Biosynthesis of a Novel Diketopiperazine Aspkyncin Incorporating a Kynurenine Unit from Aspergillus aculeatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. General DNA Manipulation Techniques
2.3. Plasmids Construction
2.4. Protoplast Preparation and Transformation of A. nidulans
2.5. Chemical Analysis and Compound Isolation and Characterization
2.6. Expression and Purification of AacA and AacB
2.7. In Vitro Enzymatic Assays of AacA and AacB
2.8. RT-PCR of acc Cluster Genes
2.9. Bioinformatics Analysis
3. Results
3.1. Bioinformatic Analysis of the Biosynthetic Gene Cluster
3.2. Heterologous Expression of the aac Cluster and Characterization of the Products
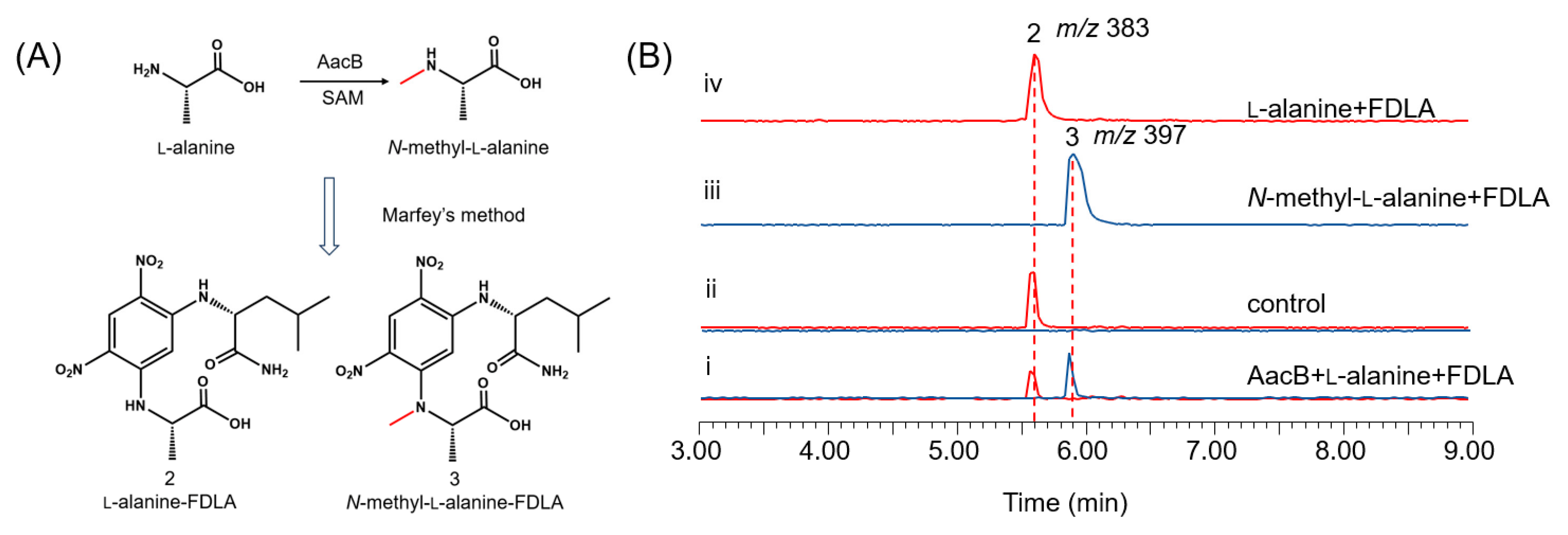
3.3. Characterization of AacB as an l-Alanine N-methyltransferase
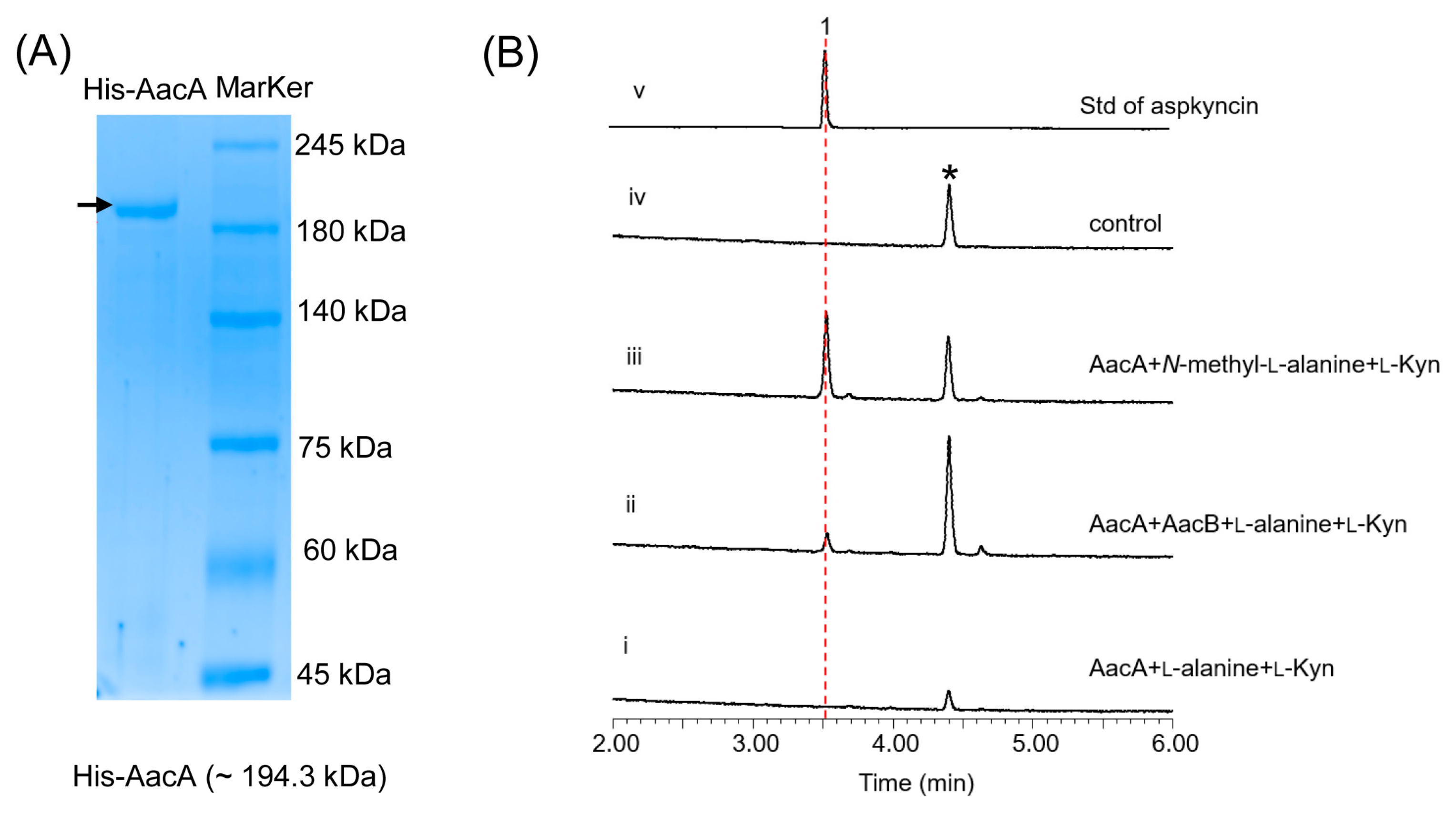
3.4. In Vitro Reconstitution of the AacA Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baccile, J.A.; Le, H.H.; Pfannenstiel, B.T.; Bok, J.W.; Gomez, C.; Brandenburger, E.; Hoffmeister, D.; Keller, N.P.; Schroeder, F.C. Diketopiperazine Formation in Fungi Requires Dedicated Cyclization and Thiolation Domains. Angew. Chem. Int. Ed. Engl. 2019, 58, 14589–14593. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.M.; Kers, J.A.; Johnson, E.G.; Song, L.; Aston, P.R.; Patel, B.; Krasnoff, S.B.; Crane, B.R.; Gibson, D.M.; Loria, R.; et al. Cytochrome P450-catalyzed L-tryptophan nitration in thaxtomin phytotoxin biosynthesis. Nat. Chem. Biol. 2012, 8, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Vior, N.M.; Lacret, R.; Chandra, G.; Dorai-Raj, S.; Trick, M.; Truman, A.W. Discovery and Biosynthesis of the Antibiotic Bicyclomycin in Distantly Related Bacterial Classes. Appl. Env. Microbiol. 2018, 84, e02828-17. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L.; Mollica, A.; Feliciani, F.; Pinnen, F. 2,5-diketopiperazines as neuroprotective agents. Mini-Rev. Med. Chem. 2012, 12, 2–12. [Google Scholar] [CrossRef]
- Canu, N.; Moutiez, M.; Belin, P.; Gondry, M. Cyclodipeptide synthases: A promising biotechnological tool for the synthesis of diverse 2,5-diketopiperazines. Nat. Prod. Rep. 2020, 37, 312–321. [Google Scholar] [CrossRef]
- Sussmuth, R.D.; Mainz, A. Nonribosomal Peptide Synthesis-Principles and Prospects. Angew. Chem. Int. Ed. Engl. 2017, 56, 3770–3821. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Goher, S.S.; Abdrabo, W.S.; Veerakanellore, G.B.; Elgendy, B. 2,5-Diketopiperazines (DKPs): Promising Scaffolds for Anticancer Agents. Curr. Pharm. Des. 2024, 30, 597–623. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Ingavat, N.; Dobereiner, J.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P.; Aspergillusol, A. An alpha-glucosidase inhibitor from the marine-derived fungus Aspergillus aculeatus. J. Nat. Prod. 2009, 72, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Ahuja, M.; Oakley, C.E.; Entwistle, R.; Asokan, A.; Zutz, C.; Wang, C.C.C.; Oakley, B.R. Development of Genetic Dereplication Strains in Aspergillus nidulans Results in the Discovery of Aspercryptin. Angew. Chem. Int. Ed. 2016, 55, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Du, L.; Lou, L. PKS and NRPS release mechanisms. Nat. Prod. Rep. 2010, 27, 255–278. [Google Scholar] [CrossRef]
- Yuasa, H.J.; Ball, H.J. Molecular evolution and characterization of fungal indoleamine 2,3-dioxygenases. J. Mol. Evol. 2011, 72, 160–168. [Google Scholar] [CrossRef]
- Skellam, E. Strategies for Engineering Natural Product Biosynthesis in Fungi. Trends Biotechnol. 2019, 37, 416–427. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.W.; Liu, Y.Y.; Matsuda, Y. Aspcandine: A Pyrrolobenzazepine Alkaloid Synthesized by a Fungal Nonribosomal Peptide Synthetase-Polyketide Synthase Hybrid. Org. Lett. 2022, 24, 4816–4819. [Google Scholar] [CrossRef]
- Li, H.; Gilchrist, C.L.M.; Phan, C.S.; Lacey, H.J.; Vuong, D.; Moggach, S.A.; Lacey, E.; Piggott, A.M.; Chooi, Y.H. Biosynthesis of a New Benzazepine Alkaloid Nanangelenin A from Aspergillus nanangensis Involves an Unusual l-Kynurenine-Incorporating NRPS Catalyzing Regioselective Lactamization. J. Am. Chem. Soc. 2020, 142, 7145–7152. [Google Scholar] [CrossRef]
- Miao, V.; Coeffet-LeGal, M.F.; Brian, P.; Brost, R.; Penn, J.; Whiting, A.; Martin, S.; Ford, R.; Parr, I.; Bouchard, M.; et al. Daptomycin biosynthesis in Streptomyces roseosporus: Cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 2005, 151 Pt 5, 1507–1523. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Ravel, J. Chapter 8 Methods for In Silico Prediction of Microbial Polyketide and Nonribosomal Peptide Biosynthetic Pathways from DNA Sequence Data. In Complex Enzymes in Microbial Natural Product Biosynthesis, Part A: Overview Articles and Peptides; Elsevier: Amsterdam, The Netherlands, 2009; pp. 181–217. [Google Scholar] [CrossRef]
- Stachelhaus, T.; Mootz, H.D.; Marahiel, M.A. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 1999, 6, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.X.; Chen, L.; Tang, M.C. Genome Mining Discovery of a New Benzazepine Alkaloid Pseudofisnin A from the Marine Fungus Neosartorya pseudofischeri F27-1. Antibiotics 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Kalb, D.; Lackner, G.; Hoffmeister, D. Fungal peptide synthetases: An update on functions and specificity signatures. Fungal Biol. Rev. 2013, 27, 43–50. [Google Scholar] [CrossRef]
- Gao, X.; Haynes, S.W.; Ames, B.D.; Wang, P.; Vien, L.P.; Walsh, C.T.; Tang, Y. Cyclization of fungal nonribosomal peptides by a terminal condensation-like domain. Nat. Chem. Biol. 2012, 8, 823–830. [Google Scholar] [CrossRef]
- Eisfeld, K. Non-Ribosomal Peptide Synthetases of Fungi. In Physiology and Genetics, 1st ed.; Anke, T., Weber, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 15, pp. 315–316. [Google Scholar]
- Maiya, S.; Grundmann, A.; Li, S.M.; Turner, G. The Fumitremorgin Gene Cluster of Aspergillus fumigatus: Identification of a Gene Encoding Brevianamide F Synthetase. ChemBioChem 2006, 7, 1062–1069. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Cozijnsen, A.J.; Wilson, L.M.; Pedras, M.S.C.; Howlett, B.J. The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus Leptosphaeria maculans. Mol. Microbiol. 2004, 53, 1307–1318. [Google Scholar] [CrossRef]
- Balibar, C.J.; Walsh, C.T.J.B. GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochemistry 2006, 45, 15029–15038. [Google Scholar] [CrossRef]
- Ogawa, H.; Gomi, T.; Takusagawa, F.; Fujioka, M. Structure, function and physiological role of glycine N-methyltransferase. Int J. Biochem. Cell Biol. 1998, 30, 13–26. [Google Scholar] [CrossRef]
- Velkov, T.; Horne, J.; Scanlon, M.J.; Capuano, B.; Yuriev, E.; Lawen, A. Characterization of the N-methyltransferase activities of the multifunctional polypeptide cyclosporin synthetase. Chem. Biol. 2011, 18, 464–475. [Google Scholar] [CrossRef]
- Shi, R.; Lamb, S.S.; Zakeri, B.; Proteau, A.; Cui, Q.; Sulea, T.; Matte, A.; Wright, G.D.; Cygler, M. Structure and function of the glycopeptide N-methyltransferase MtfA, a tool for the biosynthesis of modified glycopeptide antibiotics. Chem. Biol. 2009, 16, 401–410. [Google Scholar] [CrossRef]
- Beck, J.G.; Chatterjee, J.; Laufer, B.; Kiran, M.U.; Frank, A.O.; Neubauer, S.; Ovadia, O.; Greenberg, S.; Gilon, C.; Hoffman, A.; et al. Intestinal permeability of cyclic peptides: Common key backbone motifs identified. J. Am. Chem. Soc. 2012, 134, 12125–12133. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-methylation of peptides and proteins: An important element for modulating biological functions. Angew. Chem. Int. Ed. Engl. 2013, 52, 254–269. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Wang, X.; Liu, L. Biosynthesis of a Novel Diketopiperazine Aspkyncin Incorporating a Kynurenine Unit from Aspergillus aculeatus. J. Fungi 2025, 11, 171. https://doi.org/10.3390/jof11030171
Kong D, Wang X, Liu L. Biosynthesis of a Novel Diketopiperazine Aspkyncin Incorporating a Kynurenine Unit from Aspergillus aculeatus. Journal of Fungi. 2025; 11(3):171. https://doi.org/10.3390/jof11030171
Chicago/Turabian StyleKong, Dekun, Xin Wang, and Li Liu. 2025. "Biosynthesis of a Novel Diketopiperazine Aspkyncin Incorporating a Kynurenine Unit from Aspergillus aculeatus" Journal of Fungi 11, no. 3: 171. https://doi.org/10.3390/jof11030171
APA StyleKong, D., Wang, X., & Liu, L. (2025). Biosynthesis of a Novel Diketopiperazine Aspkyncin Incorporating a Kynurenine Unit from Aspergillus aculeatus. Journal of Fungi, 11(3), 171. https://doi.org/10.3390/jof11030171







