A New Root and Trunk Rot Disease of Grapevine Plantlets Caused by Fusarium in Four Species Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Fungal Isolation
2.2. Clustering and Identification
2.3. Pathogenicity and Virulence
2.4. Pathogenicity and Colonisation of Other Plant Species by Fusarium
2.5. Comparative Analysis of Diseased vs Healthy Tissues
3. Results
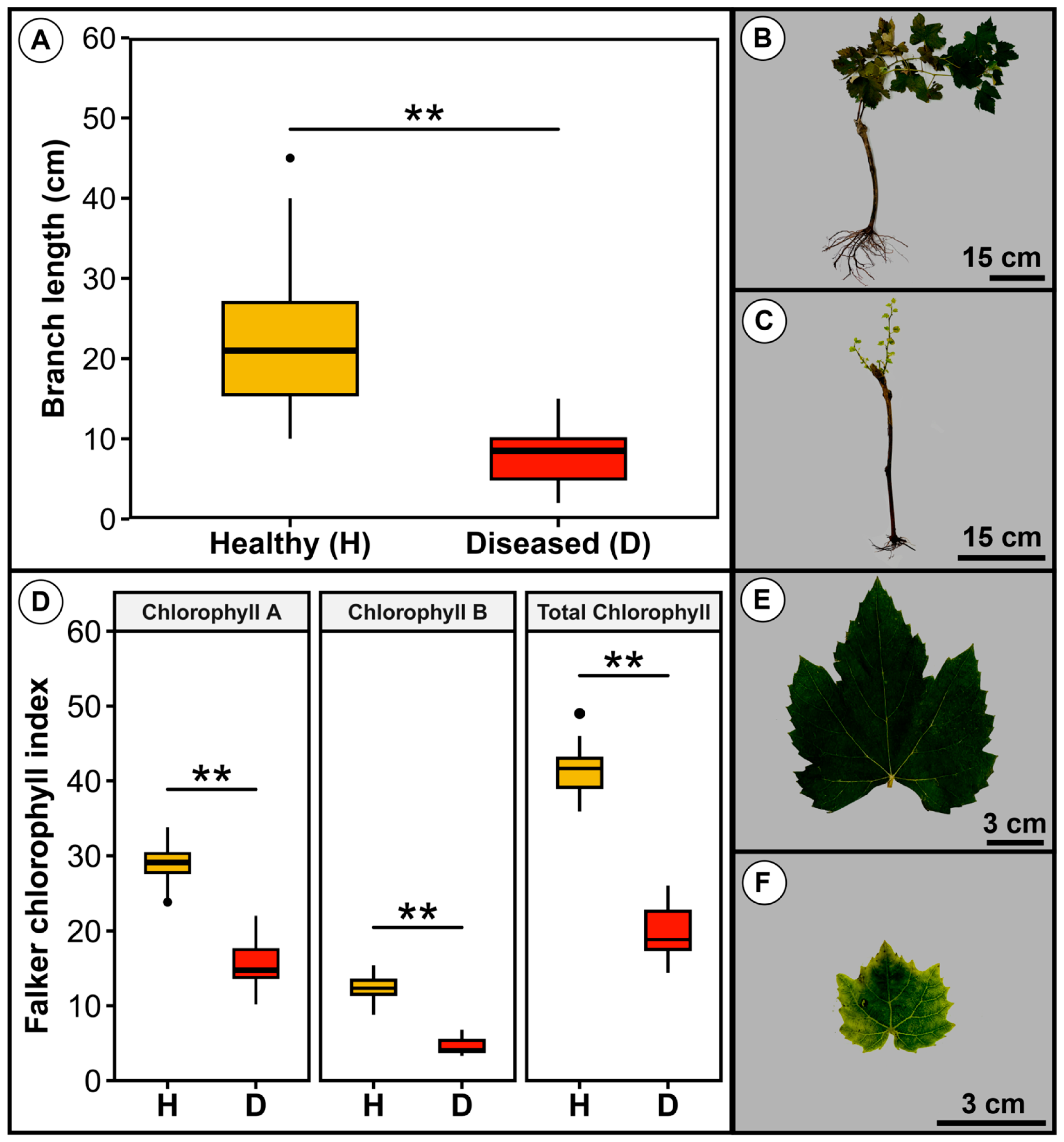
3.1. Symptoms of Root and Trunk Rot of Grapevine Plantlets Under Cold Storage
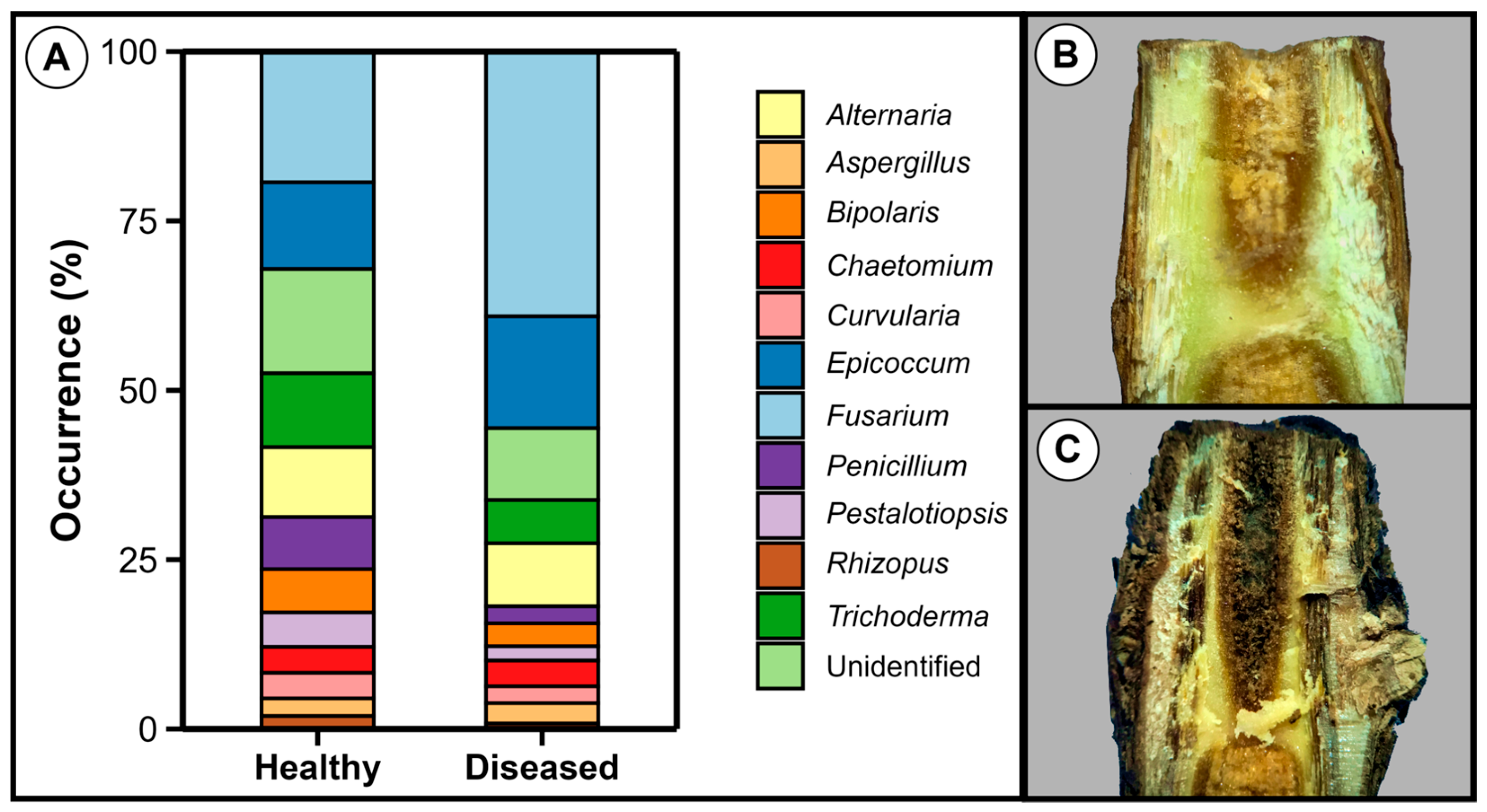
3.2. Fusarium Was the Most Prevalent Genus in Both Healthy and Diseased Plantlets
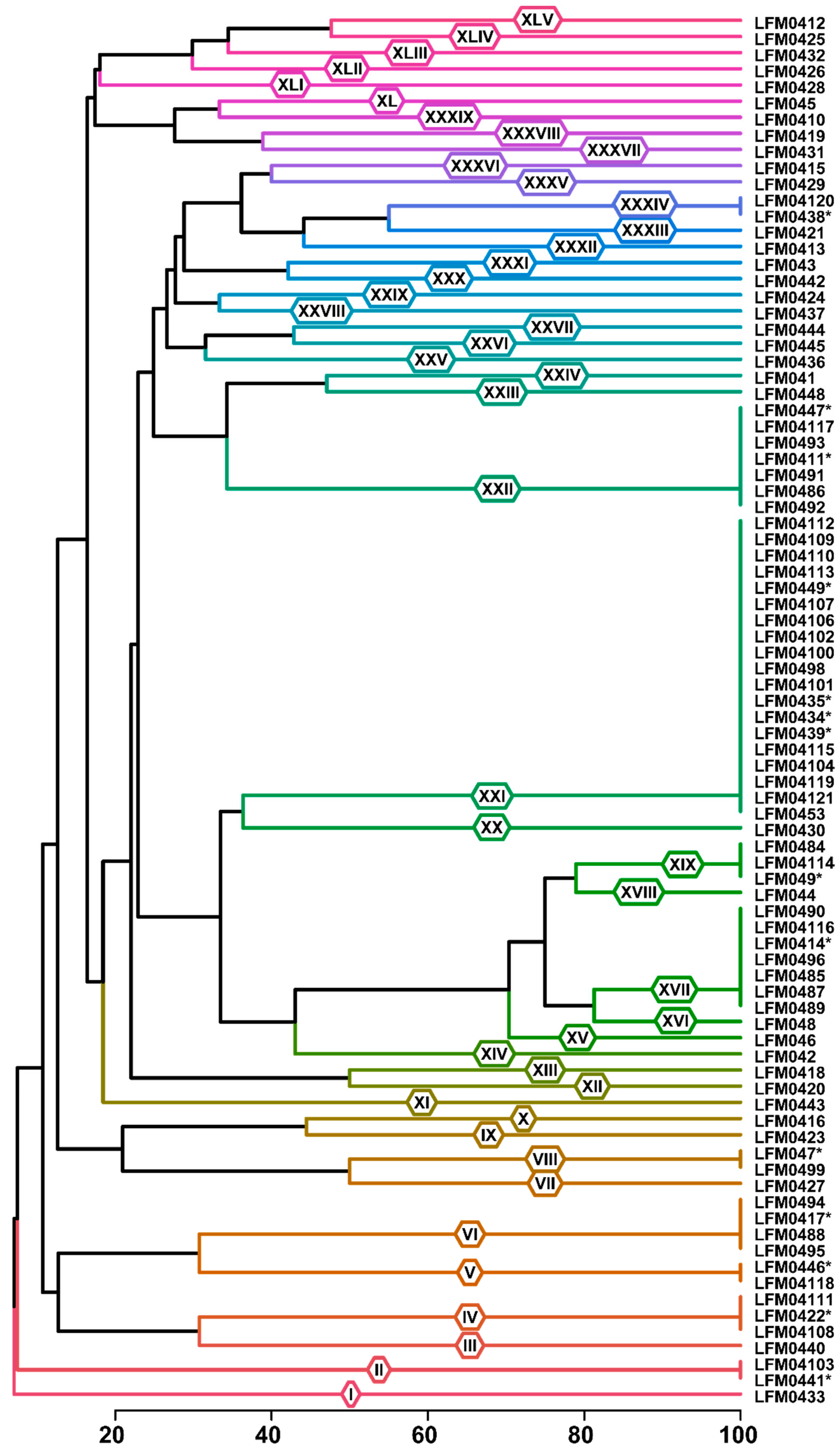
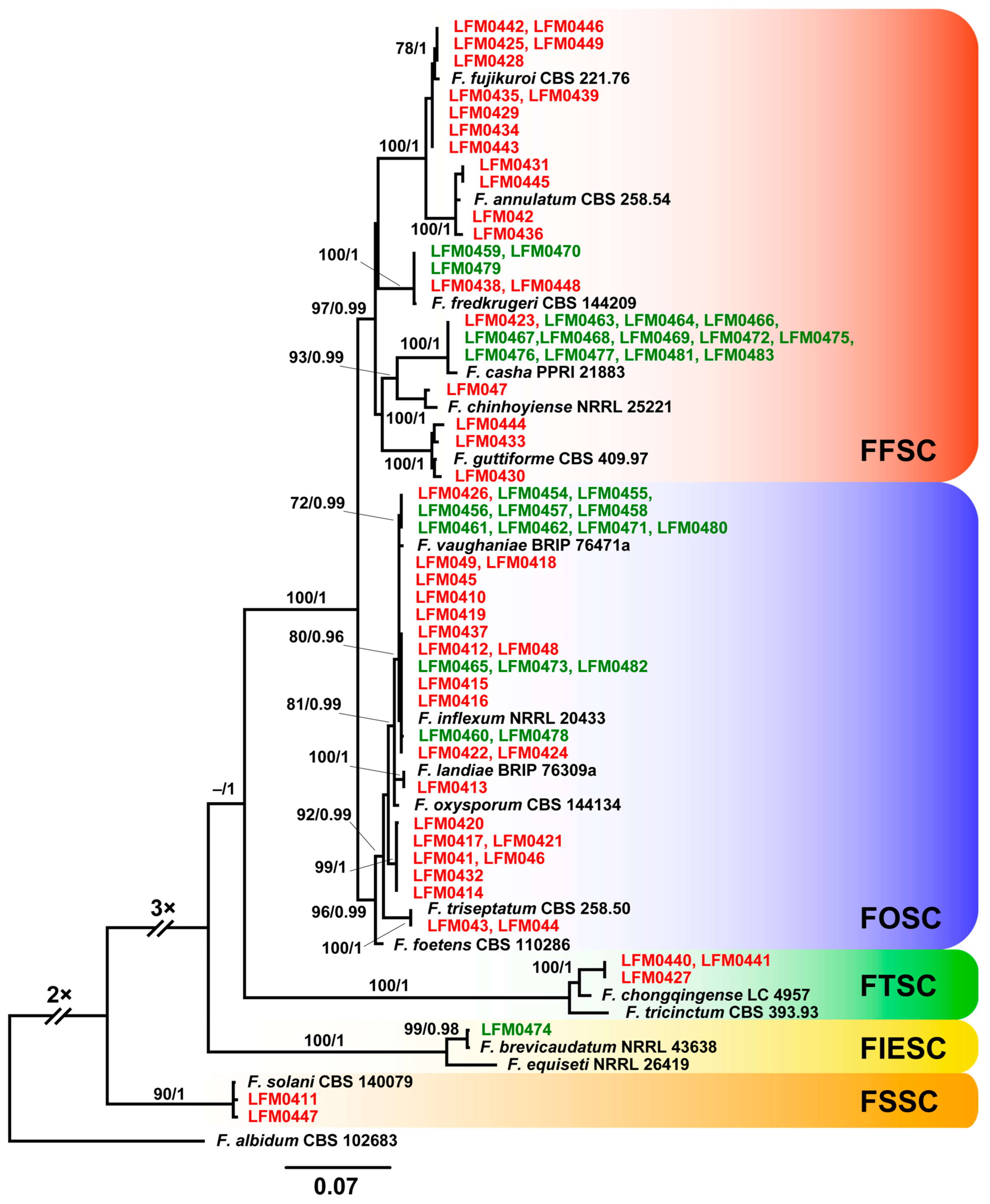
3.3. Fusarium Strains from Diseased Plantlets Were Highly Diverse
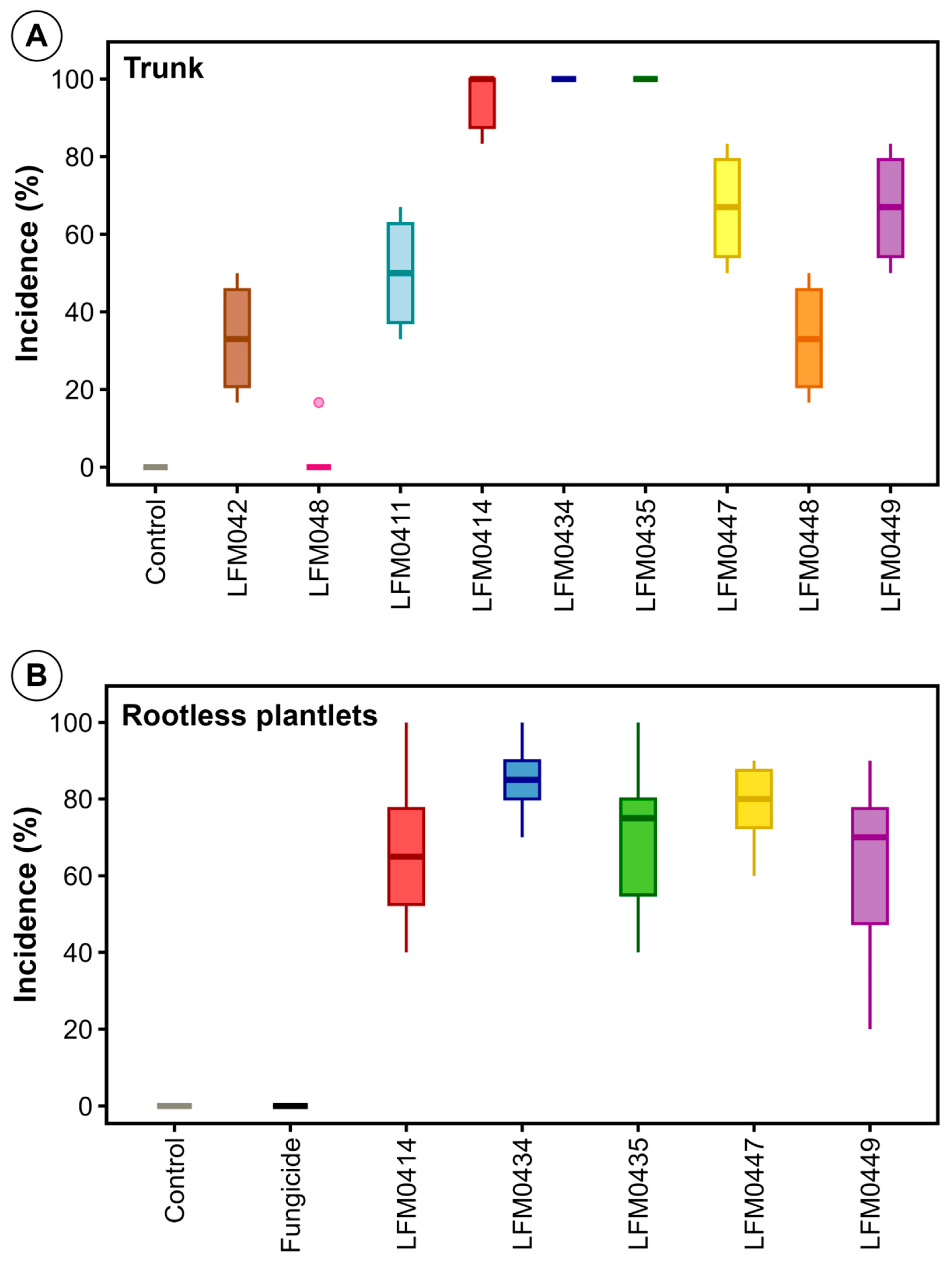
3.4. Fusarium from Diseased Plantlets Are Pathogenic to Grapevine
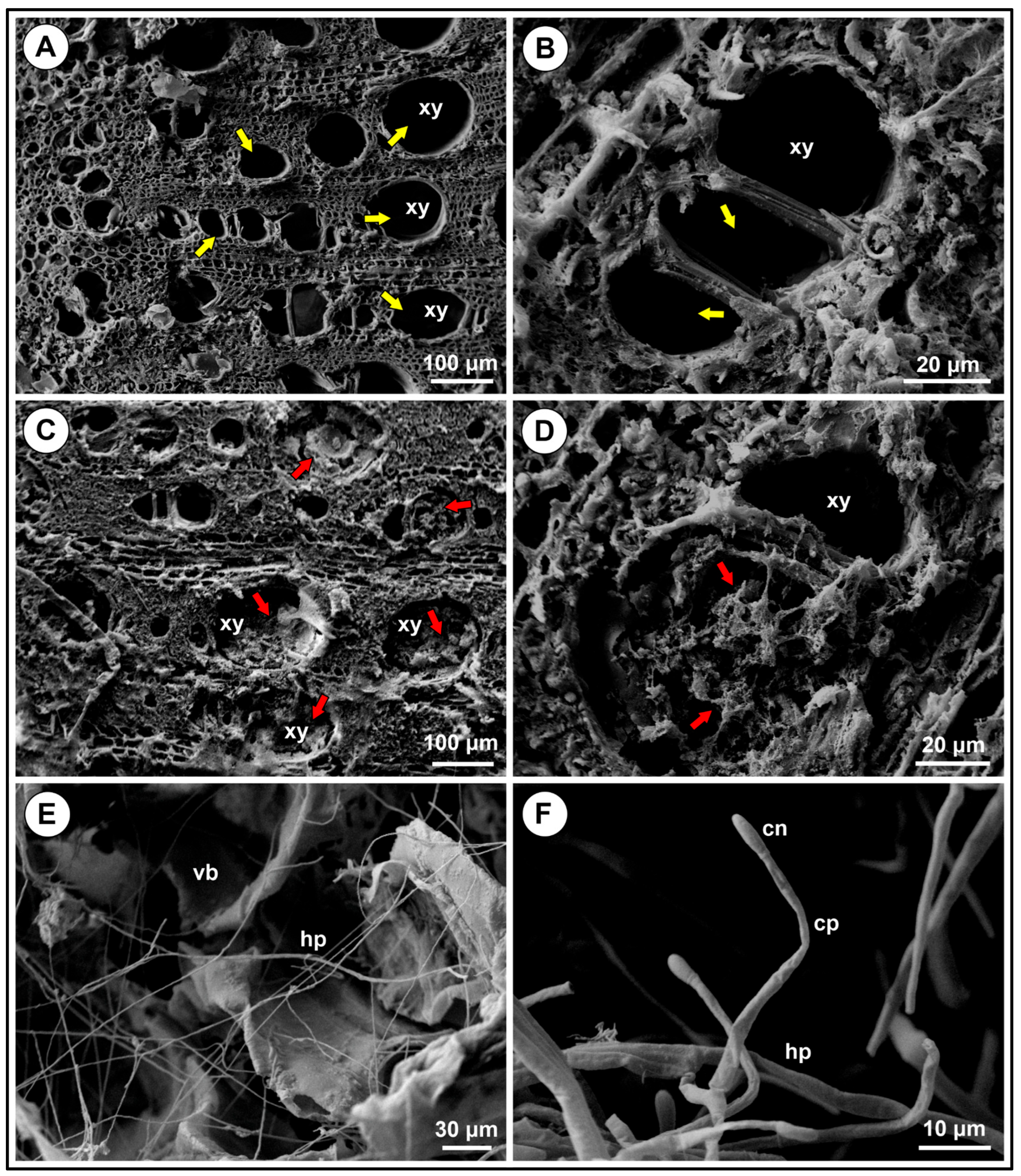
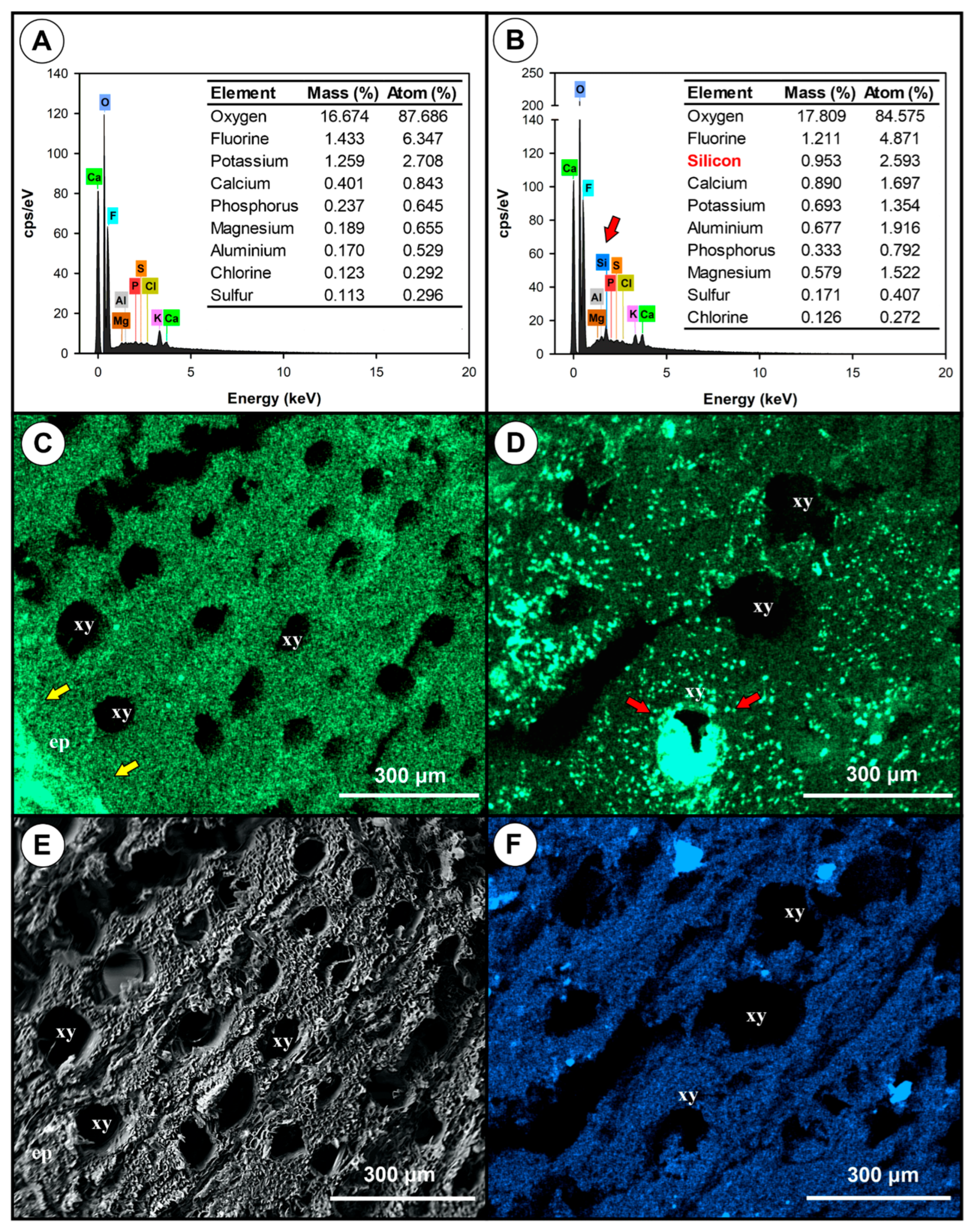
3.5. Comparative Analysis of Healthy and Diseased Tissues
3.6. Fusarium Colonises Roots of Different Species, but Does Not Show Symptoms
3.7. Fusarium Strains from Healthy Plantlets Are Also Pathogenic to Grapevine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aleixandre, J.L.; Aleixandre-Tudó, J.L.; Bolaños-Pizarro, M.; Aleixandre-Benavent, R. Viticulture and oenology scientific research: The Old World versus the New World wine-producing countries. Int. J. Inform. Manag. 2016, 36, 389–396. [Google Scholar] [CrossRef]
- Regina, M.D.A.; Mota, R.V.; Souza, C.R.; Favero, A.C. Viticulture for fine wines in Brazilian Southeast. Acta Hortic. 2011, 910, 113–120. [Google Scholar] [CrossRef]
- Almeida, L.W.; Mota, R.V.; Souza, C.R.; Silva, L.P.; Fernandes, F.P.; Mendonça, T.R.; Regina, M.A. In-row spacing and shoot thinning for ‘Chardonnay’grapevines in the south of Minas Gerais, Brazil. Sci. Agric. 2020, 77, e20180185. [Google Scholar] [CrossRef]
- Favero, A.C.; Amorim, D.A.; Mota, R.V.; Soares, A.M.; Souza, C.R.; Regina, M.A. Double-pruning of ‘Syrah’grapevines: A management strategy to harvest wine grapes during the winter in the Brazilian Southeast. VITIS-J. Grapevine Res. 2015, 50, 151. [Google Scholar]
- Santos, A.O.; Hernandes, J.L.; Pedro Junior, M.J.; Rolim, G.S. Plant parameters and microclimatic conditions for wine grapes cultivated under sequential double pruning. Rev. Bras. Eng. Agríc. Ambient. 2011, 15, 1251–1256. [Google Scholar] [CrossRef]
- Dias, F.A.N.; Mota, R.V.D.; Souza, C.R.D.; Pimentel, R.M.D.A.; Souza, L.C.D.; Souza, A.L.D.; Regina, M.D.A. Rootstock on vine performance and wine quality of ‘Syrah’under double pruning management. Sci. Agric. 2017, 74, 134–141. [Google Scholar] [CrossRef]
- Almeida Junior, O.; Souza, C.R.; Fernandes, F.P.; Torregrosa, L.; Fernandes-Brum, C.N.; Chalfun Junior, A.; Mota, R.V.; Peregrino, I.; Regina, M.A. Effect of pruning strategy on ‘Syrah’ bud necrosis and fruitfulness in Brazilian subtropical Southeast. VITIS-J. Grapevine Res. 2019, 58, 87–94. [Google Scholar] [CrossRef]
- Regina, M.D.A. Production and certification of grapevine plants in France 2: The technique of plants production by table grafting. Rev. Bras. Frutic. 2002, 24, 590–596. [Google Scholar] [CrossRef]
- Regina, M.D.A.; Souza, C.R.D.; Dias, F.A.N. Propagation of Vitis spp. by bench grafting table using different rootstocks and auxins. Rev. Bras. Frutic. 2012, 34, 897–904. [Google Scholar] [CrossRef]
- Souza, C.R.; Câmara, F.M.M.; Hernandes, J.L.; Mota, R.V.; Brant, L.A.C.; Regina, M.A. Porta enxertos e cultivares de uva para produção de vinhos de inverno. Inf. Agropecuário 2020, 41, 28–39. [Google Scholar]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine Trunk Diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Milholland, R.D. Muscadine grapes: Some important diseases and their control. Plant Dis. 1991, 75, 111–117. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Gubler, W.D. Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis. 2009, 93, 584–592. [Google Scholar] [CrossRef]
- Pitt, W.M.; Huang, R.; Steel, C.C.; Savocchia, S. Pathogenicity and epidemiology of Botryosphaeriaceae species isolated from grapevines in Australia. Australas. Plant Pathol. 2013, 42, 573–582. [Google Scholar] [CrossRef]
- Lombard, L.; Van Der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathol. Mediterr. 2014, 53, 515–532. [Google Scholar] [CrossRef]
- Carlucci, A.; Lops, F.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Mesguida, O.; Haidar, R.; Yacoub, A.; Dreux-Zigha, A.; Berthon, J.Y.; Guyoneaud, R.; Attard, E.; Rey, P. Microbial biological control of fungi associated with grapevine trunk diseases: A review of strain diversity, modes of action, and advantages and limits of current strategies. J. Fungi 2023, 9, 638. [Google Scholar] [CrossRef]
- Testempasis, S.I.; Markakis, E.A.; Tavlaki, G.I.; Soultatos, S.K.; Tsoukas, C.; Gkizi, D.; Tzima, A.T.; Paplomatas, E.; Karaoglanidis, G.S. Grapevine trunk diseases in Greece: Disease incidence and fungi involved in discrete geographical zones and varieties. J. Fungi 2024, 10, 2. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, W.; Zhang, J.; Wang, H.; Peng, J.; Wang, X.; Yan, J. Belowground microbiota analysis indicates that Fusarium spp. exacerbate grapevine trunk disease. Environ. Microbiome 2023, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Akgül, D.S.; Önder, S.; Savaş, N.G.; Yıldız, M.; Bülbül, İ.; Özarslandan, M. Molecular Identification and Pathogenicity of Fusarium Species Associated with Wood Canker, Root and Basal Rot in Turkish Grapevine Nurseries. J. Fungi 2024, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.I.; Todd, C.; Elfar, K.; Hamid, M.I.; Garcia, J.F.; Cantu, D.; Rolshausen, P.E.; Eskalen, A. Identification and pathogenicity of Fusarium species associated with young vine decline in California. Plant Dis. 2024, 108, 1053–1061. [Google Scholar] [CrossRef]
- Castellani, A. Viability of some pathogenic fungi in distilled water. J. Trop. Med. Hyg. 1939, 42, 225–226. [Google Scholar]
- Seifert, K.A.; Gams, W. The genera of Hyphomycetes–2011 update. Pers. Mol. Phylogeny Evol. Fungi 2011, 27, 119–129. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing: Ames, IA, USA, 2006; 388p. [Google Scholar]
- Inglis, P.W.; Pappas, M.D.C.R.; Resende, L.V.; Grattapaglia, D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS ONE 2018, 13, e0206085. [Google Scholar] [CrossRef]
- Versalovic, J. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell Biol. 1994, 5, 25–40. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: http://www.r-project.org (accessed on 18 March 2024).
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Benson, D.A.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 42, D32. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Seleção de blocos conservados de alinhamentos múltiplos para seu uso em análise filogenética. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, v1.4.2. Tree Figure Drawing Tool; Institute of Evolutionary Biology, Ashworth Laboratories: Edinburgh, UK, 2012; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 8 January 2025).
- Halleen, F.; Mostert, L.; Crous, P.W. Pathogenicity testing of lesser-known vascular fungi of grapevines. Australas. Plant Pathol. 2007, 36, 277–285. [Google Scholar] [CrossRef]
- Alves, E.; Lucas, G.C.; Pozza, E.A.; Alves, M.C. Scanning electron microscopy for fungal sample examination. In Laboratory Protocols in Fungal Biology; Springer: New York, NY, USA, 2013; pp. 133–150. [Google Scholar] [CrossRef]
- Chehri, K. Morphological and Molecular Characterisation of Campylocarpon fasciculare and Fusarium spp., the Cause of Black Disease of Grapevine in Iran. Pertanika J. Trop. Agric. Sci. 2017, 40, 587–600. [Google Scholar]
- Astudillo-Calderón, S.; Tello, M.L.; Alonso de Robador, J.M.; Pintos, B.; Gómez-Garay, A. First report of Fusarium equiseti causing vascular wilt disease on Vitis vinifera in Spain. Plant Dis. 2019, 103, 2471. [Google Scholar] [CrossRef]
- Rajput, N.A.; Zaman, B.; Huo, C.; Cao, J.; Atiq, M.; Lodhi, A.M.; Syed, R.N.; Khan, B.; Iqbal, O.; Zhao, Z. First report of Fusarium equiseti causing stem rot disease of grape (Vitis vinifera L.) in Afghanistan. Plant Pathol. J. 2020, 102, 1277. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Bouleé, J.; Hrycan, J.; O’Gorman, D.T. Potential role of Fusarium spp. in grapevine decline. Phytopathologia Mediterranea 2023, 62, 269–281. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.Y.; Li, X.H.; Zhang, W.; Li, Y.H.; Wang, X.D.; Yan, J.Y. First report of Fusarium commune associated with root rot of grapevine in China. Plant Dis. 2023, 107, 1238. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.; Reynolds, A.; Di Profio, F. Nonstructural carbohydrate concentrations in dormant grapevine scionwood and rootstock impact propagation success and vine growth. HortTechnology 2015, 25, 536–550. [Google Scholar] [CrossRef]
- Karabulut, B.; Çelik, H. Determination of grafting success and carbohydrate distributions of foxy grape (Vitis labrusca L.) varieties grafted on different American grape rootstocks. Horticulturae 2022, 8, 949. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Fitotoxinas produzidas por fungos associados a doenças do tronco da videira. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef]
- Dehghanpour-Farashah, S.; Taheri, P.; Falahati-Rastegar, M. Virulence factors of Fusarium spp., causing wheat crown and root rot in Iran. Phytopathol. Mediterr. 2019, 58, 115–126. [Google Scholar] [CrossRef]
- Huang, X.Q.; Lu, X.H.; Sun, M.H.; Guo, R.J.; Van Diepeningen, A.D.; Li, S.D. Transcriptome analysis of virulence-differentiated Fusarium oxysporum f. sp. cucumerinum isolates during cucumber colonisation reveals pathogenicity profiles. BMC Genom. 2019, 20, 570. [Google Scholar] [CrossRef]
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Rauwane, M.E.; Ogugua, U.V.; Kalu, C.M.; Ledwaba, L.K.; Woldesemayat, A.A.; Ntushelo, K. Pathogenicity and virulence factors of Fusarium graminearum including factors discovered using next generation sequencing technologies and proteomics. Microorganisms 2020, 8, 305. [Google Scholar] [CrossRef]
- Yang, H.; Yu, H.; Ma, L.J. Accessory chromosomes in Fusarium oxysporum. Phytopathology 2021, 110, 1488–1496. [Google Scholar] [CrossRef]
- Jangir, P.; Mehra, N.; Sharma, K.; Singh, N.; Rani, M.; Kapoor, R. Secreted in Xylem genes: Drivers of host adaptation in Fusarium oxysporum. Front. Plant Sci. 2021, 12, 628611. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Taylor, A.; Jackson, A.C.; Armitage, A.D.; Bates, H.J.; Mead, A.; Harrison, R.J.; Clarkson, J.P. Identification and expression of secreted in xylem pathogenicity genes in Fusarium oxysporum f. sp. pisi. Front. Microbiol. 2021, 12, 593140. [Google Scholar] [CrossRef] [PubMed]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, A.; Plasencia, J.; Monribot-Villanueva, J.L.; Rodríguez-Haas, B.; Ruíz-May, E.; Guerrero-Analco, J.A.; Sánchez-Rangel, D. Virulence factors of the genus Fusarium with targets in plants. Microbiol. Res. 2023, 277, 127506. [Google Scholar] [CrossRef]
- Ishikawa, F.H.; Souza, E.A.; Shoji, J.Y.; Connolly, L.; Freitag, M.; Read, N.D.; Roca, M.G. Heterokaryon incompatibility is suppressed following conidial anastomosis tube fusion in a fungal plant pathogen. PLoS ONE 2012, 7, e31175. [Google Scholar] [CrossRef]
- Kurian, S.M.; Di Pietro, A.; Read, N.D. Live-cell imaging of conidial anastomosis tube fusion during colony initiation in Fusarium oxysporum. PLoS ONE 2018, 13, e0195634. [Google Scholar] [CrossRef]
- Chang, T.H.; Lin, Y.H.; Wan, Y.L.; Chen, K.S.; Huang, J.W.; Chang, P.F.L. Degenerated virulence and irregular development of Fusarium oxysporum f. sp. niveum induced by successive subculture. J. Fungi 2020, 6, 382. [Google Scholar] [CrossRef]
- Kraus, C.; Voegele, R.T.; Fischer, M. Temporal development of the culturable, endophytic fungal community in healthy grapevine branches and occurrence of GTD-associated fungi. Microb. Ecol. 2019, 77, 866–876. [Google Scholar] [CrossRef]
- Hrycan, J.; Miranda, H.A.R.T.; Bowen, P.; Forge, T.; Urbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar] [CrossRef]
- González, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42. [Google Scholar] [CrossRef]
- Veloso, J.; Van Kan, J.A. Many shades of grey in Botrytis–host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Benemann, D.P.; Nohato, A.M.; Vargas, L.; Avila, L.A.; Agostinetto, D. Identification and validation of reference genes for the normalization in real-time RT-qPCR on rice and red rice in competition, under different nitrogen doses. Planta Daninha 2017, 35, e017161319. [Google Scholar] [CrossRef]
- Marek, S.M.; Yaghmour, M.A.; Bostock, R.M. Fusarium spp., Cylindrocarpon spp., and environmental stress in the etiology of a canker disease of cold-stored fruit and nut tree seedlings in California. Plant Dis. 2013, 97, 259–270. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae speciales and races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Afordoanyi, D.M.; Diabankana, R.G.C.; Akosah, Y.A.; Validov, S.Z. Are formae speciales pathogens really host specific? A broadened host specificity in Fusarium oxysporum f. sp. radicis-cucumerinum. Braz. J. Microbiol. 2022, 53, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium-induced diseases of tropical, perennial crops. Phytopathology 2006, 96, 648–652. [Google Scholar] [CrossRef]
- Embrapa. Cultivares de Uva e Porta-Enxertos de Alta Sanidade. 2015. Available online: https://www.embrapa.br/en/uva-e-vinho/cultivares-e-porta-enxertos (accessed on 4 February 2025).
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Zhang, L.; Du, L.; Poovaiah, B.W. Calcium signaling and biotic defense responses in plants. Plant Signal Behav. 2014, 9, e973818. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Greger, M. A review on Si uptake and transport system. Plants 2019, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking intruders: Inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot. 2021, 72, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Bouamama-Gzara, B.; Zemni, H.; Sleimi, N.; Ghorbel, A.; Gzara, L.; Mahfoudhi, N. Diversification of Vascular Occlusions and Crystal Deposits in the Xylem Sap Flow of Five Tunisian Grapevines. Plants 2022, 11, 2177. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant–microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef]
- Lazcano, C.; Boyd, E.; Holmes, G.; Hewavitharana, S.; Pasulka, A.; Ivors, K. The rhizosphere microbiome plays a role in the resistance to soil-borne pathogens and nutrient uptake of strawberry cultivars under filed conditions. Sci. Rep. 2021, 11, 3188. [Google Scholar] [CrossRef]
- Chastagner, G.A.; Vassey, W.E. Occurrence of iprodione-tolerant Fusarium nivale under field conditions. Plant Dis. 1982, 66, 112–113. [Google Scholar] [CrossRef]

| N° Diseased Plantlets | Pathogenicity/Virulence |
|---|---|
| 0 | - |
| <5 | + |
| 6–10 | ++ |
| >11 | +++ |
| Strains | BOX-PCR Group | Closest Species (Type Material) | tef1-α (bp) | Accession Number | Identity (%) | Species Complex | Pathogenicity/Virulence 1 |
|---|---|---|---|---|---|---|---|
| LFM0433 | I | F. guttiforme | 650 | PV081890 | 99.23 | FFSC | +++ |
| LFM0441 | II | F. chongqingense | 645 | PV081913 | 97.51 | FTSC | + |
| LFM0440 | III | F. chongqingense | 645 | PV081913 | 97.51 | FTSC | + |
| LFM0422 | IV | F. inflexum | 651 | PV081876 | 99.68 | FOSC | ++ |
| LFM0446 | V | F. fujikuroi | 648 | PV081893 | 99.53 | FFSC | ++ |
| LFM0417 | VI | F. foetens | 658 | PV081883 | 97.88 | FOSC | +++ |
| LFM0427 | VII | F. chongqingense | 639 | PV081914 | 97.48 | FTSC | + |
| LFM047 | VIII | F. chinhoyiense | 656 | PV081900 | 98.92 | FFSC | - |
| LFM0423 | IX | F. casha | 630 | PV081901 | 99.21 | FFSC | ++ |
| LFM0416 | X | F. inflfexum | 669 | PV081877 | 100 | FOSC | +++ |
| LFM0443 | XI | F. fujikuroi | 639 | PV081894 | 99.37 | FFSC | +++ |
| LFM0420 | XII | F. foetens | 668 | PV081884 | 97.91 | FOSC | ++ |
| LFM0418 | XIII | F. vaughaniae | 661 | PV081870 | 99.54 | FOSC | ++ |
| LFM042 | XIV | F. annulatum | 654 | PV081904 | 99.54 | FFSC | + |
| LFM046 | XV | F. foetens | 667 | PV081885 | 98.20 | FOSC | +++ |
| LFM048 | XVI | F. inflexum | 671 | PV081878 | 99.69 | FOSC | + |
| LFM0414 | XVII | F. foetens | 673 | PV081886 | 97.93 | FOSC | +++ |
| LFM044 | XVIII | F. triseptatum | 655 | PV081888 | 99.51 | FOSC | +++ |
| LFM049 | XIX | F. vaughaniae | 661 | PV081870 | 99.54 | FOSC | ++ |
| LFM0430 | XX | F. guttiforme | 651 | PV081891 | 99.23 | FFSC | ++ |
| LFM0439 | XXI | F. fujikuroi | 656 | PV081895 | 99.39 | FFSC | ++ |
| LFM0434 | XXI | F. fujikuroi | 656 | PV081896 | 99.39 | FFSC | +++ |
| LFM0435 | XXI | F. fujikuroi | 656 | PV081895 | 99.39 | FFSC | +++ |
| LFM0449 | XXI | F. fujikuroi | 693 | PV081897 | 99.54 | FFSC | +++ |
| LFM0411 | XXII | F. solani | 688 | PV081912 | 99.41 | FSSC | ++ |
| LFM0447 | XXII | F. solani | 706 | PV081911 | 99.86 | FSSC | ++ |
| LFM0448 | XXIII | F. fredkrugeri | 706 | PV081908 | 98.67 | FFSC | +++ |
| LFM041 | XXIV | F. foetens | 667 | PV081885 | 98.20 | FOSC | ++ |
| LFM0436 | XXV | F. annulatum | 654 | PV081905 | 98.93 | FFSC | ++ |
| LFM0445 | XXVI | F. annulatum | 658 | PV081906 | 99.09 | FFSC | + |
| LFM0444 | XXVII | F. guttiforme | 653 | PV081892 | 98.47 | FFSC | +++ |
| LFM0437 | XXVIII | F. inflexum | 651 | PV081879 | 99.68 | FOSC | ++ |
| LFM0424 | XXIX | F. inflexum | 651 | PV081876 | 99.68 | FOSC | ++ |
| LFM0442 | XXX | F. fujikuroi | 648 | PV081893 | 99.53 | FFSC | +++ |
| LFM043 | XXXI | F. triseptatum | 655 | PV081888 | 99.51 | FOSC | ++ |
| LFM0413 | XXXII | F. landiae | 664 | PV081889 | 100 | FOSC | ++ |
| LFM0421 | XXXIII | F. foetens | 658 | PV081883 | 97.88 | FOSC | ++ |
| LFM0438 | XXXIV | F. fredkrugeri | 706 | PV081908 | 98.67 | FFSC | + |
| LFM0429 | XXXV | F. fujikuroi | 644 | PV081898 | 99.38 | FFSC | ++ |
| LFM0415 | XXXVI | F. inflexum | 653 | PV081880 | 100 | FOSC | + |
| LFM0431 | XXXVII | F. annulatum | 645 | PV081907 | 99.07 | FFSC | + |
| LFM0419 | XXXVIII | F. vaughaniae | 661 | PV081871 | 99.69 | FOSC | ++ |
| LFM0410 | XXXIX | F. vaughaniae | 639 | PV081872 | 99.69 | FOSC | + |
| LFM045 | XL | F. vaughaniae | 650 | PV081873 | 99.69 | FOSC | +++ |
| LFM0428 | XLI | F. fujikuroi | 639 | PV081899 | 99.53 | FFSC | + |
| LFM0426 | XLII | F. vaughaniae | 660 | PV081869 | 99.54 | FOSC | +++ |
| LFM0432 | XLIII | F. foetens | 655 | PV081887 | 97.87 | FOSC | + |
| LFM0425 | XLIV | F. fujikuroi | 693 | PV081897 | 99.54 | FFSC | + |
| LFM0412 | XLV | F. inflexum | 671 | PV081878 | 99.69 | FOSC | - |
| Strains 1 | BOX-PCR Group | Closest Species (Type Material) | Grapevine | Tomato | Common Bean | |||
|---|---|---|---|---|---|---|---|---|
| Re-Isolation (%) | Symptoms | Re-Isolation (%) | Symptoms | Re-Isolation (%) | Symptoms | |||
| LFM042 | XIV | F. annulatum | 100.0 | – | 94.0 | – | 88.0 | – |
| LFM048 | XVI | F. inflexum | 91.6 | – | 92.0 | – | 89.0 | – |
| LFM0411 | XXII | F. solani | 75.0 | – | 95.0 | – | 97.0 | – |
| LFM0414 | XVII | F. foetens | 91.6 | – | 93.0 | – | 94.0 | – |
| LFM0434 | XXI | F. fujikuroi | 100.0 | – | 96.0 | – | 93.0 | – |
| LFM0435 | XXI | F. fujikuroi | 100.0 | – | 100.0 | – | 91.0 | – |
| LFM0447 | XXII | F. solani | 75.0 | – | 92.0 | – | 92.0 | – |
| LFM0448 | XXIII | F. fredkrugeri | 91.6 | – | 96.0 | – | 87.0 | – |
| LFM0449 | XXI | F. fujikuroi | 91.6 | – | 90.0 | – | 85.0 | – |
| Control | - | - | 0.0 | – | 0.0 | – | 0.0 | – |
| Strains | Isolation Source | Species (Type Material) | tef1-α (bp) | Accession Number | Identity (%) | Species Complex | Pathogenicity/Virulence 1 |
|---|---|---|---|---|---|---|---|
| LFM0464 | Trunk | F. casha | 657 | PV081902 | 99.39 | FFSC | ++ |
| LFM0466 | Trunk | F. casha | 657 | 99.39 | FFSC | + | |
| LFM0468 | Root | F. casha | 657 | 99.39 | FFSC | ++ | |
| LFM0469 | Trunk | F. casha | 657 | 99.39 | FFSC | + | |
| LFM0472 | Root | F. casha | 657 | 99.39 | FFSC | +++ | |
| LFM0475 | Trunk | F. casha | 657 | 99.39 | FFSC | - | |
| LFM0476 | Root | F. casha | 657 | 99.39 | FFSC | ++ | |
| LFM0477 | Root | F. casha | 657 | 99.39 | FFSC | ++ | |
| LFM0481 | Trunk | F. casha | 657 | 99.39 | FFSC | + | |
| LFM0483 | Trunk | F. casha | 657 | 99.39 | FFSC | + | |
| LFM0463 | Root | F. casha | 654 | PV081903 | 99.38 | FFSC | +++ |
| LFM0467 | Trunk | F. casha | 654 | 99.38 | FFSC | - | |
| LFM0459 | Trunk | F. fredkrugeri | 658 | PV081909 | 98.62 | FFSC | +++ |
| LFM0470 | Trunk | F. fredkrugeri | 658 | 98.62 | FFSC | ++ | |
| LFM0479 | Trunk | F. fredkrugeri | 655 | PV081910 | 98.61 | FFSC | ++ |
| LFM0460 | Root | F. inflexum | 581 | PV081881 | 99.66 | FOSC | ++ |
| LFM0478 | Root | F. inflexum | 581 | 99.66 | FOSC | ++ | |
| LFM0462 | Root | F. vaughaniae | 660 | PV081874 | 99.54 | FOSC | - |
| LFM0454 | Root | F. vaughaniae | 657 | PV081875 | 99.54 | FOSC | - |
| LFM0455 | Root | F. vaughaniae | 657 | 99.54 | FOSC | ++ | |
| LFM0456 | Root | F. vaughaniae | 657 | 99.54 | FOSC | - | |
| LFM0457 | Root | F. vaughaniae | 657 | 99.54 | FOSC | + | |
| LFM0458 | Root | F. vaughaniae | 657 | 99.54 | FOSC | - | |
| LFM0461 | Root | F. vaughaniae | 657 | 99.54 | FOSC | ++ | |
| LFM0471 | Root | F. vaughaniae | 657 | 99.54 | FOSC | ++ | |
| LFM0480 | Root | F. vaughaniae | 657 | 99.54 | FOSC | ++ | |
| LFM0465 | Root | F. inflexum | 613 | PV081882 | 100 | FOSC | + |
| LFM0473 | Root | F. inflexum | 613 | 100 | FOSC | + | |
| LFM0482 | Root | F. inflexum | 613 | 100 | FOSC | +++ | |
| LFM0474 | Trunk | F. brevicaudatum | 651 | PV081915 | 99.68 | FIESC | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.M.F.d.L.; Farias, O.R.d.; Araújo, B.C.L.; Rivera, A.V.; de Souza, C.R.; de Souza, J.T. A New Root and Trunk Rot Disease of Grapevine Plantlets Caused by Fusarium in Four Species Complexes. J. Fungi 2025, 11, 230. https://doi.org/10.3390/jof11030230
Cruz JMFdL, Farias ORd, Araújo BCL, Rivera AV, de Souza CR, de Souza JT. A New Root and Trunk Rot Disease of Grapevine Plantlets Caused by Fusarium in Four Species Complexes. Journal of Fungi. 2025; 11(3):230. https://doi.org/10.3390/jof11030230
Chicago/Turabian StyleCruz, José Manoel Ferreira de Lima, Otília Ricardo de Farias, Brunno Cassiano Lemos Araújo, Alejandra Valencia Rivera, Cláudia Rita de Souza, and Jorge Teodoro de Souza. 2025. "A New Root and Trunk Rot Disease of Grapevine Plantlets Caused by Fusarium in Four Species Complexes" Journal of Fungi 11, no. 3: 230. https://doi.org/10.3390/jof11030230
APA StyleCruz, J. M. F. d. L., Farias, O. R. d., Araújo, B. C. L., Rivera, A. V., de Souza, C. R., & de Souza, J. T. (2025). A New Root and Trunk Rot Disease of Grapevine Plantlets Caused by Fusarium in Four Species Complexes. Journal of Fungi, 11(3), 230. https://doi.org/10.3390/jof11030230







