strategies to Prevent Spread. J. Clin. Med. 2024, 13, 6675. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Abbasi, A.F.; Prakash, S.; Mangat, J.; Hosein, Z.; Haider, N.; Chan, J. Candida auris: An Overview of the Emerging Drug-Resistant Fungal Infection. Infect. Chemother. 2022, 54, 236–246. [Google Scholar] [CrossRef]
- Bienvenu, A.-L.; Pradat, P.; Guerin, C.; Aubrun, F.; Fellahi, J.-L.; Friggeri, A.; Guichon, C.; Hernu, R.; Menotti, J.; Monard, C.; et al. Evaluation of first-line therapies for the treatment of candidemia in ICU patients: A propensity score analysis. Int. J. Infect. Dis. 2020, 93, 15–21. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Aldejohann, A.M.; Herz, M.; Martin, R.; Walther, G.; Kurzai, O. Emergence of resistant Candida glabrata in Germany. JAC Antimicrob. Resist. 2021, 3, dlab122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Paul, R.A.; Rudramurthy, S.M.; Kashyap, N.; Bhattacharya, S.; Soman, R.; Shankarnarayan, S.A.; Chavan, D.; Singh, S.; Das, P.; et al. Impact of FKS1 Genotype on Echinocandin In Vitro Susceptibility in Candida auris and In Vivo Response in a Murine Model of Infection. Antimicrob. Agents Chemother. 2022, 66, e0165221. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement. CLSI Guideline M27-S4 2012. Available online: https://webstore.ansi.org/standards/clsi/clsim27s4?srsltid=AfmBOorXfW5_ngh6gbIMm1__fmnJSAOy7uKEagcWIeaOXTgtuq4fvpfa (accessed on 7 August 2024).
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 4th ed. CLSI Guideline M27. 2017. Available online: https://clsi.org/standards/products/microbiology/documents/m27/ (accessed on 7 August 2024).
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 3rd ed. CLSI Guideline M27M44S. 2022. Available online: https://clsi.org/standards/products/microbiology/documents/m27m44s/ (accessed on 7 August 2024).
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 4th ed. CLSI Guideline M57S. 2022. Available online: https://clsi.org/media/tbvf5qr2/m57sed4e_sample.pdf (accessed on 7 August 2024).
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia-Four Sites, United States, 2012-2016. MMWR Surveill. Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Resistance to Fluconazole of Candida albicans in Vaginal Isolates: A 10-Year Study in a Clinical Referral Center. Antimicrob. Agents Chemother. 2023, 67, e0018123. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hilmioğlu-Polat, S.; Fang, W.; Yaşar, M.; Polat, F.; Metin, D.Y.; Rigole, P.; Coenye, T.; Ilkit, M.; et al. First Report of Candidemia Clonal Outbreak Caused by Emerging Fluconazole-Resistant Candida parapsilosis Isolates Harboring Y132F and/or Y132F+K143R in Turkey. Antimicrob. Agents Chemother. 2020, 64, e01001-20. [Google Scholar] [CrossRef]
- Thomaz, D.Y.; de Almeida, J.N.; Sejas, O.N.E.; Del Negro, G.M.B.; Carvalho, G.O.M.H.; Gimenes, V.M.F.; de Souza, M.E.B.; Arastehfar, A.; Camargo, C.H.; Motta, A.L.; et al. Environmental Clonal Spread of Azole-Resistant Candida parapsilosis with Erg11-Y132F Mutation Causing a Large Candidemia Outbreak in a Brazilian Cancer Referral Center. J. Fungi 2021, 7, 259. [Google Scholar] [CrossRef]
- Fan, X.; Tsui, C.K.M.; Chen, X.; Wang, P.; Liu, Z.-J.; Yang, C.-X. High prevalence of fluconazole resistant Candida tropicalis among candiduria samples in China: An ignored matter of concern. Front. Microbiol. 2023, 14, 1125241. [Google Scholar] [CrossRef]
- Favarello, L.M.; Nucci, M.; Queiroz-Telles, F.; Guimarães, T.; Salles, M.J.; Sukiennik, T.C.T.; da Matta, D.A.; Melo, A.S.A.; Colombo, A.L. Trends towards lower azole susceptibility among 200 Candida tropicalis bloodstream isolates from Brazilian medical centres. J. Glob. Antimicrob. Resist. 2021, 25, 199–201. [Google Scholar] [CrossRef]
- Kilburn, S.; Innes, G.; Quinn, M.; Southwick, K.; Ostrowsky, B.; Greenko, J.A.; Lutterloh, E.; Greeley, R.; Magleby, R.; Chaturvedi, V.; et al. Antifungal Resistance Trends of Candida auris Clinical Isolates in New York and New Jersey from 2016 to 2020. Antimicrob. Agents Chemother. 2022, 66, e0224221. [Google Scholar] [CrossRef]
- Cerqueira, F.M.; Bertsch, J.; DeMaet, M.A.; York, T.; McDougal, A.; Patel, J.A.; Ren, P. Enhancing Candida auris Surveillance in High-Risk Settings by Implementing a High-Throughput Molecular Assay on the Hologic Fusion Open Access Platform. J. Fungi 2024, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Chezzi, C. MALDI-TOF MS: A Reliable Tool in the Real Life of the Clinical Microbiology Laboratory. Microorganisms 2024, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.D.; Ren, P. Trends in Antifungal Resistance Among Candida Species: An Eight-Year Retrospective Study in the Galveston–Houston Gulf Coast Region. J. Fungi 2025, 11, 232. https://doi.org/10.3390/jof11030232
Nguyen MD, Ren P. Trends in Antifungal Resistance Among Candida Species: An Eight-Year Retrospective Study in the Galveston–Houston Gulf Coast Region. Journal of Fungi. 2025; 11(3):232. https://doi.org/10.3390/jof11030232
Chicago/Turabian StyleNguyen, Michael D., and Ping Ren. 2025. "Trends in Antifungal Resistance Among Candida Species: An Eight-Year Retrospective Study in the Galveston–Houston Gulf Coast Region" Journal of Fungi 11, no. 3: 232. https://doi.org/10.3390/jof11030232
APA StyleNguyen, M. D., & Ren, P. (2025). Trends in Antifungal Resistance Among Candida Species: An Eight-Year Retrospective Study in the Galveston–Houston Gulf Coast Region. Journal of Fungi, 11(3), 232. https://doi.org/10.3390/jof11030232





