Mr-lac3 and Mr-lcc2 in Metarhizium robertsii Regulate Conidiation and Maturation, Enhancing Tolerance to Abiotic Stresses and Pathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Bacterial Strains
2.1.1. Gene Deletion, Complementation, and Overexpression of Mr-lac3 and Mr-lcc2
2.1.2. Assays of Conidial Yield and Tolerance to Abiotic Stress
2.1.3. Assays of Trehalose Content
2.2. Bioassays
2.3. qRT-PCR
3. Results
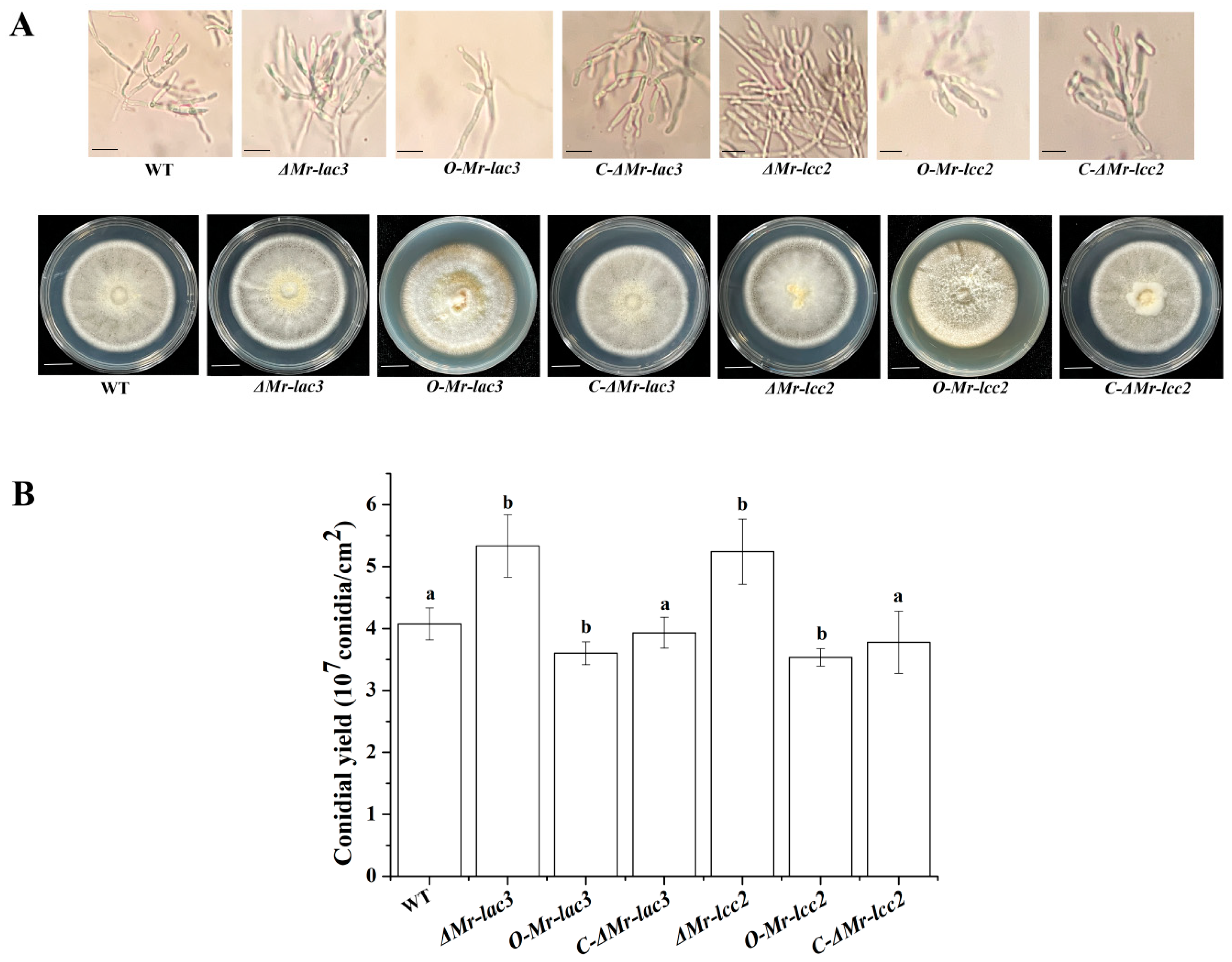
3.1. Construction of Mr-lac3 and Mr-lcc2 Disruption, Complementation, and Overexpression Strains
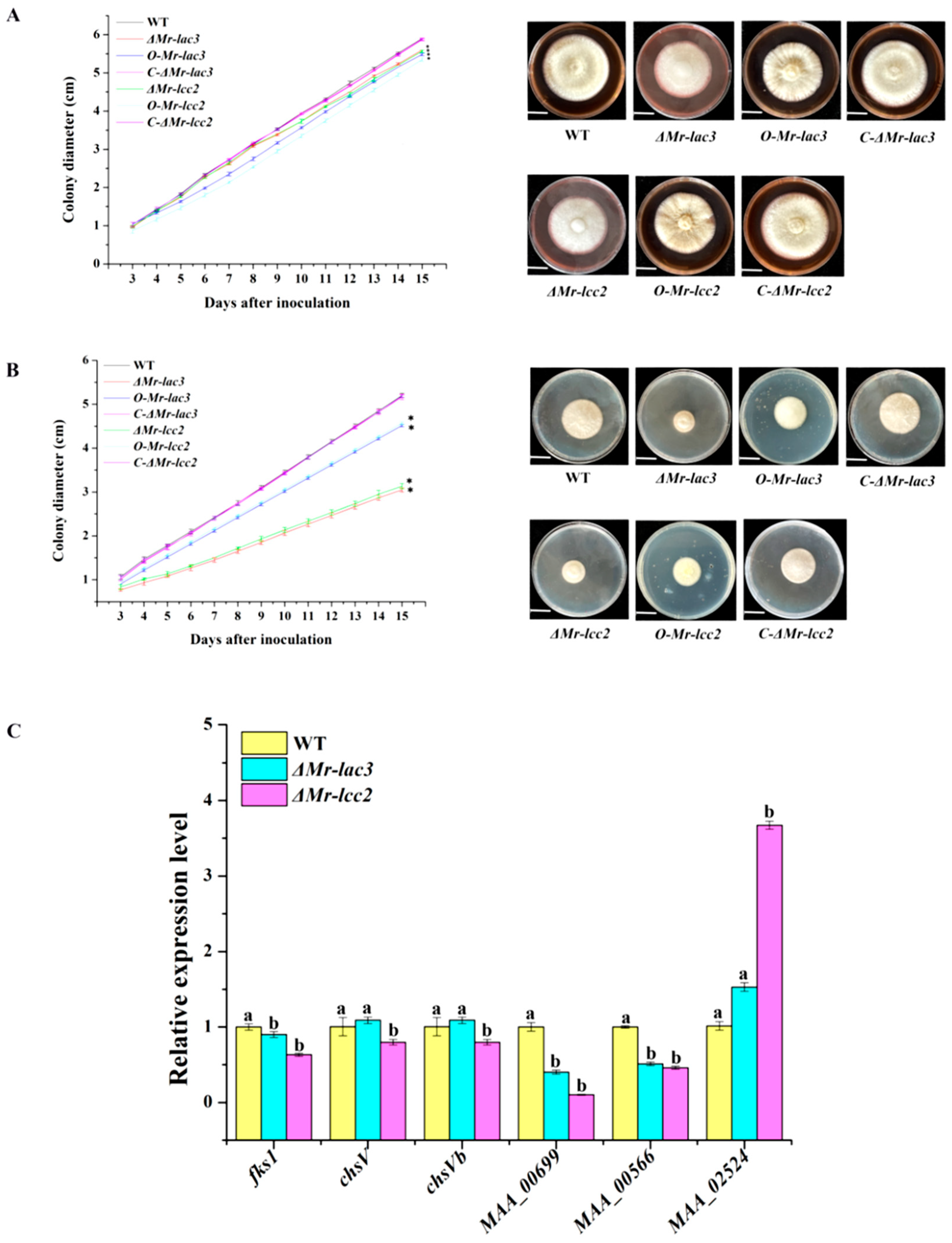
3.2. The Conidiophores and Conidial Yields of Mr-lac3 and Mr-lcc2 Disruption, Overexpression, and Complementation Strains
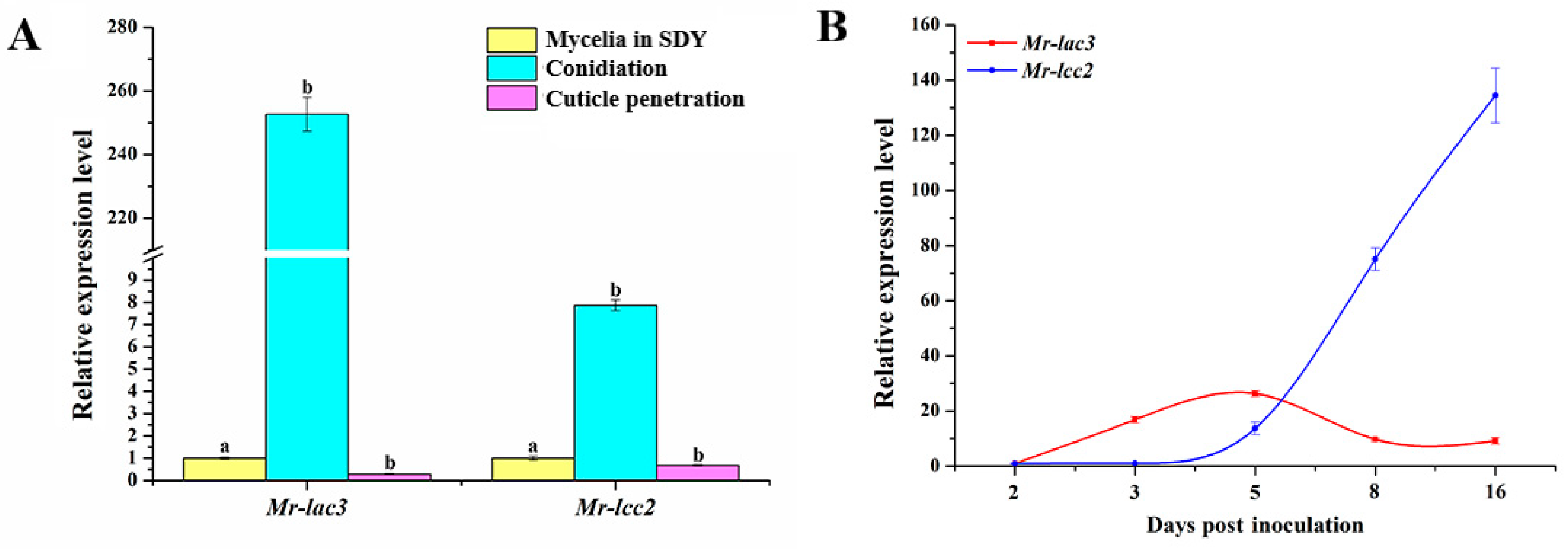
3.3. Expression Patterns of Mr-lac3 and Mr-lcc2 in M. robertsii
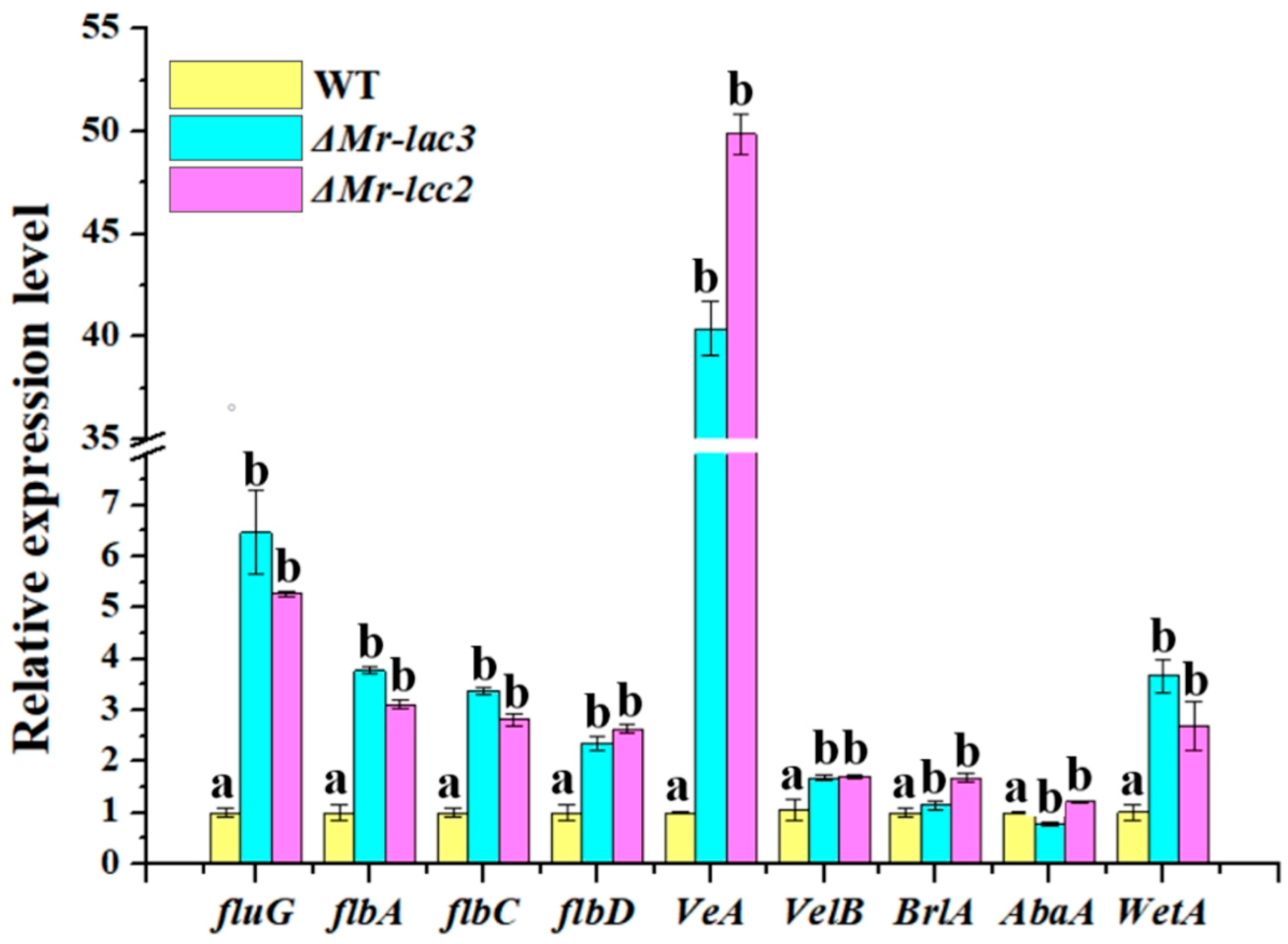
3.4. Mr-lac3 and Mr-lcc2 Negatively Regulated Conidiation
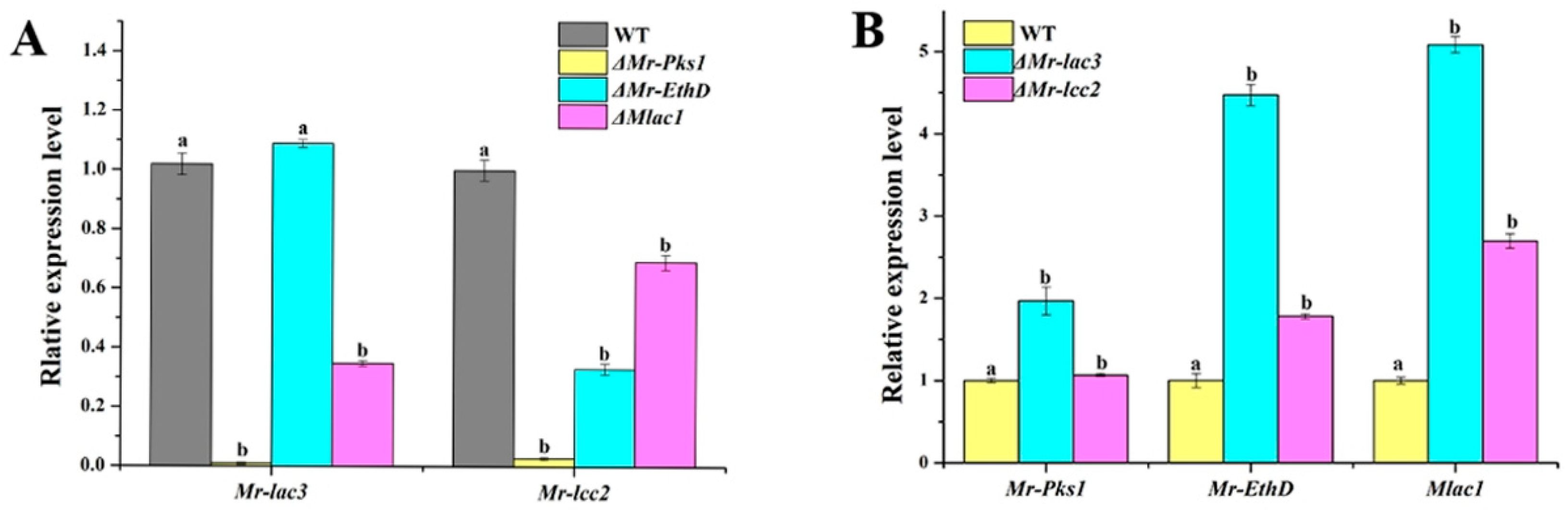
3.5. Mr-lac3 and Mr-lcc2 Participated in Synthesizing Conidial Pigment
3.6. Mr-lac3 and Mr-lcc2 Affect Cell Wall Integrity
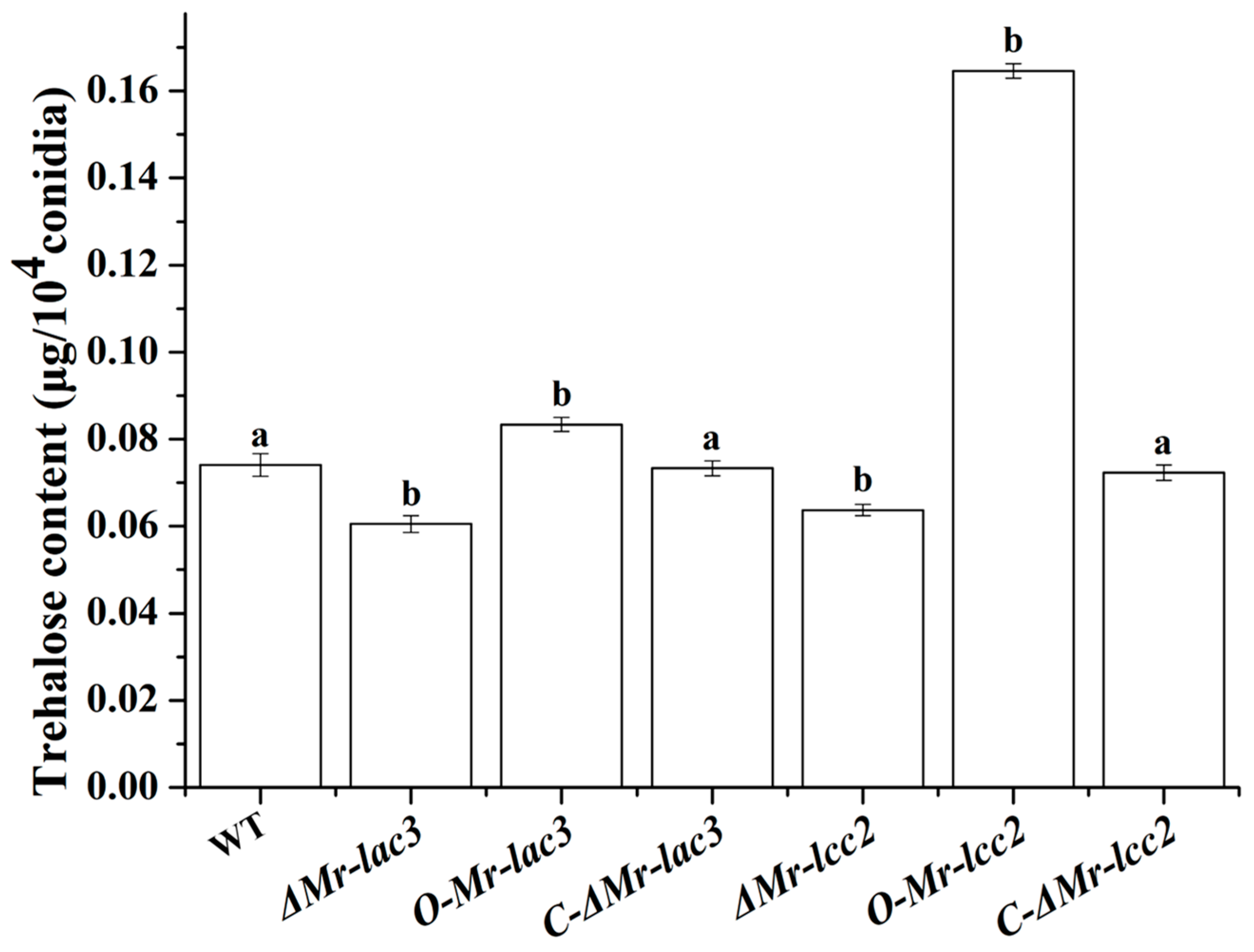
3.7. Mr-lac3 and Mr-lcc2 Are Involved in Conidial Trehalose Synthesis
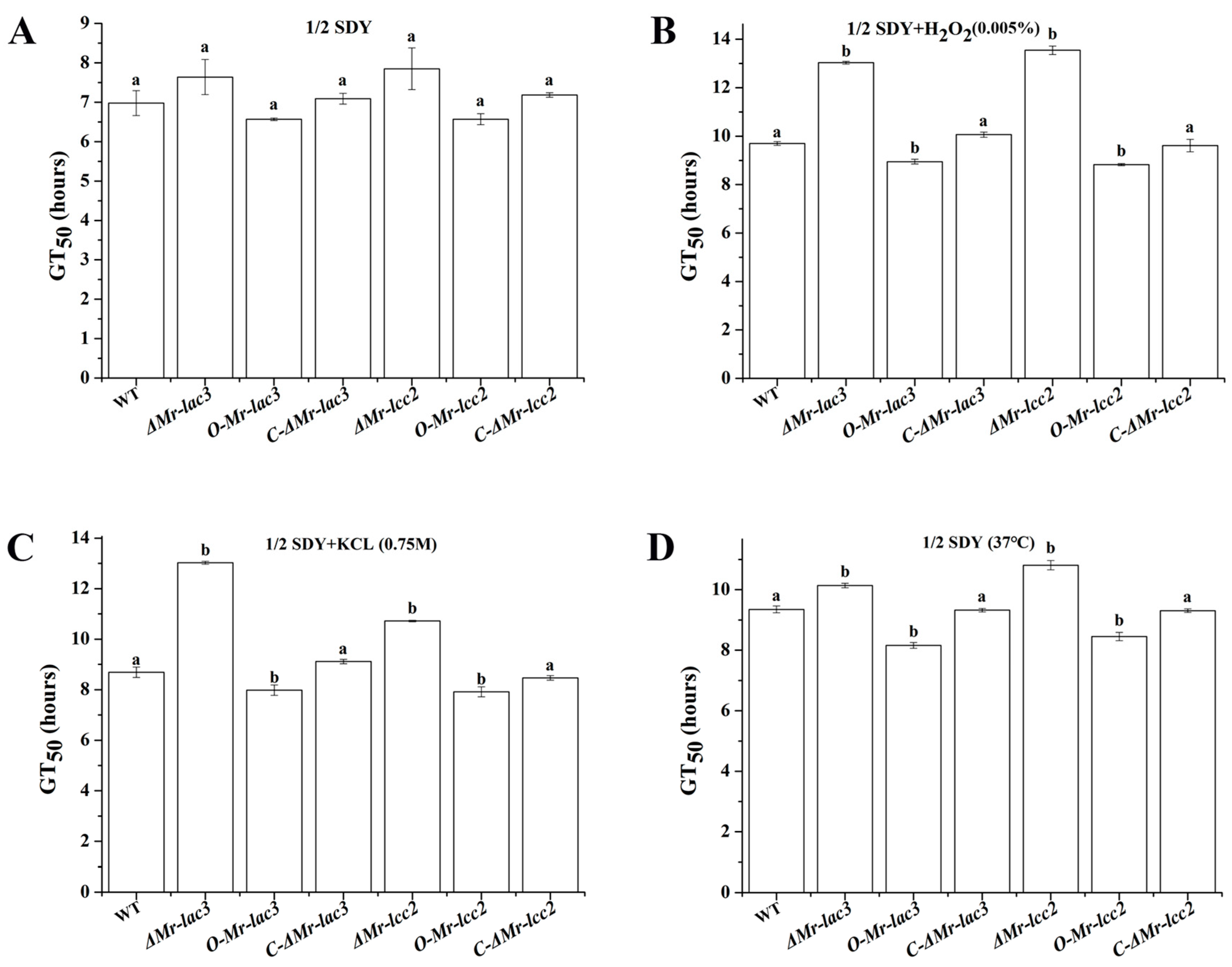
3.8. Tolerance of ΔMr-lac3 and ΔMr-lcc2 to Abiotic Stresses
3.9. Roles of Mr-lac3 and Mr-lcc2 in Pathogenicity
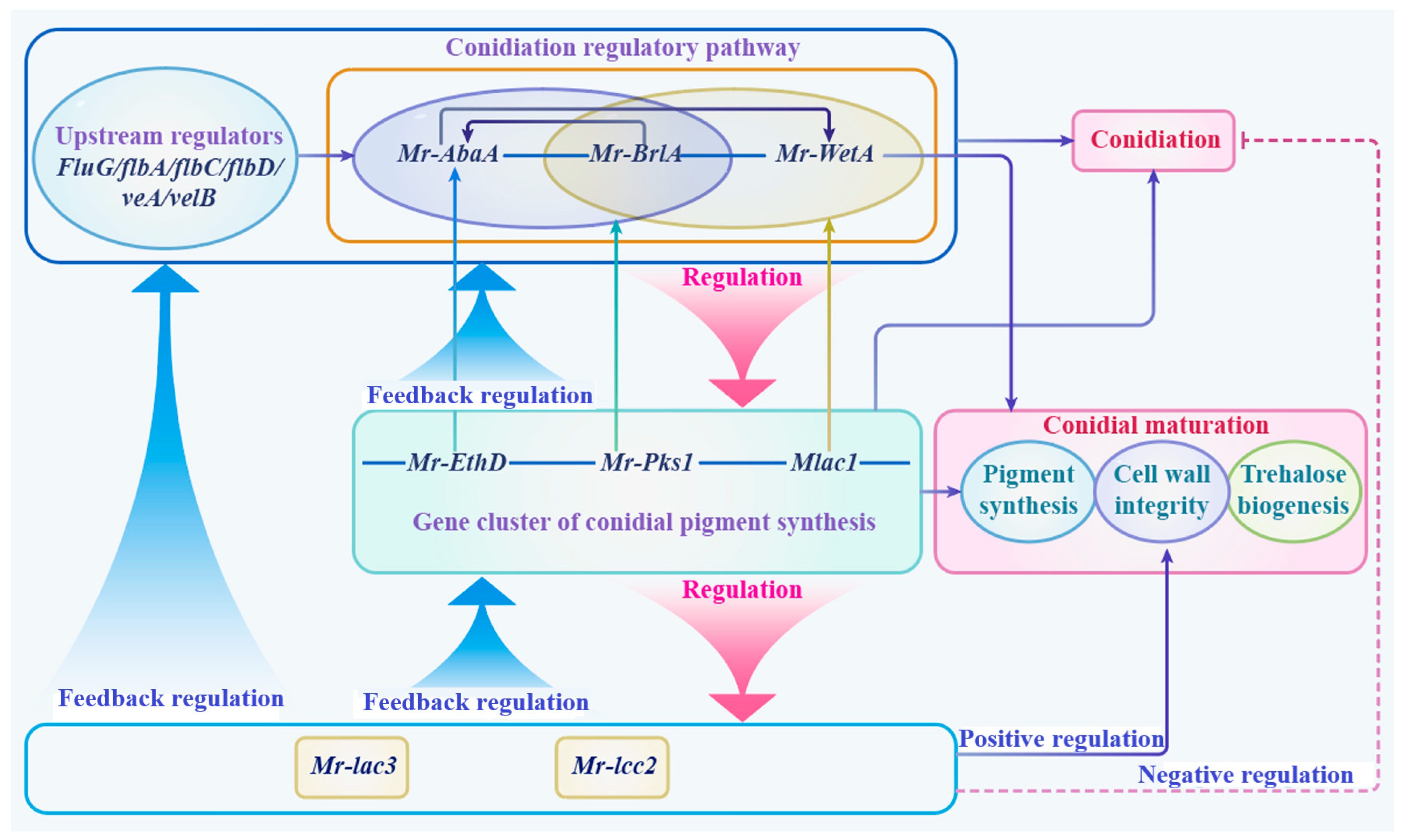
3.10. A Schematic Model of Function and the Regulation of Mr-lac3 and Mr-lcc2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A. Paszczyński Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Komori, H.; Higuchi, Y. Structural insights into the O2 reduction mechanism of multicopper oxidase. Biochemistry 2015, 158, 293–298. [Google Scholar]
- Ruiz-Duenas, F.J.; Martinez, A.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microbiol. Biot. 2009, 2, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Divya, L.; Sadasivan, C. Trichoderma viride laccase plays a crucial role in defense mechanism against antagonistic organisms. Front. Microbiol. 2016, 7, 741. [Google Scholar] [CrossRef] [PubMed]
- Schouten, A.; Maksimova, O.; Cuesta-Arenas, Y.; Van Den Berg, G.; Raaijmakers, J.M. Involvement of the ABC transporter BcAtrB and the laccase BcLCC2 in defence of Botrytis cinerea against the broad-spectrum antibiotic 2,4-diacetylphloroglucinol. Environ. Microbiol. 2008, 10, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J Bacteriol. 1994, 176, 656–664. [Google Scholar] [CrossRef]
- Salas, S.D.; Bennett, J.E.; won-Chung, K.J.; Perfect, K.J.; Williamson, R.P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef]
- Sapmak, A.; Kaewmalakul, J.; Nosanchuk, J.D.; Vanittanakom, N.; Andrianopoulos, A.; Pruksaphon, K.; Youngchim, S. Talaromyces marneffei laccase modifies THP-1 macrophage responses. Virulence 2016, 7, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Fan, L.; Gao, X.; Li, X.X.; Lin, M.; Luo, Q.; Li, H.L.; Wang, Z.K.; Wu, G.X. Mrlac1, an extracellular laccase, is required for conidial morphogenesis as well as the well adaptability in field of Metarhizium rileyi. Pestic. Biochem. Physiol. 2024, 205, 106161. [Google Scholar] [CrossRef]
- Wei, Y.; Pu, J.; Zhang, H.; Liu, Y.; Zhou, F.; Zhang, K.; Liu, X. The laccase gene (LAC1) is essential for Colletotrichum gloeosporioides development and virulence on mango leaves and fruits. Physiol. Mol. Plant Pathol. 2017, 99, 55–64. [Google Scholar] [CrossRef]
- Guo, N.; Qian, Y.; Zhang, Q. Alternative transcription start site selection in Mr-OPY2 controls lifestyle transitions in the fungus Metarhizium robertsii. Nat. Commun. 2017, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Azimzadeh, P.; St Leger, R.J. Strain improvement of fungal insecticides for controlling insect pests and vector-borne diseases. Curr. Opin. Microbiol. 2012, 15, 232–238. [Google Scholar] [CrossRef]
- Fang, W.; St Leger, R.J. RNA binding proteins mediate the ability of a fungus to adapt to the cold. Environ. Microbiol. 2010, 12, 810–820. [Google Scholar] [CrossRef]
- Fang, W.; St Leger, R.J. Enhanced UV resistance and improved killing of Malaria Mosquitoes by photolyase transgenic entomopathogenic fungi. PLoS ONE 2012, 7, e43069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; St Leger, R.J.; Fang, W. Pyruvate accumulation is the first line of cell defense against heat stress in a fungus. mBio 2017, 5, e01284-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; St Leger, R.J.; Fang, W. Stress-induced pyruvate accumulation contributes to cross protection in a fungus. Environ. Microbiol. 2018, 20, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Fernandes, E.K.K.; Roberts, D.W.; Bidochka, M.J.; St Leger, R.J. A laccase exclusively expressed by Metarhizium anisopliae during isotropic growth is involved in pigmentation, tolerance to abiotic stresses, and virulence. Fungal Genet Biol. 2010, 47, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Chen, X.; Zhang, X.; Zhang, Q.; Xu, C.; Mi, W.; Guo, N.; Zhao, H.; You, Y.; Dryburgh, F.J.; et al. Genome-wide identification of pathogenicity, conidiation and colony sectorization genes in Metarhizium robertsii. Environ. Microbiol. 2017, 19, 3896–3908. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Qian, Y.; Chen, X.; Liu, R.; Zeng, G.; Zhao, H.; Fang, W. A high-throughput gene disruption methodology for the entomopathogenic fungus Metarhizium robertsii. PLoS ONE 2014, 9, e107657. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Pei, Y.; Bidochka, M.J. A regulator of a G protein signalling (RGS) gene, Cag8, from the insect-pathogenic fungus Metarhizium anisopliae is involved in conidiation, virulence and hydrophobin synthesis. Microbiology 2007, 153, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, C.; Qian, Y.; Liu, R.; Zhang, Q.; Zeng, G.; Zhang, X.; Zhao, H.; Fang, W. MAPK cascade-mediated regulation of pathogenicity, conidiation and tolerance to abiotic stresses in the entomopathogenic fungus Metarhizium robertsii. Environ. Microbiol. 2016, 18, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Zhang, P.; Zhang, Q.; Zhao, H.; Li, Z.; Zhang, X. Duplication of a Pks gene cluster and subsequent functional diversification to facilitate environmental adaptation in Metarhizium species. PLoS Genet. 2018, 14, e1007472. [Google Scholar] [CrossRef]
- d’Enfert, C.; Fontaine, T. Molecular characterization of the Aspergillus nidulans treA gene encoding an acid trehalase required for growth on trehalose. Mol. Microbiol. 1997, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, C.; Lu, H.L.; Chen, X.; St Leger, R.J.; Fang, W. Host-to-pathogen gene transfer facilitated infection of insects by a pathogenic fungus. PLoS Pathog. 2014, 10, e1004009. [Google Scholar] [CrossRef]
- Park, H.S.; Yu, J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.F. Chapter two-fungal cell wall organization and biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar]
- Boylan, M.T.; Mirabito, P.M.; Willett, C.E.; Zimmerman, C.R.; Timberlake, W.E. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. J. Mol. Cell Biol. 1987, 7, 3113–3118. [Google Scholar]
- Mirabito, P.M.; Adams, T.H.; Timberlake, W.E. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell 1989, 57, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Gao, N.; Kwon, N.J.; Shin, K.S.; Yu, J.H. Regulation of Aspergillus conidiation. Cell. Mol. Biol. Filamentous Fungi 2010, 559–576. [Google Scholar] [CrossRef]
- Li, F.; Shi, H.; Ying, S.; Feng, M. WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen. Appl. Microbiol. Biot. 2015, 23, 10069–10081. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.; Butler, M.J.; Torabinejad, J.; Anderson, A.J.; Braga, G.U.; Day, A.W.; Roberts, D.W. Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. J. Invertebr. Pathol. 2006, 93, 170–182. [Google Scholar] [CrossRef] [PubMed]
- d’Enfert, C.; Bonini, B.M.; Zapella, P.D.A.; Fontaine, T.; da Silva, A.M.; Terenzi, H.F. Neutral trehalases catalyse intracellular trehalose breakdown in the filamentous fungi Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 1999, 32, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Suderman, R.J.; Dittmer, N.T.; Kanost, M.R.; Kramer, K.J. Model reactions for insect cuticle sclerotization: Cross-linking of recombinant cuticular proteins upon their laccase-catalyzed oxidative conjugation with catechols. Insect. Biochem. Mol. Biol. 2006, 36, 353–365. [Google Scholar] [CrossRef]
- Roncal, T.; Ugalde, U. Conidiation induction in Penicillium. Res. Microbiol. 2003, 154, 539–546. [Google Scholar] [CrossRef] [PubMed]

| Strains | Relative Germination Inhibition | ||
|---|---|---|---|
| H2O2 Stress | KCL Stress | Heat Stress | |
| WT | 0.39 ± 0.01 a | 0.25 ± 0.03 a | 0.34 ± 0.02 a |
| ΔMr-lac3 | 0.71 ± 0.01 b | 0.71 ± 0.01 b | 0.33 ± 0.01 a |
| O-Mr-lac3 | 0.36 ± 0.02 b | 0.22 ± 0.03 b | 0.24 ± 0.01 b |
| C-ΔMr-lac3 | 0.40 ± 0.01 a | 0.27 ± 0.01 a | 0.32 ± 0.01 a |
| ΔMr-lcc2 | 0.73 ± 0.02 b | 0.37 ± 0.01 b | 0.38 ± 0.02 b |
| O-Mr-lcc2 | 0.34 ± 0.01 b | 0.21 ± 0.03 b | 0.29 ± 0.02 b |
| C-ΔMr-lcc2 | 0.37 ± 0.01 a | 0.26 ± 0.01 a | 0.30 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Ye, Y.; Liu, Y.; He, Y.; Li, X.; Yang, S.; Xu, T.; Hu, X.; Zeng, G. Mr-lac3 and Mr-lcc2 in Metarhizium robertsii Regulate Conidiation and Maturation, Enhancing Tolerance to Abiotic Stresses and Pathogenicity. J. Fungi 2025, 11, 176. https://doi.org/10.3390/jof11030176
Wu Q, Ye Y, Liu Y, He Y, Li X, Yang S, Xu T, Hu X, Zeng G. Mr-lac3 and Mr-lcc2 in Metarhizium robertsii Regulate Conidiation and Maturation, Enhancing Tolerance to Abiotic Stresses and Pathogenicity. Journal of Fungi. 2025; 11(3):176. https://doi.org/10.3390/jof11030176
Chicago/Turabian StyleWu, Qiaoyun, Yingying Ye, Yiran Liu, Yufan He, Xing Li, Siqi Yang, Tongtong Xu, Xiufang Hu, and Guohong Zeng. 2025. "Mr-lac3 and Mr-lcc2 in Metarhizium robertsii Regulate Conidiation and Maturation, Enhancing Tolerance to Abiotic Stresses and Pathogenicity" Journal of Fungi 11, no. 3: 176. https://doi.org/10.3390/jof11030176
APA StyleWu, Q., Ye, Y., Liu, Y., He, Y., Li, X., Yang, S., Xu, T., Hu, X., & Zeng, G. (2025). Mr-lac3 and Mr-lcc2 in Metarhizium robertsii Regulate Conidiation and Maturation, Enhancing Tolerance to Abiotic Stresses and Pathogenicity. Journal of Fungi, 11(3), 176. https://doi.org/10.3390/jof11030176






