Epigenetic Regulation of Fungal Secondary Metabolism
Abstract
1. Introduction
2. Secondary Metabolism in Fungi
3. Epigenetic Regulation
3.1. DNA Methylation
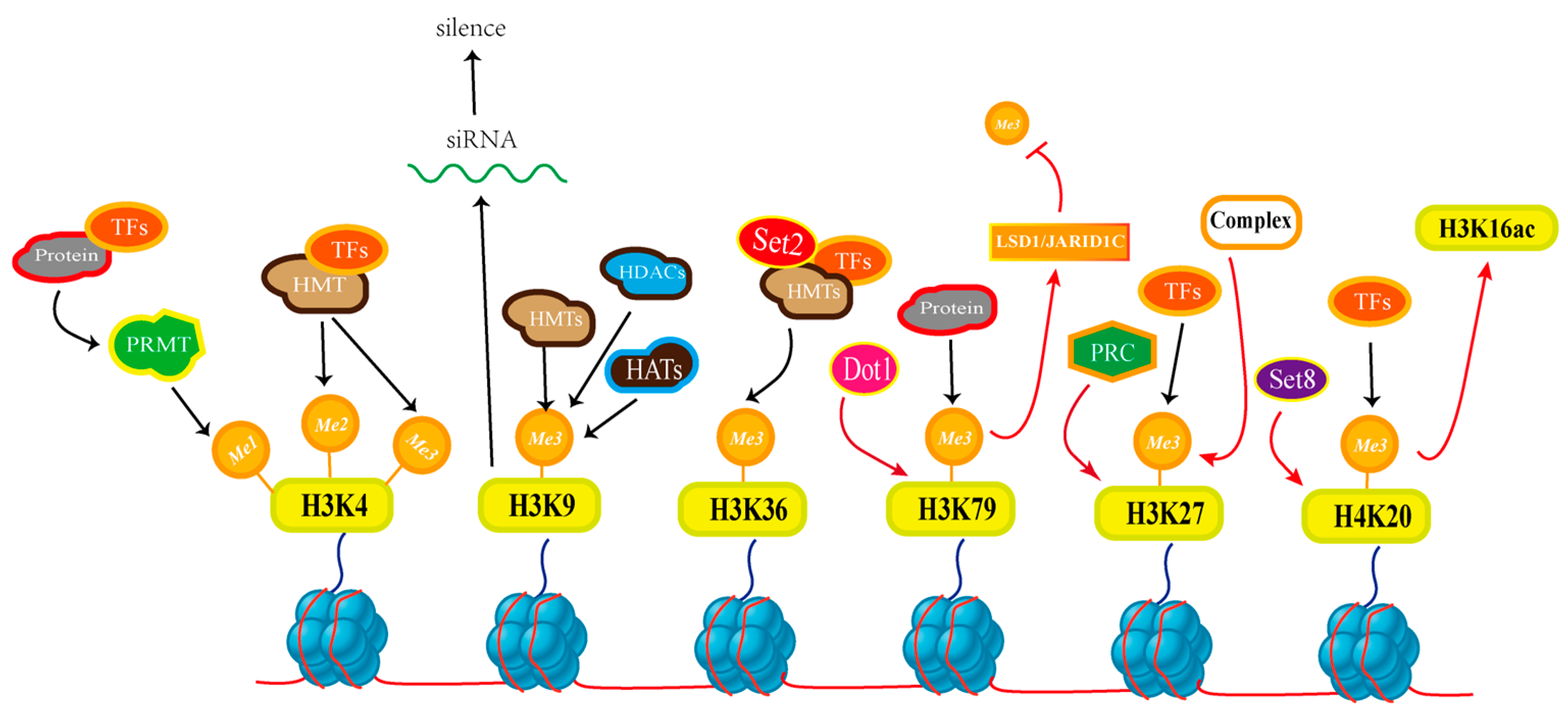
3.2. Histone Methylation
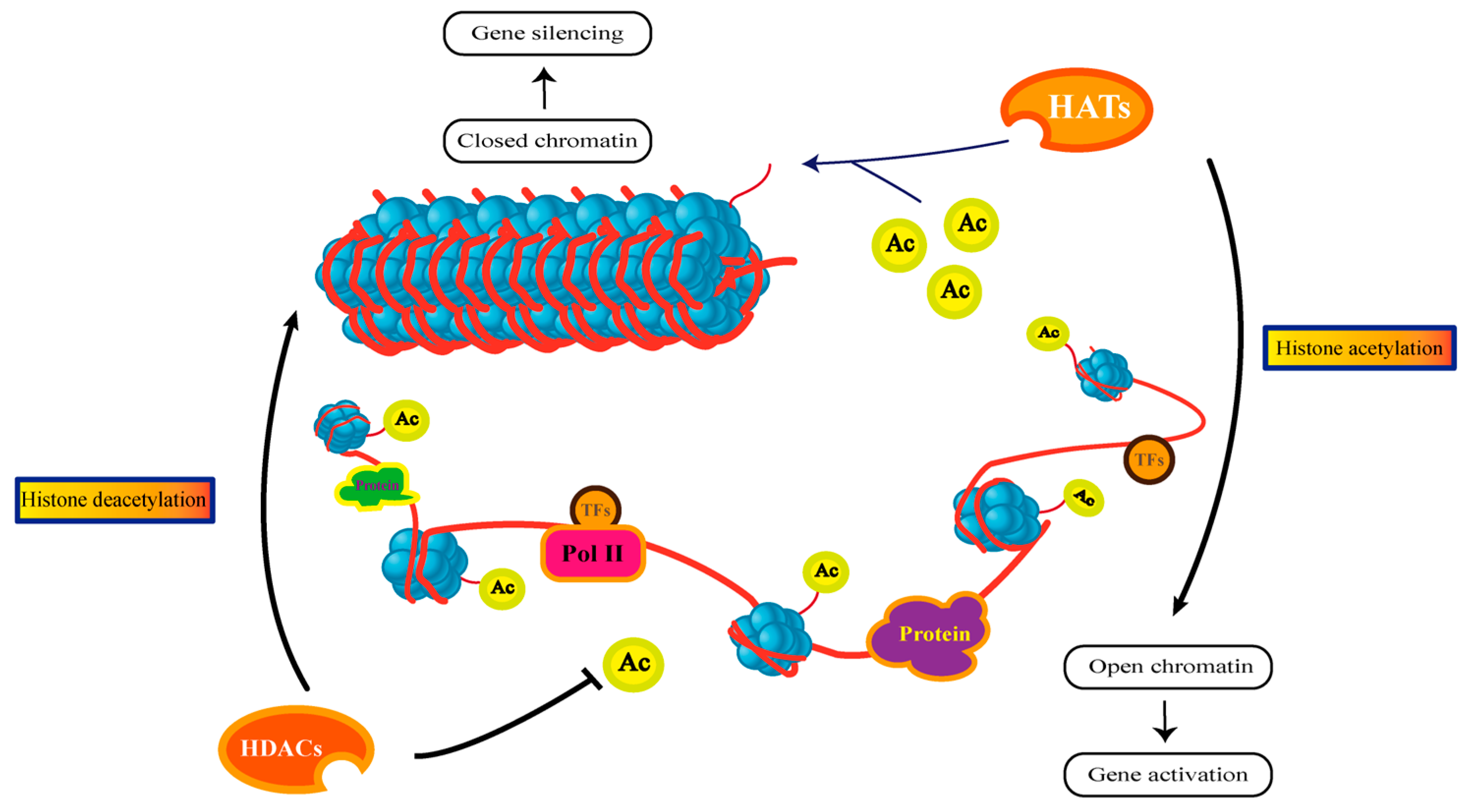
3.3. Histone Acetylation
3.4. Other Epigenetic Regulation
4. Cross-Regulation of Secondary Metabolism by Epigenetic and Global Regulation
4.1. LaeA
4.2. GcnE
4.3. SirE/Hst4, SirB/Hst2
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tebbi, C.K. Mycoviruses in Fungi: Carcinogenesis of Fungal Agents May Not Always Be Mycotoxin Related. J. Fungi 2023, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.R.; Nielsen, J.B.; Klitgaard, A.; Petersen, L.M.; Zachariasen, M.; Hansen, T.J.; Blicher, L.H.; Gotfredsen, C.H.; Larsen, T.O.; Nielsen, K.F.; et al. Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc. Natl. Acad. Sci. USA 2013, 110, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, Z.; Zhu, W.; Liu, Y.; Bai, X.; Zhang, H. Fusarium-Derived Secondary Metabolites with Antimicrobial Effects. Molecules 2023, 28, 3424. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Yu, W.; Pei, R.; Zhou, J.; Zeng, B.; Tu, Y.; He, B. Molecular regulation of fungal secondary metabolism. World J. Microbiol. Biotechnol. 2023, 39, 204. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Q. Secondary Metabolites of Purpureocilliumlilacinum. Molecules 2021, 27, 18. [Google Scholar] [CrossRef]
- Negreiros de Carvalho, P.L.; Silva, E.d.O.; Chagas-Paula, D.A.; Hortolan Luiz, J.H.; Ikegaki, M. Importance and Implications of the Production of Phenolic Secondary Metabolites by Endophytic Fungi: A Mini-Review. Mini-Rev. Med. Chem. 2015, 16, 259–271. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, S.; Sarma, S.J. Challenges and advancements in bioprocess intensification of fungal secondary metabolite: Kojic acid. World J. Microbiol. Biotechnol. 2023, 39, 140. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Wang, B.-G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016, 80, 301–342. [Google Scholar] [CrossRef]
- Dourou, M.; La Porta, C.A.M. A Pipeline to Investigate Fungal–Fungal Interactions: Trichoderma Isolates against Plant-Associated Fungi. J. Fungi 2023, 9, 461. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a Potential Source of Pigments: Harnessing Filamentous Fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2007, 7, 89–123. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 1, 992. [Google Scholar] [CrossRef]
- Panwar, N.; Szczepaniec, A. Endophytic entomopathogenic fungi as biological control agents of insect pests. Pest Manag. Sci. 2024; ahead of print. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.-Y.; Nong, X.-H.; Qi, S.-H. New Furanone Derivatives and Alkaloids from the Co-Culture of Marine-Derived Fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2016, 14, e1600327. [Google Scholar] [CrossRef]
- Moubasher, H.; Elkholy, A.; Sherif, M.; Zahran, M.; Elnagdy, S. In Vitro Investigation of the Impact of Bacterial–Fungal Interaction on Carbapenem-Resistant Klebsiella pneumoniae. Molecules 2022, 27, 2541. [Google Scholar] [CrossRef] [PubMed]
- Lynch, F.; Lewis, S.; Macciocca, I.; Craig, J.M. Public knowledge and opinion of epigenetics and epigenetic concepts. J. Dev. Orig. Health Dis. 2021, 13, 431–440. [Google Scholar] [CrossRef]
- Gina, F.L.; Avril, M.H.; Mekala, S.; Tonia, S.S.; Mark, R.C.; DeWoody, J.A.; Janna, R.W. Epigenetics in Ecology, Evolution, and Conservation. Front. Ecol. Evol. 2022, 10, 871791. [Google Scholar] [CrossRef]
- Li, C.-Y.; Chung, Y.-M.; Wu, Y.-C.; Hunyadi, A.; Wang, C.C.C.; Chang, F.-R. Natural products development under epigenetic modulation in fungi. Phytochem. Rev. 2020, 19, 1323–1340. [Google Scholar] [CrossRef]
- Mishra, R.; Kushveer, J.S.; Majumder, D.; Sarma, V.V. Stimulation of secondary metabolite production in Hypoxylon anthochroum by naturally occurring epigenetic modifiers. J. Food Meas. Charact. 2019, 14, 946–962. [Google Scholar] [CrossRef]
- Qadri, M.; Nalli, Y.; Jain, S.K.; Chaubey, A.; Ali, A.; Strobel, G.A.; Vishwakarma, R.A.; Riyaz-Ul-Hassan, S. An Insight into the Secondary Metabolism of Muscodor yucatanensis: Small-Molecule Epigenetic Modifiers Induce Expression of Secondary Metabolism-Related Genes and Production of New Metabolites in the Endophyte. Microb. Ecol. 2016, 73, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, Z.; Tang, J.; Wu, J.; Tong, J.; Li, M.; Ju, J.; Chen, H.; Wang, L. Identification and Biological Evaluation of Secondary Metabolites from Marine Derived Fungi-Aspergillus sp. SCSIOW3, Cultivated in the Presence of Epigenetic Modifying Agents. Molecules 2017, 22, 1302. [Google Scholar] [CrossRef]
- VanderMolen, K.M.; Darveaux, B.A.; Chen, W.-L.; Swanson, S.M.; Pearce, C.J.; Oberlies, N.H. Epigenetic Manipulation of a Filamentous Fungus by the Proteasome-Inhibitor Bortezomib Induces the Production of an Additional Secondary Metabolite. RSC Adv. 2014, 4, 18329–18335. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zou, Z.-M. Discovery of New Secondary Metabolites by Epigenetic Regulation and NMR Comparison from the Plant Endophytic Fungus Monosporascus eutypoides. Molecules 2020, 25, 4192. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lo, I.W.; Hsueh, Y.P.; Chung, Y.M.; Wang, S.W.; Korinek, M.; Tsai, Y.H.; Cheng, Y.B.; Hwang, T.L.; Wang, C.C.C.; et al. Epigenetic Manipulation Induces the Production of Coumarin-Type Secondary Metabolite from Arthrobotrys foliicola. Isr. J. Chem. 2019, 59, 432–438. [Google Scholar] [CrossRef]
- Conte, M.; Fontana, E.; Nebbioso, A.; Altucci, L. Marine-Derived Secondary Metabolites as Promising Epigenetic Bio-Compounds for Anticancer Therapy. Mar. Drugs 2020, 19, 15. [Google Scholar] [CrossRef]
- Yin, W.; Keller, N.P. Transcriptional regulatory elements in fungal secondary metabolism. J. Microbiol. 2011, 49, 329–339. [Google Scholar] [CrossRef]
- Shabana, S.; Lakshmi, K.R.; Satya, A.K. An Updated Review of Secondary Metabolites from Marine Fungi. Mini-Rev. Med. Chem. 2021, 21, 602–642. [Google Scholar] [CrossRef]
- Asare, M.O.; Száková, J.; Tlustoš, P. The fate of secondary metabolites in plants growing on Cd-, As-, and Pb-contaminated soils—A comprehensive review. Environ. Sci. Pollut. Res. 2022, 30, 11378–11398. [Google Scholar] [CrossRef]
- Wang, J.-T.; Shi, T.-T.; Ding, L.; Xie, J.; Zhao, P.-J. Multifunctional Enzymes in Microbial Secondary Metabolic Processes. Catalysts 2023, 13, 581. [Google Scholar] [CrossRef]
- Marfil-Santana, M.D.; Martínez-Cárdenas, A.; Ruíz-Hernández, A.; Vidal-Torres, M.; Márquez-Velázquez, N.A.; Figueroa, M.; Prieto-Davó, A. A Meta-Omics Analysis Unveils the Shift in Microbial Community Structures and Metabolomics Profiles in Mangrove Sediments Treated with a Selective Actinobacterial Isolation Procedure. Molecules 2021, 226, 7332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, H.; Chen, B.; Dai, H.; Sun, J.; Han, J.; Liu, H. Discovery of Anti-MRSA Secondary Metabolites from a Marine-Derived Fungus Aspergillus fumigatus. Mar. Drugs 2022, 20, 302. [Google Scholar] [CrossRef] [PubMed]
- Anh, C.V.; Yoon, Y.D.; Kang, J.S.; Lee, H.-S.; Heo, C.-S.; Shin, H.J. Nitrogen-Containing Secondary Metabolites from a Deep-Sea Fungus Aspergillus unguis and Their Anti-Inflammatory Activity. Mar. Drugs 2022, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhang, J.-L.; Li, C.; Mu, X.-G.; Liu, X.-L.; Wang, L.; Zhao, Y.-C.; Zhang, P.; Li, X.-D.; Zhang, X.-X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules 2019, 20, 4596. [Google Scholar] [CrossRef] [PubMed]
- Kianfé, B.Y.; Tchamgoue, J.; Narmani, A.; Teponno, R.B.; Njouonkou, A.-L.; Stadler, M.; Fogue Kouam, S. Bioactive Secondary Metabolites from Fungi of the Genus Cytospora Ehrenb. (Ascomycota). Molecules 2023, 228, 3120. [Google Scholar] [CrossRef]
- Saad, M.M.G.; Abdelgaleil, S.A.M.; Shiono, Y. Antibacterial and herbicidal properties of secondary metabolites from fungi. Nat. Prod. Res. 2020, 35, 5446–5451. [Google Scholar] [CrossRef]
- Kälvö, D.; Menkis, A.; Broberg, A. Secondary Metabolites from the Root Rot Biocontrol Fungus Phlebiopsis gigantea. Molecules 2018, 23, 1417. [Google Scholar] [CrossRef]
- Zhang, L.; Fasoyin, O.E.; Molnár, I.; Xu, Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020, 37, 1181–1206. [Google Scholar] [CrossRef]
- Iida, Y.; Higashi, Y.; Nishi, O.; Kouda, M.; Maeda, K.; Yoshida, K.; Asano, S.; Kawakami, T.; Nakajima, K.; Kuroda, K.; et al. Entomopathogenic fungus Beauveria bassiana–based bioinsecticide suppresses severity of powdery mildews of vegetables by inducing the plant defense responses. Front. Plant Sci. 2023, 14, 1211825. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2021, 369, 130927. [Google Scholar] [CrossRef]
- Kim, K.; Yim, W.; Trivedi, P.; Madhaiyan, M.; Deka Boruah, H.P.; Islam, M.R.; Lee, G.; Sa, T. Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacterium oryzae strains on growth and nutrient uptake of red pepper (Capsicum annuum L.). Plant Soil 2009, 327, 429–440. [Google Scholar] [CrossRef]
- Valiante, V. The Cell Wall Integrity Signaling Pathway and Its Involvement in Secondary Metabolite Production. J. Fungi 2018, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, T.; Nepal, B.; Lorber, S.; Puel, O.; Calvo, A.M. The Transcriptional Regulator HbxA Governs Development, Secondary Metabolism, and Virulence in Aspergillus fumigatus. Appl. Environ. Microbiol. 2020, 18, e01779-19. [Google Scholar] [CrossRef]
- Patra, S.; Raney, M.; Pareek, A.; Kaur, R. Epigenetic Regulation of Antifungal Drug Resistance. J. Fungi 2022, 8, 875. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Pan, L.; Wang, B.; Pan, L. CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite. Microbiol. Res. 2021, 245, 126694. [Google Scholar] [CrossRef]
- Strauss, J.; Reyes-Dominguez, Y. Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet. Biol. 2010, 48, 62–69. [Google Scholar] [CrossRef]
- Han, M.; Jia, L.; Lv, W.; Wang, L.; Cui, W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kulkarni, M.G.; White, J.F.; Van Staden, J. Epigenetic-based developments in the field of plant endophytic fungi. South Afr. J. Bot. 2020, 134, 394–400. [Google Scholar] [CrossRef]
- Bind, S.; Bind, S.; Sharma, A.K.; Chaturvedi, P. Epigenetic Modification: A Key Tool for Secondary Metabolite Production in Microorganisms. Front. Microbiol. 2022, 13, 78410. [Google Scholar] [CrossRef]
- Ahrodia, T.; Kandiyal, B.; Das, B. Microbiota and epigenetics: Health impact. Prog. Mol. Biol. Transl. Sci. 2023, 198, 93–117. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef] [PubMed]
- Vanheer, L.N.; Zhang, H.; Lin, G.; Kafsack, B.F.C. Activity of Epigenetic Inhibitors against Plasmodium falciparum Asexual and Sexual Blood Stages. Antimicrob. Agents Chemother. 2020, 64, e02523-19. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Jeon, J. Epigenetic regulation of development and pathogenesis in fungal plant pathogens. Mol. Plant Pathol. 2016, 18, 887–898. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, D.; Miao, Y. Epigenetic control of plant senescence and cell death and its application in crop improvement. Front. Plant Sci. 2023, 14, 1258487. [Google Scholar] [CrossRef]
- Chen, X.; Moran Torres, J.P.; Li, Y.; Lugones, L.G.; Wösten, H.A.B. Inheritable CRISPR based epigenetic modification in a fungus. Microbiol. Res. 2023, 272, 127397. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, Y.; Liu, J.; Liu, W.; Xing, Y.; Xiu, Z.; Dong, Y. Metabolomic Strategy to Characterize the Profile of Secondary Metabolites in Aspergillus aculeatus DL1011 Regulated by Chemical Epigenetic Agents. Molecules 2022, 28, 218. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Wei, C.; Deng, X.; Xu, J. Chemical epigenetic modifiers enhance the production of immunosuppressants from the endophytic fungus Aspergillus fumigatus isolated from Cynodon dactylon. Nat. Prod. Res. 2021, 36, 4481–4485. [Google Scholar] [CrossRef]
- Adpressa, D.A.; Stalheim, K.J.; Proteau, P.J.; Loesgen, S. Unexpected Biotransformation of the HDAC Inhibitor Vorinostat Yields Aniline-Containing Fungal Metabolites. ACS Chem. Biol. 2017, 12, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.; Chi, X.; Jia, Z.; Shi, C.; Zhang, X.; Li, Q.; Gao, T.; Zhang, L.; Liu, Z. NSD3: Advances in cancer therapeutic potential and inhibitors research. Eur. J. Med. Chem. 2023, 256, 115440. [Google Scholar] [CrossRef]
- Ye, F.; Huang, J.; Wang, H.; Luo, C.; Zhao, K. Targeting epigenetic machinery: Emerging novel allosteric inhibitors. Pharmacol. Ther. 2019, 204, 107406. [Google Scholar] [CrossRef]
- Terranova-Barberio, M.; Thomas, S.; Munster, P.N. Epigenetic modifiers in immunotherapy: A focus on checkpoint inhibitors. Immunotherapy 2016, 8, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Karaman Mayack, B.; Valente, S.; Battistelli, C. Editorial: Natural Product Epigenetic Modulators and Inhibitors. Front. Pharmacol. 2021, 12, 651395. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, N.; Li, C.; Tu, J.; Li, Z.; Sheng, C. Discovery of Novel Fungal Lanosterol 14α-Demethylase (CYP51)/Histone Deacetylase Dual Inhibitors to Treat Azole-Resistant Candidiasis. J. Med. Chem. 2020, 63, 5341–5359. [Google Scholar] [CrossRef]
- Gryzinska, M.; Wlazlo, L.; Nowakowicz-Debek, B.; Jezewska-Witkowska, G.; Jakubczak, A. DNA Methylation in Yeast-Like Fungi of the Species Candida albicans Induced by Different Lengths of Exposure to Ozone. Russ. J. Genet. 2019, 55, 396–398. [Google Scholar] [CrossRef]
- So, K.-K.; Ko, Y.-H.; Chun, J.; Bal, J.; Jeon, J.; Kim, J.-M.; Choi, J.; Lee, Y.-H.; Huh, J.H.; Kim, D.-H. Global DNA Methylation in the Chestnut Blight Fungus Cryphonectria parasitica and Genome-Wide Changes in DNA Methylation Accompanied with Sectorization. Front. Plant Sci. 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhu, J.; Wang, Z.; Tang, G.; Huang, B. Differential DNA methylation may contribute to temporal and spatial regulation of gene expression and the development of mycelia and conidia in entomopathogenic fungus Metarhizium robertsii. Fungal Biol. 2017, 121, 293–303. [Google Scholar] [CrossRef]
- Xin, X.; Yin, J.; Zhang, B.; Li, Z.; Zhao, S.; Gui, Z. Genome-wide analysis of DNA methylation in subcultured Cordyceps militaris. Arch. Microbiol. 2019, 201, 369–375. [Google Scholar] [CrossRef]
- Aramayo, R.; Selker, E.U. Neurospora crassa, a model system for epigenetics research. Cold Spring Harb. Perspect. Biol. 2013, 5, a017921. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, J.; Kovalchuk, A.; Raffaello, T.; Wen, Z.; Liu, M.; Asiegbu, F.O. Genome-wide DNA methylation and transcriptomic profiles in the lifestyle strategies and asexual development of the forest fungal pathogen Heterobasidion parviporum. Epigenetics 2019, 14, 16–40. [Google Scholar] [CrossRef]
- Zhuang, Z.; Pan, X.; Zhang, M.; Liu, Y.; Huang, C.; Li, Y.; Hao, L.; Wang, S. Set2 family regulates mycotoxin metabolism and virulence via H3K36 methylation in pathogenic fungus Aspergillus flavus. Virulence 2022, 13, 1358–1378. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, Z.; Sun, X.; Ji, T.; Huang, H.; Yang, Y.; Zhang, H.; Tahir, H.A.S.; Wu, L.; Wu, H.; et al. FvSet2 regulates fungal growth, pathogenicity, and secondary metabolism in Fusarium verticillioides. Fungal Genet. Biol. 2017, 107, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dallery, J.-F.; Adelin, É.; Le Goff, G.; Pigné, S.; Auger, A.; Ouazzani, J.; O’Connell, R.J. H3K4 trimethylation by CclA regulates pathogenicity and the production of three families of terpenoid secondary metabolites in Colletotrichum higginsianum. Mol. Plant Pathol. 2019, 20, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.M.; Bok, J.W.; Lee, S.; Dagenais, T.R.T.; Andes, D.R.; Kontoyiannis, D.P.; Keller, N.P. Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 2013, 1, e4. [Google Scholar] [CrossRef]
- Chen, X.; Wu, L.; Lan, H.; Sun, R.; Wen, M.; Ruan, D.; Zhang, M.; Wang, S. Histone acetyltransferases MystA and MystB contribute to morphogenesis and aflatoxin biosynthesis by regulating acetylation in fungus Aspergillus flavus. Environ. Microbiol. 2021, 24, 1340–1361. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Han, N.; Zheng, H.; Wang, L. The Histone Deacetylase HstD Regulates Fungal Growth, Development and Secondary Metabolite Biosynthesis in Aspergillus terreus. Int. J. Mol. Sci. 2023, 24, 12569. [Google Scholar] [CrossRef]
- Wen, M.; Lan, H.; Sun, R.; Chen, X.; Zhang, X.; Zhu, Z.; Tan, C.; Yuan, J.; Wang, S. Histone deacetylase SirE regulates development, DNA damage response and aflatoxin production in Aspergillus flavus. Environ. Microbiol. 2022, 24, 5596–5610. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, J.; Liu, L.; Song, A.; Liao, Y.; Guan, Z.; Fang, W.; Chen, F. Ubiquitin E3 Ligase AaBre1 Responsible for H2B Monoubiquitination Is Involved in Hyphal Growth, Conidiation and Pathogenicity in Alternaria alternata. Genes 2020, 11, 229. [Google Scholar] [CrossRef]
- Zhu, W.; Fan, X.; Zhao, Q.; Xu, Y.; Wang, X.; Chen, J. Bre1 and Ubp8 regulate H2B mono-ubiquitination and the reversible yeast-hyphae transition in Candida albicans. Mol. Microbiol. 2020, 115, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.; Han, Q.; Nair, P.; Stacey, L.; Gaynier, H.; Mosley, M.; Huang, Q.Q.; Pearson, J.K.; Hsieh, T.-F.; An, Y.-Q.C.; et al. Dynamic DNA Methylation in Plant Growth and Development. Int. J. Mol. Sci. 2018, 19, 2144. [Google Scholar] [CrossRef]
- Acharjee, S.; Chauhan, S.; Pal, R.; Tomar, R.S. Mechanisms of DNA methylation and histone modifications. Prog. Mol. Biol. Transl. Sci. 2023, 197, 51–92. [Google Scholar] [CrossRef]
- Bailey, M.T.; Scott, B.R. Mechanisms of DNA methylation regulatory function and crosstalk with histone lysine methylation. J. Mol. Biol. 2024, 436, 168394. [Google Scholar] [CrossRef]
- Sergeeva, A.; Davydova, K.; Perenkov, A.; Vedunova, M. Mechanisms of human DNA methylation, alteration of methylation patterns in physiological processes and oncology. Gene 2023, 875, 147487. [Google Scholar] [CrossRef]
- Li, S.; Tollefsbol, T.O. DNA methylation methods: Global DNA methylation and methylomic analyses. Methods 2020, 187, 28–43. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Li, B.; Tian, S. The Pattern and Function of DNA Methylation in Fungal Plant Pathogens. Microorganisms 2020, 8, 227. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, Z.-X.; Liu, C.; Wang, S.-B.; Huang, B. Genome-wide analysis of DNA methylation in the sexual stage of the insect pathogenic fungus Cordyceps militaris. Fungal Biol. 2015, 119, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Deleris, A.; Halter, T.; Navarro, L. DNA Methylation and Demethylation in Plant Immunity. Annu. Rev. Phytopathol. 2016, 54, 579–603. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, H.; Bradai, M.; Zhao, C.; You, Y.; Ma, J.; Zhao, L.; Lozano-Durán, R.; Zhu, J.-K. DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development. Nat. Commun. 2022, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Doblas, J.T.; Roldán-Arjona, T.; Ariza, R.R.; Córdoba-Cañero, D. Active DNA Demethylation in Plants. Int. J. Mol. Sci. 2019, 20, 4683. [Google Scholar] [CrossRef]
- Huang, R.; Ding, Q.; Xiang, Y.; Gu, T.; Li, Y. Comparative Analysis of DNA Methyltransferase Gene Family in Fungi: A Focus on Basidiomycota. Front. Plant Sci. 2016, 7, 1556. [Google Scholar] [CrossRef]
- Rountree, M.R.; Selker, E.U. DNA methylation and the formation of heterochromatin in Neurospora crassa. Heredity 2010, 105, 38–44. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chang, J.-C.; Yen, M.-R.; Huang, Y.-F.; Chen, T.-H.; Chen, L.-H.; Nai, Y.-S. Whole-genome DNA methylome analysis of different developmental stages of the entomopathogenic fungus Beauveria bassiana NCHU-157 by nanopore sequencing. Front. Genet. 2023, 14, 1085631. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhang, L.; Hu, J.; Zhou, J.; Lin, H.-W.; He, C.; Chen, H.-Z.; Zhang, L. A fungal dioxygenase CcTet serves as a eukaryotic 6mA demethylase on duplex DNA. Nat. Chem. Biol. 2022, 18, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Nai, Y.-S.; Huang, Y.-C.; Yen, M.-R.; Chen, P.-Y. Diversity of Fungal DNA Methyltransferases and Their Association with DNA Methylation Patterns. Front. Microbiol. 2021, 11, 616922. [Google Scholar] [CrossRef] [PubMed]
- Ličytė, J.; Kvederavičiūtė, K.; Rukšėnaitė, A.; Godliauskaitė, E.; Gibas, P.; Tomkutė, V.; Petraitytė, G.; Masevičius, V.; Klimašauskas, S.; Kriukienė, E. Distribution and regulatory roles of oxidized 5-methylcytosines in DNA and RNA of the basidiomycete fungi Laccaria bicolor and Coprinopsis cinerea. Open Biol. 2022, 12, 210302. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; Krueger, C.; Mellado-Lopez, M.; Hemberger, M.; Dean, W.; Perez-Garcia, V.; Hanna, C.W. Mechanisms and function of de novo DNA methylation in placental development reveals an essential role for DNMT3B. Nat. Commun. 2023, 14, 371. [Google Scholar] [CrossRef]
- Liu, G.; Xia, Y.; Liu, T.; Dai, S.; Hou, X. The DNA Methylome and Association of Differentially Methylated Regions with Differential Gene Expression during Heat Stress in Brassica rapa. Int. J. Mol. Sci. 2018, 19, 1414. [Google Scholar] [CrossRef]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA Methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef]
- Long, J.; Liu, J.; Xia, A.; Springer, N.M.; He, Y. Maize Decrease in DNA methylation 1 targets RNA-directed DNA methylation on active chromatin. Plant Cell 2021, 33, 2183–2196. [Google Scholar] [CrossRef]
- Furuya, K.; Ikura, M.; Ikura, T. Epigenetic interplays between DNA demethylation and histone methylation for protecting oncogenesis. J. Biochem. 2019, 165, 297–299. [Google Scholar] [CrossRef]
- Yi, X.; Jiang, X.; Li, X.; Jiang, D.-S. Histone lysine methylation and congenital heart disease: From bench to bedside (Review). Int. J. Mol. Med. 2017, 40, 953–964. [Google Scholar] [CrossRef]
- Charidemou, E.; Koufaris, C.; Louca, M.; Kirmizis, A.; Rubio-Tomás, T. Histone methylation in pre-cancerous liver diseases and hepatocellular carcinoma: Recent overview. Clin. Transl. Oncol. 2023, 25, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Miller, K.M. Histone methylation and the DNA damage response. Mutat. Res. Mol. Mech. Mutagen. 2017, 780, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Wang, Z.; Li, S.S.-C. SnapShot: Lysine Methylation beyond Histones. Mol. Cell 2017, 68, 1016–1016.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Long, Y.; Paucek, R.D.; Gooding, A.R.; Lee, T.; Burdorf, R.M.; Cech, T.R. Regulation of histone methylation by automethylation of PRC2. Genes Dev. 2019, 33, 1416–1427. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, J.; Zheng, S. The Regulation and Function of Histone Methylation. J. Plant Biol. 2018, 61, 347–357. [Google Scholar] [CrossRef]
- Binda, O.; LeRoy, G.; Bua, D.J.; Garcia, B.A.; Gozani, O.; Richard, S. Trimethylation of histone H3 lysine 4 impairs methylation of histone H3 lysine 9: Regulation of lysine methyltransferases by physical interaction with their substrates. Epigenetics 2010, 5, 767–775. [Google Scholar] [CrossRef]
- Freitag, M. Histone Methylation by SET Domain Proteins in Fungi. Annu. Rev. Microbiol. 2017, 71, 413–439. [Google Scholar] [CrossRef]
- Al Temimi, A.H.K.; Reddy, Y.V.; White, P.B.; Guo, H.; Qian, P.; Mecinović, J. Lysine Possesses the Optimal Chain Length for Histone Lysine Methyltransferase Catalysis. Sci. Rep. 2017, 7, 16148. [Google Scholar] [CrossRef]
- Al Temimi, A.H.K.; Teeuwen, R.S.; Tran, V.; Altunc, A.J.; Lenstra, D.C.; Ren, W.; Qian, P.; Guo, H.; Mecinović, J. Importance of the main chain of lysine for histone lysine methyltransferase catalysis. Org. Biomol. Chem. 2019, 17, 5693–5697. [Google Scholar] [CrossRef]
- Cheng, K.; Xu, Y.; Yang, C.; Ouellette, L.; Niu, L.; Zhou, X.; Chu, L.; Zhuang, F.; Liu, J.; Wu, H.; et al. Histone tales: Lysine methylation, a protagonist in Arabidopsis development. J. Exp. Bot. 2020, 71, 793–807. [Google Scholar] [CrossRef]
- Ho, C.-H.; Takizawa, Y.; Kobayashi, W.; Arimura, Y.; Kimura, H.; Kurumizaka, H. Structural basis of nucleosomal histone H4 lysine 20 methylation by SET8 methyltransferase. Life Sci. Alliance 2021, 4, e202000919. [Google Scholar] [CrossRef] [PubMed]
- Haws, S.A.; Miller, L.J.; La Luz, D.R.; Kuznetsov, V.I.; Trievel, R.C.; Craciun, G.; Denu, J.M. Intrinsic catalytic properties of histone H3 lysine-9 methyltransferases preserve monomethylation levels under low S-adenosylmethionine. J. Biol. Chem. 2023, 299, 104938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jing, L.; Li, M.; He, L.; Guo, Z. Regulation of histone arginine methylation/demethylation by methylase and demethylase (Review). Mol. Med. Rep. 2019, 19, 3963–3971. [Google Scholar] [CrossRef]
- Fulton, M.D.; Brown, T.; Zheng, Y.G. Mechanisms and Inhibitors of Histone Arginine Methylation. Chem. Rec. 2018, 18, 1792–1807. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bedford, M.T. Histone arginine methylation. FEBS Lett. 2010, 585, 2024–2031. [Google Scholar] [CrossRef]
- Fulton, M.D.; Zhang, J.; He, M.; Ho, M.-C.; Zheng, Y.G. Intricate Effects of α-Amino and Lysine Modifications on Arginine Methylation of the N-Terminal Tail of Histone H4. Biochemistry 2017, 56, 3539–3548. [Google Scholar] [CrossRef]
- Al-Hamashi, A.A.; Diaz, K.; Huang, R. Non-Histone Arginine Methylation by Protein Arginine Methyltransferases. Curr. Protein Pept. Sci. 2020, 21, 699–712. [Google Scholar] [CrossRef]
- Raveendran, V.V.; Al-Haffar, K.; Kunhi, M.; Belhaj, K.; Al-Habeeb, W.; Al-Buraiki, J.; Eyjolsson, A.; Poizat, C. Protein arginine methyltransferase 6 mediates cardiac hypertrophy by differential regulation of histone H3 arginine methylation. Heliyon 2020, 6, e03864. [Google Scholar] [CrossRef] [PubMed]
- Spannhoff, A.; Heinke, R.; Bauer, I.; Trojer, P.; Metzger, E.; Gust, R.; Schüle, R.; Brosch, G.; Sippl, W.; Jung, M. arget-based approach to inhibitors of histone arginine methyltransferases. J. Med. Chem. 2007, 50, 2319–2325. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Abraham, L.N.; Reyes-Avila, C.S.; Corrêa, B.; Croll, D. Histone H3K27 Methylation Perturbs Transcriptional Robustness and Underpins Dispensability of Highly Conserved Genes in Fungi. Mol. Biol. Evol. 2021, 39, msab323. [Google Scholar] [CrossRef]
- Seymour, M.; Ji, L.; Santos, A.M.; Kamei, M.; Sasaki, T.; Basenko, E.Y.; Schmitz, R.J.; Zhang, X.; A Lewis, Z. Histone H1 Limits DNA Methylation in Neurospora crassa. G3 Genes Genomes Genet. 2016, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Z.; Wang, E.; Dong, L.; Bai, J.; Wang, D.; Zhu, J.; Zhang, C. Potential antifungal targets based on histones post-translational modifications against invasive aspergillosis. Front. Microbiol. 2022, 13, 980615. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, W.; Liu, Y.; Li, W.; Wang, Y.; Liu, N.; Sheng, C. Jumonji Histone Demethylase Inhibitor JIB-04 as a Broad-Spectrum Antifungal Agent. ACS Infect. Dis. 2022, 8, 1316–1323. [Google Scholar] [CrossRef]
- Ye, M.; Jiang, H.; Fu, X.; Xu, J.-R.; Jiang, C. Fng1 is involved in crosstalk between histone acetylation and methylation. Curr. Genet. 2021, 67, 535–538. [Google Scholar] [CrossRef]
- Bachleitner, S.; Sørensen, J.L.; Gacek-Matthews, A.; Sulyok, M.; Studt, L.; Strauss, J. Evidence of a Demethylase-Independent Role for the H3K4-Specific Histone Demethylases in Aspergillus nidulans and Fusarium graminearum Secondary Metabolism. Front. Microbiol. 2019, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, X.; Zhao, F.; Jiang, Y.; Lu, Z.; Ji, H.; Feng, Y.; Li, J.; Zhang, H.; Zheng, J.; et al. The COMPASS Complex Regulates Fungal Development and Virulence through Histone Crosstalk in the Fungal Pathogen Cryptococcus neoformans. J. Fungi 2023, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, N.; Yin, Y.; Chen, Y.; Jiang, J.; Ma, Z. Histone H3K4 methylation regulates hyphal growth, secondary metabolism and multiple stress responses in Fusarium graminearum. Environ. Microbiol. 2015, 17, 4615–4630. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Kwon, S.; Lee, E.-J.; Lee, J.-S. Set1-mediated H3K4 methylation is required for Candida albicans virulence by regulating intracellular level of reactive oxygen species. Virulence 2021, 12, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, K.; McNaught, K.J.; Ormsby, T.; Leggett, N.A.; Honda, S.; Selker, E.U. Japan Telomere repeats induce domains of H3K27 methylation in Neurospora. eLife 2018, 7, e31216. [Google Scholar] [CrossRef]
- Ling, H.; Mengjuan, Z.; Chi, Y.; Xiaohua, P.; Dandan, W.; Hong, L.; Dongmei, M.; Yanfang, Y.; Wangzhuo, F.; Jiarui, C.; et al. The epigenetic regulator Set9 harmonizes fungal development, secondary metabolism, and colonization capacity of Aspergillus flavus. Int. J. Food Microbiol. 2023, 403, 110298. [Google Scholar] [CrossRef]
- Sebastián, C.; Mostoslavsky, R. The Various Metabolic Sources of Histone Acetylation. Trends Endocrinol. Metab. 2017, 28, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Parbin, S.; Kar, S.; Shilpi, A.; Sengupta, D.; Deb, M.; Rath, S.K.; Patra, S.K. Histone deacetylases: A saga of perturbed acetylation homeostasis in cancer. J. Histochem. Cytochem. 2013, 62, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, X.; Gao, Y.; Zhou, C.; Chiang, V.L.; Li, W. Histone Acetylation Changes in Plant Response to Drought Stress. Genes 2021, 12, 1409. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Schueller, E.; Paiva, I.; Blanc, F.; Wang, X.-L.; Cassel, J.-C.; Boutillier, A.-L.; Bousiges, O. Dysregulation of histone acetylation pathways in hippocampus and frontal cortex of Alzheimer’s disease patients. Eur. Neuropsychopharmacol. 2020, 33, 101–116. [Google Scholar] [CrossRef] [PubMed]
- McCann, K.E.; Rosenhauer, A.M.; Jones, G.M.; Norvelle, A.; Choi, D.C.; Huhman, K.L. Histone deacetylase and acetyltransferase inhibitors modulate behavioral responses to social stress. Psychoneuroendocrinology 2017, 75, 100–109. [Google Scholar] [CrossRef]
- Chang, L.; Takada, S. Structural Dynamics of Tri-Nuleosome by Coarse-Grained Simulations: Effects of Histone Tail Acetylation. Biophys. J. 2016, 110, 69a. [Google Scholar] [CrossRef]
- Klein, B.J.; Jang, S.M.; Lachance, C.; Mi, W.; Lyu, J.; Sakuraba, S.; Krajewski, K.; Wang, W.W.; Sidoli, S.; Liu, J.; et al. Histone H3K23-specific acetylation by MORF is coupled to H3K14 acylation. Nat. Commun. 2019, 10, 4724. [Google Scholar] [CrossRef]
- Chen, X.; Duan, Y.; Qiao, F.; Liu, H.; Huang, J.; Luo, C.; Chen, X.; Li, G.; Xie, K.; Hsiang, T.; et al. A secreted fungal effector suppresses rice immunity through host histone hypoacetylation. New Phytol. 2022, 235, 1977–1994. [Google Scholar] [CrossRef]
- Elsner, V.R.; de Menezes, M.F.; Nicola, F.; da Silva, I.R.V.; Vizuete, A.; Xavier, L.L.; Gonçalves, C.A.S.; Netto, C.A.; Mestriner, R.G. Glial fibrillary acidic protein levels are associated with global histone H4 acetylation after spinal cord injury in rats. Neural Regen. Res. 2018, 13, 1945–1952. [Google Scholar] [CrossRef]
- Cai, Q.; Tong, S.-M.; Shao, W.; Ying, S.-H.; Feng, M.-G. Pleiotropic effects of the histone deacetylase Hos2 linked to H4-K16 deacetylation, H3-K56 acetylation, and H2A-S129 phosphorylation in Beauveria bassiana. Cell. Microbiol. 2018, 20, e12839. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Li, X.; Yang, X.; Li, Y.; Chen, X.; Sun, S.; Jia, S. Histone acetylation/deacetylation in Candida Albicans and their potential as antifungal targets. Futur. Microbiol. 2020, 15, 1075–1090. [Google Scholar] [CrossRef]
- Dubey, A.; Lee, J.; Kwon, S.; Lee, Y.; Jeon, J. A MYST family histone acetyltransferase, MoSAS3, is required for development and pathogenicity in the rice blast fungus. Mol. Plant Pathol. 2019, 20, 1491–1505. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, G.; Li, H.; Liu, N.; Zhang, W.; Sheng, C. Discovery of Janus Kinase 2 (JAK2) and Histone Deacetylase (HDAC) Dual Inhibitors as a Novel Strategy for the Combinational Treatment of Leukemia and Invasive Fungal Infections. J. Med. Chem. 2018, 61, 6056–6074. [Google Scholar] [CrossRef]
- Navarrete, B.; Ibeas, J.I.; Barrales, R.R. Systematic characterization of Ustilago maydis sirtuins shows Sir2 as a modulator of pathogenic gene expression. Front. Microbiol. 2023, 14, 1157990. [Google Scholar] [CrossRef]
- Wang, G.; Song, L.; Bai, T.; Liang, W. BcSas2-Mediated Histone H4K16 Acetylation Is Critical for Virulence and Oxidative Stress Response of Botrytis cinerea. Mol. Plant-Microbe Interact. 2020, 33, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Yang, S.-Y.; Hsu, L.-H.; Yu, S.-J.; Chen, Y.-L. The Gcn5-Ada2-Ada3 histone acetyltransferase module has divergent roles in pathogenesis of Candida glabrata. Med. Mycol. 2023, 61, myad004. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, Z.; Shao, W.; Ying, S.; Feng, M. Essential role of Rpd3-dependent lysine modification in the growth, development and virulence of Beauveria bassiana. Environ. Microbiol. 2018, 20, 1590–1606. [Google Scholar] [CrossRef]
- Fan, A.; Mi, W.; Liu, Z.; Zeng, G.; Zhang, P.; Hu, Y.; Fang, W.; Yin, W.-B. Deletion of a Histone Acetyltransferase Leads to the Pleiotropic Activation of Natural Products in Metarhizium robertsii. Org. Lett. 2017, 19, 1686–1689. [Google Scholar] [CrossRef]
- Li, C.; Tu, J.; Han, G.; Liu, N.; Sheng, C. Heat shock protein 90 (Hsp90)/Histone deacetylase (HDAC) dual inhibitors for the treatment of azoles-resistant Candida albicans. Eur. J. Med. Chem. 2022, 227, 113961. [Google Scholar] [CrossRef]
- Qi, S.; He, L.; Zhang, Q.; Dong, Q.; Wang, Y.; Yang, Q.; Tian, C.; He, Q.; Wang, Y. Cross-pathway control gene CPC1/GCN4 coordinates with histone acetyltransferase GCN5 to regulate catalase-3 expression under oxidative stress in Neurospora crassa. Free Radic. Biol. Med. 2018, 117, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, Y.; Liu, B.; Tang, F.; Shao, Y. Histone deacetylase MrHos3 negatively regulates the production of citrinin and pigments in Monascus ruber. J. Basic Microbiol. 2023, 63, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Betim, K.; Brandon, T.P.; Özlem, S.-B.; Zhiqiang, D.; Koon Ho, W.; Alastair, B.F.; Nancy, P.K.; Özgür, B. The KdmB-EcoA-RpdA-SntB (KERS) chromatin regulatory complex controls development, secondary metabolism and pathogenicity in Aspergillus flavus. Fungal Genet. Biol. 2023, 169, 103836. [Google Scholar] [CrossRef]
- Etier, A.; Dumetz, F.; Chéreau, S.; Ponts, N. Post-Translational Modifications of Histones Are Versatile Regulators of Fungal Development and Secondary Metabolism. Toxins 2022, 14, 317. [Google Scholar] [CrossRef]
- Li, J.; Mahata, B.; Escobar, M.; Goell, J.; Wang, K.; Khemka, P.; Hilton, I.B. Programmable human histone phosphorylation and gene activation using a CRISPR/Cas9-based chromatin kinase. Nat. Commun. 2021, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Loginova, D.B.; Silkova, O.G. H3Ser10 histone phosphorylation in plant cell division. Russ. J. Genet. Appl. Res. 2017, 7, 46–56. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Krapivinsky, L.; Renthal, N.E.; Santa-Cruz, A.; Manasian, Y.; Clapham, D.E. Histone phosphorylation by TRPM6’s cleaved kinase attenuates adjacent arginine methylation to regulate gene expression. Proc. Natl. Acad. Sci. USA 2017, 114, E7092–E7100. [Google Scholar] [CrossRef]
- Ju, J.; Chen, A.; Deng, Y.; Liu, M.; Wang, Y.; Wang, Y.; Nie, M.; Wang, C.; Ding, H.; Yao, B.; et al. NatD promotes lung cancer progression by preventing histone H4 serine phosphorylation to activate Slug expression. Nat. Commun. 2017, 8, 928. [Google Scholar] [CrossRef]
- Chen, J.J.; Stermer, D.; Tanny, J.C. Decoding histone ubiquitylation. Front. Cell Dev. Biol. 2022, 10, 968398. [Google Scholar] [CrossRef]
- Zarreen, F.; Karim, M.J.; Chakraborty, S. The diverse roles of histone 2B monoubiquitination in the life of plants. J. Exp. Bot. 2022, 73, 3854–3865. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Z.; Yuan, J.; Zou, R.; Wang, Y.; Peng, X.; Xu, S.; Xie, C. Functional Role of RING Ubiquitin E3 Ligase VdBre1 and VdHrd1 in the Pathogenicity and Penetration Structure Formation of Verticillium dahliae. J. Fungi 2023, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Aquila, L.; Atanassov, B.S. Regulation of Histone Ubiquitination in Response to DNA Double Strand Breaks. Cells 2020, 9, 1699. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Lee, Y.-H.; Lin, C.-J.; Hsu, L.-H.; Chen, Y.-L. Deubiquitination module is critical for oxidative stress response and biofilm formation in Candida glabrata. Med. Mycol. 2023, 61, myad099. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, W.A. Ubiquitylation: How Nucleosomes Use Histones to Evict Histones. Trends Cell Biol. 2019, 29, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, F.; Zhang, J.; Dijke, P.T.; Zhou, F. SUMO-triggered ubiquitination of NR4A1 controls macrophage cell death. Cell Death Differ. 2017, 24, 1530–1539. [Google Scholar] [CrossRef]
- Zalzman, M.; Meltzer, W.A.; Portney, B.A.; Brown, R.A.; Gupta, A. The Role of Ubiquitination and SUMOylation in Telomere Biology. Curr. Issues Mol. Biol. 2019, 35, 85–98. [Google Scholar] [CrossRef]
- Grau, M.F.; Entwistle, R.; Oakley, C.E.; Wang, C.C.C.; Oakley, B.R. Overexpression of an LaeA-like Methyltransferase Upregulates Secondary Metabolite Production in Aspergillus nidulans. ACS Chem. Biol. 2019, 14, 1643–1651. [Google Scholar] [CrossRef]
- Luo, Q.; Li, N.; Xu, J.-W. A methyltransferase LaeA regulates ganoderic acid biosynthesis in Ganoderma lingzhi. Front. Microbiol. 2022, 13, 1025983. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhu, Y.; Yang, L.; Li, Y.; Qu, J.; Wang, L.; Zhao, J.; Qu, Y.; Qin, Y. Penicillium oxalicum putative methyltransferase Mtr23B has similarities and differences with LaeA in regulating conidium development and glycoside hydrolase gene expression. Fungal Genet. Biol. 2020, 143, 103445. [Google Scholar] [CrossRef]
- Zhao, Z.; Gu, S.; Liu, D.; Liu, D.; Chen, B.; Li, J.; Tian, C. The putative methyltransferase LaeA regulates mycelium growth and cellulase production in Myceliophthora thermophila. Biotechnol. Biofuels 2023, 16, 58. [Google Scholar] [CrossRef]
- Shi, J.-C.; Shi, W.-L.; Zhou, Y.-R.; Chen, X.-L.; Zhang, Y.-Z.; Zhang, X.; Zhang, W.-X.; Song, X.-Y. The Putative Methyltransferase TlLAE1 Is Involved in the Regulation of Peptaibols Production in the Biocontrol Fungus Trichoderma longibrachiatum SMF2. Front. Microbiol. 2020, 11, 1267. [Google Scholar] [CrossRef]
- Lin, C.-J.; Hou, Y.-H.; Chen, Y.-L. The histone acetyltransferase GcnE regulates conidiation and biofilm formation in Aspergillus fumigatus. Med. Mycol. 2019, 58, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Nossmann, M.; Boysen, J.M.; Krüger, T.; König, C.C.; Hillmann, F.; Munder, T.; Brakhage, A.A. Yeast two-hybrid screening reveals a dual function for the histone acetyltransferase GcnE by controlling glutamine synthesis and development in Aspergillus fumigatus. Curr. Genet. 2018, 65, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Yu, D.; Chen, X.; Tabudravu, J.; Deng, H.; Pan, L. Deletion of the epigenetic regulator GcnE in Aspergillus niger FGSC A1279 activates the production of multiple polyketide metabolites. Microbiol. Res. 2018, 217, 101–107. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Park, S.-H.; Kim, S.-S.; Lee, M.-W.; Yu, J.-H.; Shin, K.-S. Functional Characterization of the GNAT Family Histone Acetyltransferase Elp3 and GcnE in Aspergillus fumigatus. Int. J. Mol. Sci. 2023, 24, 2179. [Google Scholar] [CrossRef] [PubMed]
- Nützmann, H.-W.; Reyes-Dominguez, Y.; Scherlach, K.; Schroeckh, V.; Horn, F.; Gacek, A.; Schümann, J.; Hertweck, C.; Strauss, J.; Brakhage, A.A. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA 2011, 108, 14282–14287. [Google Scholar] [CrossRef]
- Lan, H.; Sun, R.; Fan, K.; Yang, K.; Zhang, F.; Nie, X.Y.; Wang, X.; Zhuang, Z.; Wang, S. The Aspergillus flavus Histone Acetyltransferase AflGcnE Regulates Morphogenesis, Aflatoxin Biosynthesis, and Pathogenicity. Front. Microbiol. 2016, 7, 1324. [Google Scholar] [CrossRef]
- Cánovas, D.; Marcos, A.T.; Gacek, A.; Ramos, M.S.; Gutiérrez, G.; Reyes-Domínguez, Y.; Strauss, J. The histone acetyltransferase GcnE (GCN5) plays a central role in the regulation of Aspergillus asexual development. Genetics 2014, 197, 1175–1189. [Google Scholar] [CrossRef]
- Itoh, E.; Odakura, R.; Oinuma, K.-I.; Shimizu, M.; Masuo, S.; Takaya, N. Sirtuin E is a fungal global transcriptional regulator that determines the transition from the primary growth to the stationary phase. J. Biol. Chem. 2017, 292, 11043–11054. [Google Scholar] [CrossRef]
- Wierman, M.B.; Smith, J.S. Yeast sirtuins and the regulation of aging. FEMS Yeast Res. 2013, 14, 73–88. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B.; Duan, G.; Wang, J.; Pan, Z.; Ou, M.; Bai, X.; Wang, P.; Zhao, D.; Nan, N.; et al. Histone Deacetylase UvHST2 Is a Global Regulator of Secondary Metabolism in Ustilaginoidea virens. J. Agric. Food Chem. 2023, 71, 13124–13136. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, M.; Nishiura, M.; Iwashita, K. Fungus-specific sirtuin HstD coordinates secondary metabolism and development through control of LaeA. Eukaryot. Cell 2013, 12, 1087–1096. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, L.; Nan, Y.; Chu, J. Efficient gene deletion and replacement in Aspergillus niger by modified in vivo CRISPR/Cas9 systems. Bioresour. Bioprocess. 2019, 6, 4. [Google Scholar] [CrossRef]
- Arentshorst, M.; Valappil, P.K.; Mózsik, L.; Regensburg-Tuïnk, T.J.G.; Seekles, S.J.; Tjallinks, G.; Fraaije, M.W.; Visser, J.; Ram, A.F.J. A CRISPR/Cas9-based multicopy integration system for protein production in Aspergillus niger. FEBS J. 2023, 290, 5127–5140. [Google Scholar] [CrossRef] [PubMed]


| Species | Epigenetic Type | Secondary Metabolic Effects | Key Genes or Enzymes | Other Physiological | References |
|---|---|---|---|---|---|
| Candida albicans | DNA methylation | Ergosterol | Lanosterol 14α-demethylase | The repression of gene transcription or expression, and loss of product function | [64,65] |
| Cryphonectria parasititica | DNA methylation | sectored progeny | CpDmt1/CpDmt2 | Robust mycelial growth, reduced conidiation, and restricted pigmentation | [66] |
| Metarhizium robertsii | DNA methylation | Regulates energy synthesis and metabolic activity | MrDIM-2/MrRID | Genes with moderately methylated promoter regions are likely to have enhanced transcription | [67] |
| Cordyceps militaris | DNA methylation | 3′-deoxyadenosine | CmDMTA/CmDIM-2 | Methylation modification and DNA recombination can alter a strain’s genotype and thus induce strain degeneration | [68] |
| Neurospora crassa | DNA methylation | Meiosis is silent | DIM-2 | Silencing of the transgene as well as its natural homologues | [69] |
| Heterobasidion parviporum | DNA methylation | The expression level of TEs was silenced | SAP-specific genes/NECT-specific gene | saprotrophic growth (SAP) and necrotrophic growth (NECT) | [70] |
| Aspergillus flavus | H3K36me | aflatoxin B1 | AshA | Involved in morphogenesis and mycotoxin synthesis | [71] |
| Fusarium verticillioides | H3K36me | FB1 biosynthesis | FvSet2 | Defects in vegetative growth, pigmentation, and fungal virulence | [72] |
| Colletotrichum higginsianum | H3K4me | colletochlorins, higginsianins, and sclerosporide | CclA | Significant reductions in virulence and wall penetration ability | [73] |
| Aspergillus fumigatus | H3K4me | gliotoxin | CclA | A slow-growing strain is produced | [74] |
| Aspergillus flavus | H3K14ac/H3K18ac/H3K23ac | aflatoxin B1 | MystB | Significant defects in conidiation, sclerotia formation, and aflatoxin production | [75] |
| Aspergillus terreus | H3K27ac/H3K56ac | lovastatin | HstD | Ablation of HstD resulted in decreased mycelial growth, reduced hyphalization, and a significant increase in tylosin biosynthesis | [76] |
| Aspergillus flavus | H4K16ac | aflatoxin B1 | MystA | Decreased conidiation, increased sclerotia formation and aflatoxin production | [75] |
| Aspergillus niger | H3K9ac | fumonisin B2 | GcnE | Synthesis of more secondary metabolites | [46] |
| Aspergillus flavus | H3K56ac | aflatoxin B1 | SirE | Highly sensitive to DNA damage and oxidative stress | [77] |
| Alternaria alternata | H2Bub | Macromolecular complex generation | AaBre1 | Mycelial growth, conidial formation and pathogenicity | [78] |
| Candida albicans | H2Bub | antibiotics | Ubp8 | Activation of the mycelial program | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, W.; Lu, Y.; Wu, Y.; Ouyang, Z.; Tu, Y.; He, B. Epigenetic Regulation of Fungal Secondary Metabolism. J. Fungi 2024, 10, 648. https://doi.org/10.3390/jof10090648
Zhang Y, Yu W, Lu Y, Wu Y, Ouyang Z, Tu Y, He B. Epigenetic Regulation of Fungal Secondary Metabolism. Journal of Fungi. 2024; 10(9):648. https://doi.org/10.3390/jof10090648
Chicago/Turabian StyleZhang, Yufei, Wenbin Yu, Yi Lu, Yichuan Wu, Zhiwei Ouyang, Yayi Tu, and Bin He. 2024. "Epigenetic Regulation of Fungal Secondary Metabolism" Journal of Fungi 10, no. 9: 648. https://doi.org/10.3390/jof10090648
APA StyleZhang, Y., Yu, W., Lu, Y., Wu, Y., Ouyang, Z., Tu, Y., & He, B. (2024). Epigenetic Regulation of Fungal Secondary Metabolism. Journal of Fungi, 10(9), 648. https://doi.org/10.3390/jof10090648






