Dynamics of Physiological Properties and Endophytic Fungal Communities in the Xylem of Aquilaria sinensis (Lour.) with Different Induction Times
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Materials
2.2. Experimental Design
2.3. Measurement of Physio-Biochemical Indices of Xylem Samples
2.4. DNA Extraction and PCR Amplification of Xylem Endogenous Fungi
2.5. Library Construction, Quantification and Sequencing
2.6. Bioinformatic Analyses of Sequencing Data
2.7. Statistical Analysis
3. Results
3.1. Physio-Biochemical Properties of Xylem Samples
3.2. Endophytic Fungal Community Diversity and Composition with Induction Time
3.2.1. The Alpha Diversity of Endophytic Fungi
3.2.2. Composition of Endophytic Fungal Community
3.3. Changes of Endophytic Fungal Community Structure
3.4. Endophytic Fungal Co-Occurrence Network Analysis
3.5. Predicted Functions of Fungal Communities
3.6. The Correlation Analysis between Endophytic Fungi and Physio-Biochemical Indicators in the Xylem
4. Discussion
4.1. Physiological Properties of Xylem with Induction Time
4.2. Diversity and Compositional Characteristics of Endophytic Fungi
4.3. Change in Endophytic Fungal Community Structure and Function with Induction Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turjaman, M.; Hidayat, A.; Santoso, E. Agarwood Science behind the Fragrance; Mohamed, R., Ed.; Springer: Singapore, 2016; pp. 1–2. [Google Scholar]
- Ma, S.; Yan, T.T.; Chen, Y.; Li, G.Y. Chemical composition and bioactivity variability of two-step extracts derived from traditional and “QiNan” agarwood (Aquilaris spp.). Fitoterapia 2024, 176, 106012. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, R.; Sarma, N.; Begum, T.; Chanda, S.K.; Lekhak, H.; Sastry, G.N.; Lal, M. Agarwood (Aquilaria malaccensis L.) a quality fragrant and medicinally significant plant based essential oil with pharmacological potentials and genotoxicity. Ind. Crops Prod. 2023, 197, 116535. [Google Scholar] [CrossRef]
- Singh, B.R.; Sinha, D.K.; Or, V.K.; Vadhana, P.; Bhardwaj, M.; Saraf, A.; Dubey, S.; Pawde, A.M.; De, U.K.; Gupta, V.K. Antimicrobial activity of agarwood oil against multiple-drug-resistant (MDR) microbes of clinical, food and environmental origin. Curr. Drug Discov. Technol. 2020, 17, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.H.; Yu, Z.X.; Wu, C.M.; Peng, D.Q.; Liu, X.M.; Liu, Y.Y.; Yang, Y.; Guo, P.; Wei, J.H. Agarwood essential oil ameliorates restrain stress-induced anxiety and depression by inhibiting HPA axis hyperactivity. Int. J. Mol. Sci. 2018, 19, 3468. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Soehartono, T. CITES and the conservation of tree species: The case of Aquilaria in Indonesia. Int. For. Rev. 2001, 3, 27–33. [Google Scholar]
- Wang, S.; Yu, Z.X.; Wang, C.H.; Wu, C.M.; Guo, P.; Wei, J.H. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef]
- Chen, H.Q.; Yang, Y.; Xue, J.; Wei, J.H.; Zhang, Z.; Chen, H.J. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules 2011, 16, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Liu, Y.X.; Fu, Y.L.; Qiao, M.J.; Wei, P.L.; Liu, Z.G.; Li, Y.J. Structural, defense enzyme activity and chemical composition changes in the xylem of Aquilaria sinensis during fungus induction. Ind. Crops Prod. 2024, 208, 117804. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Jiang, C.; Zhou, J.H.; Zhao, Y.Y.; Huang, L.Q. Volatile organic compound and endogenous phytohormone characteristics during callus browning in Aquilaria sinensis. Ind. Crops Prod. 2021, 168, 113605. [Google Scholar] [CrossRef]
- Li, X.F.; Cui, Z.Y.; Liu, X.J.; Hong, Z.; Zhang, P.; Xu, D.P. Comparative morphological, anatomical and physiological analyses explain the difference of wounding-induced agarwood formation between ordinary agarwood nongrafted plants and five grafted Qi-Nan clones (Aquilaria sinensis). Forests 2022, 13, 1618. [Google Scholar] [CrossRef]
- Fang, X.Y.; Li, X.F.; Zhang, Q.L.; Hu, H.Z.; Hong, Z.; Liu, X.J.; Cui, Z.Y.; Xu, D.P. Physiological and endophytic fungi changes in grafting seedlings of Qi-Nan Clones (Aquilaria sinensis). Forests 2024, 15, 106. [Google Scholar] [CrossRef]
- Mohamed, R.; Jong, P.L.; Zali, M.S. Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers. 2010, 43, 67–74. [Google Scholar] [CrossRef]
- Xu, D.P.; Ma, H.B.; Zeng, J.; Zeng, B.S.; Yang, J.C.; Xin, K.; Li, M.; Guo, J.J. Genetic Improvement and Efficient Cultivation of Major Afforestation Species in Tropical South Subtropics; China Forestry Publishing House: Beijing, China, 2022; pp. 37–38. [Google Scholar]
- Azren, P.D.; Lee, S.Y.; Emang, D.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Thanh, L.V.; Do, T.V.; Son, N.H.; Sato, T.; Kozan, O. Impacts of biological, chemical and mechanical treatments on sesquiterpene content in stems of planted Aquilaria crassna trees. Agrofor. Syst. 2015, 89, 973–981. [Google Scholar] [CrossRef]
- Zhang, N.N.; Xue, S.Y.; Song, J.; Zhou, X.R.; Zhou, D.H.; Liu, X.J.; Hong, Z.; Xu, D.P. Effects of various artificial agarwood-induction techniques on the metabolome of Aquilaria sinensis. BMC Plant Biol. 2021, 21, 591. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Dong, W.H.; Kong, F.D.; Liao, G.; Wang, J.; Wei, L.; Mei, W.L.; Dai, H.F. Characterization and analysis of 2-(2-phenylethy)-chromone derivatives from agarwood (Aquilaria crassna) by artificial holing for different times. Molecules 2016, 21, 911. [Google Scholar] [CrossRef] [PubMed]
- Yagura, T.; Shibayama, N.; Ito, M.; Kiuchi, F.; Honda, G. Three novel diepoxy tetrahydrochromones from agarwood artificially produced by intentional wounding. Tetrahedron Lett. 2005, 46, 4395–4398. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, H.Q.; Yang, Y.; Zhang, Z.; Wei, J.H.; Meng, H.; Chen, W.P.; Feng, J.D.; Gan, B.C.; Chen, X.Y.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3087–3106. [Google Scholar] [CrossRef] [PubMed]
- Faizal, A.; Esyanti, R.R.; Aulianisa, E.N. Formation of agarwood from Aquilaria malaccensis in response to inoculation of local strains of Fusarium solani. Trees Struct. Funct. 2016, 31, 189–197. [Google Scholar] [CrossRef]
- Zhao, W.W.; Song, X.C.; Zhou, Z.Z.; Liu, G.F.; Zhang, Q.Q.; Pang, S.J. Effects of different levels of physical damage combined with fungal induction on agarwood formation. Forests 2024, 15, 168. [Google Scholar] [CrossRef]
- Naziz, P.S.; Das, R.; Sen, S. The scent of stress: Evidence from the unique fragrance of agarwood. Front. Plant Sci. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.Q.; Li, T.Z.; Liu, X.; Shi, S.P.; Wang, X.H.; Tu, P.F. AsTal1 from Aquilaria sinensis regulates ABA signaling-mediated seed germination and root growth in Nicotiana benthamiana. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 147, 97–106. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.F.; Cui, Z.Y.; Xu, D.P. Morphological, physiological, biochemical and molecular analyses reveal wounding-induced agarwood formation mechanism in two types of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 178, 114603. [Google Scholar] [CrossRef]
- Liu, Y.X.; Qiao, M.J.; Fu, Y.L.; Wei, P.L.; Li, Y.J.; Liu, Z.G. Tissue structure changes of Aquilaria sinensis xylem after fungus induction. Forests 2022, 13, 43. [Google Scholar] [CrossRef]
- Saw, P.C.; Abdul, R.K.; Awang, M.R. Histology study of the Aquilaria malaccensis and agarwood resin formation under light microscope. J. Agrobiotech. 2014, 35, 77–83. [Google Scholar]
- Emmenegger, B.; Massoni, J.; Pestalozzi, C.M.; Bortfeld-Miller, M.; Maier, B.A.; Vorholt, J.A. Identifying microbiota community patterns important for plant protection using synthetic communities and machine learning. Nat. Commun. 2023, 14, 7983. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef] [PubMed]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White Jr, J.F. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; de Bruin, S.; Luckerhoff, L.; van Logtestijn, R.S.P.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Godara, H.; Ramakrishna, W. Endophytes as nature’s gift to plants to combat abiotic stresses. Lett. Appl. Microbiol. 2023, 76, ovac067. [Google Scholar] [CrossRef]
- Pathak, P.; Rai, V.K.; Can, H.; Singh, S.K.; Kumar, D.; Bhardwaj, N.; Roychowdhury, R.; de Azevedo, L.C.B.; Kaushalendra; Verma, H.; et al. Plant-endophyte interaction during biotic stress management. Plants 2022, 11, 2203. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbio. Res. 2023, 271, 127368. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 2022, 13, 933017. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. CSH Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fu, Y.L.; Li, Y.J.; Wei, P.L.; Liu, Z.G. The formation and quality evaluation of agarwood induced by the fungi in Aquilaria sinensis. Ind. Crops Prod. 2021, 173, 114129. [Google Scholar] [CrossRef]
- Du, T.Y.; Karunarathna, S.C.; Zhang, X.; Dai, D.Q.; Mapook, A.; Suwannarach, N.; Xu, J.C.; Stephenson, S.L.; Elgorban, A.M.; Al-Rejaie, S.; et al. Endophytic fungi associated with Aquilaria sinensis (agarwood) from China show antagonism against bacterial and fungal pathogens. J. Fungi 2022, 8, 1197. [Google Scholar] [CrossRef]
- Monggoot, S.; Popluechai, S.; Gentekaki, E.; Pripdeevech, P. Fungal endophytes: An alternative source for production of volatile compounds from agarwood oil of Aquilaria subintegra. Microb. Ecol. 2017, 74, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jong, P.L.; Kamziah, A.K. Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J. For. Res. 2014, 25, 201–204. [Google Scholar] [CrossRef]
- Chhipa, H.; Kaushik, N. Fungal and bacterial diversity isolated from Aquilaria malaccensis tree and soil, induces agarospirol formation within 3 months after artificial infection. Front. Microbiol. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Huang, M.Q.; Ma, S.; Qiao, M.J.; Fu, Y.L.; Li, Y.J. Quality similarity between induced agarwood by fungus and wild agarwood. J. Agric. Food Chem. 2023, 71, 15620–15631. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Fang, X.Y.; Cui, Z.Y.; Hong, Z.; Liu, X.J.; Li, G.Y.; Hu, H.Z.; Xu, D.P. Anatomical, chemical and endophytic fungal diversity of a Qi-Nan clone of Aquilaria sinensis (Lour.) Spreng with different induction times. Front. Plant Sci. 2024, 15, 1320226. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.W.; Zhang, Y.X.; Yang, Y.; Lv, F.F.; Wei, J.H. Programmed cell death might involve in progress of wounding induced agarwood formation in stems of Aquilaria sinensis. Microsc. Res. Tech. 2022, 85, 2904–2912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wei, J.H.; Han, X.M.; Liang, L.; Yang, Y.; Meng, H.; Xu, Y.H.; Gao, Z.H. The sesquiterpene biosynthesis and vessel-occlusion formation in stems of Aquilaria sinensis (Lour.) Gilg trees induced by wounding treatments without variation of microbial communities. Int. J. Mol. Sci. 2014, 15, 23589–23603. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Ma, J.Y.; Liu, Z.D.; Dai, N.F.; Yang, H.Q.; Yang, L.; Wang, Y.H.; Shen, S.K. Gene co-expression network and regression analysis identify the transcriptomic, physiological, and biochemical indicators of the response of alpine woody plant Rhododendron rex to drought stress. Front. Plant Sci. 2022, 13, 896691. [Google Scholar] [CrossRef]
- Herrera-Ramríez, D.; Hartmann, H.; Römermann, C.; Trumbore, S.; Muhr, J.; Maracahipes-Santos, L.; Brando, P.; Silvério, D.; Huang, J.B.; Kuhlmann, I.; et al. Anatomical distribution of starch in the stemwood influences carbon dynamics and suggests storage-growth trade-offs in some tropical trees. J. Ecol. 2023, 111, 2532–2548. [Google Scholar] [CrossRef]
- Shen, H.F.; Zhao, B.; Xu, J.J.; Liang, W.; Huang, W.M.; Li, H.H. Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron. S. Afr. J. Bot. 2017, 112, 338–345. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Hinojosa, A.; Strauss, S.L.; González-López, J.; Bedmar, E. Changes in the diversity and predicted functional composition of the bulk and rhizosphere soil bacterial microbiomes of tomato and common bean after inorganic N-fertilization. Rhizosphere 2021, 18, 100362. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Wang, X.B.; Lin, M.P.; Li, K.L.; Han, Q.; Pan, D.K.; Ma, H.B.; Huang, G.H. Effects of intercropping teak with Alpinia katsumadai Hayata and Amomum longiligulare T.L. Wu on rhizosphere soil nutrients and bacterial community diversity; structure; and network. Front. Microbiol. 2024, 15, 1328772. [Google Scholar]
- Xie, G.R.; Xu, R.; Chong, L.; Zhu, Y.F. Understanding drought stress response mechanisms in tomato. Veg. Res. 2024, 4, e001. [Google Scholar] [CrossRef]
- Begum, K.; Das, A.; Ahmed, R.; Akhtar, S.; Kulkarni, R.; Banu, S. Genome-wide analysis of respiratory burst oxidase homolog (Rboh) genes in Aquilaria species and insight into ROS-mediated metabolites biosynthesis and resin deposition. Front. Plant Sci. 2024, 14, 1326080. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.S.; Liu, S.C.; Li, R.X.; Li, Z.B.; Piao, J.Z.; Zhou, R.J. Calcium enhanced the resistance against Phoma arachidicola by improving cell membrane stability and regulating reactive oxygen species metabolism in peanut. BMC Plant Biol. 2024, 24, 501. [Google Scholar] [CrossRef]
- Zhou, X.F.; Chen, S.L.; Wu, H.; Yang, Y.; Xu, H.W. Biochemical and proteomics analyses of antioxidant enzymes reveal the potential stress tolerance in Rhododendron chrysanthum Pall. Biol. Direct. 2017, 12, 10. [Google Scholar] [CrossRef]
- Rathoreehakur, D.; Chawla, A. Seasonal variations coupled with elevation gradient drives significant changes in eco-physiological and biogeochemical traits of a high altitude evergreen broadleaf shrub; Rhododendron anthopogon. Plant Physiol. Biochem. 2018, 132, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.S.; Zhang, S.H.; Li, B.W.; Li, J.L.; Wang, X.Y.; Zhang, J.Q.; Guan, C.F.; Ji, J. The combined use of a plant growth promoting Bacillus sp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system; enhancing photosynthesis and improving soil enzyme activities. Microbiol. Res. 2023, 266, 127225. [Google Scholar] [CrossRef] [PubMed]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- Yousaf, M.I.; Riaz, M.W.; Jiang, Y.R.; Yasir, M.; Aslam, M.Z.; Hussain, S.; Shah, S.A.S.; Shehzad, A.; Riasat, G.; Manzoor, M.A.; et al. Concurrent effects of drought and heat stresses on physio-chemical attributes; antioxidant status and kernel quality traits in maize (Zea mays L.) hybrids. Front. Plant Sci. 2022, 13, 898823. [Google Scholar] [CrossRef]
- Cui, J.L.; Guo, S.X.; Fu, S.B.; Xiao, P.G.; Wang, M.L. Effects of inoculating fungi on agilawood formation in Aquilaria sinensis. Chin. Sci. Bull. 2013, 58, 3280–3287. [Google Scholar] [CrossRef]
- Liu, P.W.; Zhang, X.L.; Yang, Y.; Sui, C.; Xu, Y.H.; Wei, J.H. Interxylary phloem and xylem rays are the structural foundation of agarwood resin formation in the stems of Aquilaria sinesis. Trees Struct. Funct. 2018, 33, 533–542. [Google Scholar] [CrossRef]
- Jacob, J.K.S.; Witzel, K.; dela Cruz, T.E.E. Comparative diversity and functional traits of fungal endophytes in response to elevated mineral content in a Mangrove ecosystem. J. Fungi 2023, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Siburian, R.H.; Siregar, U.J.; Siregar, I.Z.; Santoso, E.; Wahyudi, I. Identification of anatomical characteristics of Aquilaria macrocarpa in its interaction with Fusarium solani. Biotropia 2013, 20, 104–111. [Google Scholar]
- Liu, J.; Li, T.X.; Chen, T.; Gao, J.Q.; Zhang, X.; Jiang, C.; Yang, J.; Zhou, J.H.; Wang, T.L.; Chi, X.L.; et al. Integrating multiple omics identifies Phaeoacremonium rubrigenum acting as Aquilaria sinensis marker fungus to promote agarwood sesquiterpene accumulation by inducing plant host phosphorylation. Microbiol. Spectr. 2022, 10, e02722-21. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Meng, D.Y.; Li, W.Y.; Li, X.N.; He, X.L. Dynamics of endophytic fungal communities associated with cultivated medicinal plants in farmland ecosystem. J. Fungi 2023, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Li, T.X.; Qiu, Z.D.; Lee, S.Y.; Li, X.; Gao, J.Q.; Jiang, C.; Huang, L.Q.; Liu, J. Biodiversity and application prospects of fungal endophytes in the agarwood-producing genera; Aquilaria and Gyrinops (Thymelaeaceae): A review. Arab. J. Chem. 2023, 16, 104435. [Google Scholar] [CrossRef]
- Bertini, L.; Perazzolli, M.; Proietti, S.; Capaldi, G.; Savatin, D.V.; Bigini, V.; Longa, C.M.O.; Basaglia, M.; Favaro, L.; Casella, S.; et al. Biodiversity and bioprospecting of fungal endophytes from the Antarctic plant Colobanthus quitensis. J. Fungi 2022, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere microbiology with special reference to diversity and plant genotype. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr. J. Biotechnol. 2009, 8, 731–736. [Google Scholar]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- De Vries, F.T.; Wallenstein, M.D. Belowground connections underlying aboveground food production: A framework for optimizing ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; et al. Co–occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Zhao, W.W.; Zhou, Z.Z.; Huang, G.H.; Wang, X.B.; Han, Q.; Liu, G.F. The application of mixed organic and inorganic fertilizers drives soil nutrient and bacterial community changes in teak plantations. Microorganisms 2022, 10, 958. [Google Scholar] [CrossRef]
- Kogel, K.H.; Franken, P.; Hückelhoven, R. Endophyte or parasite—What decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
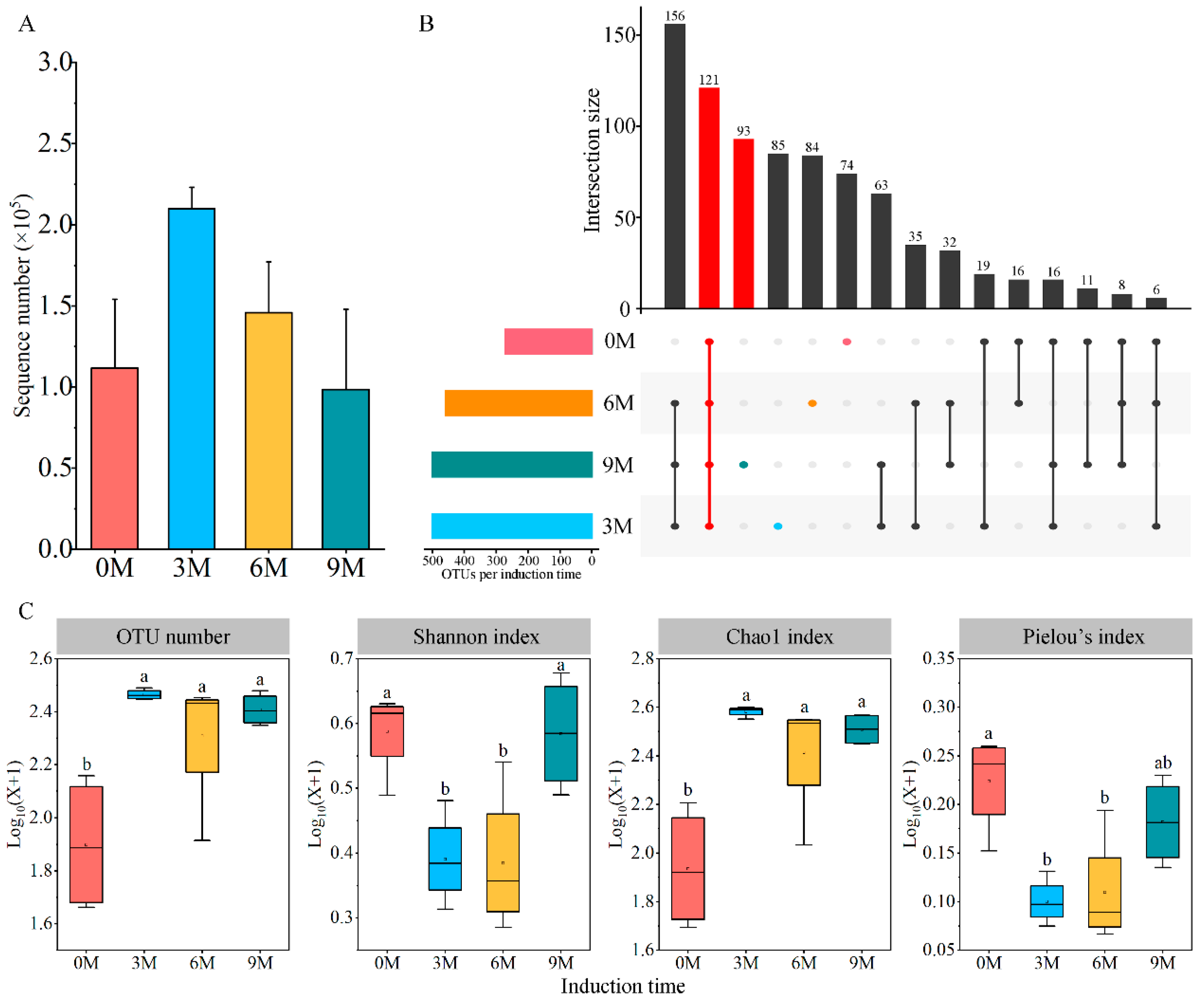
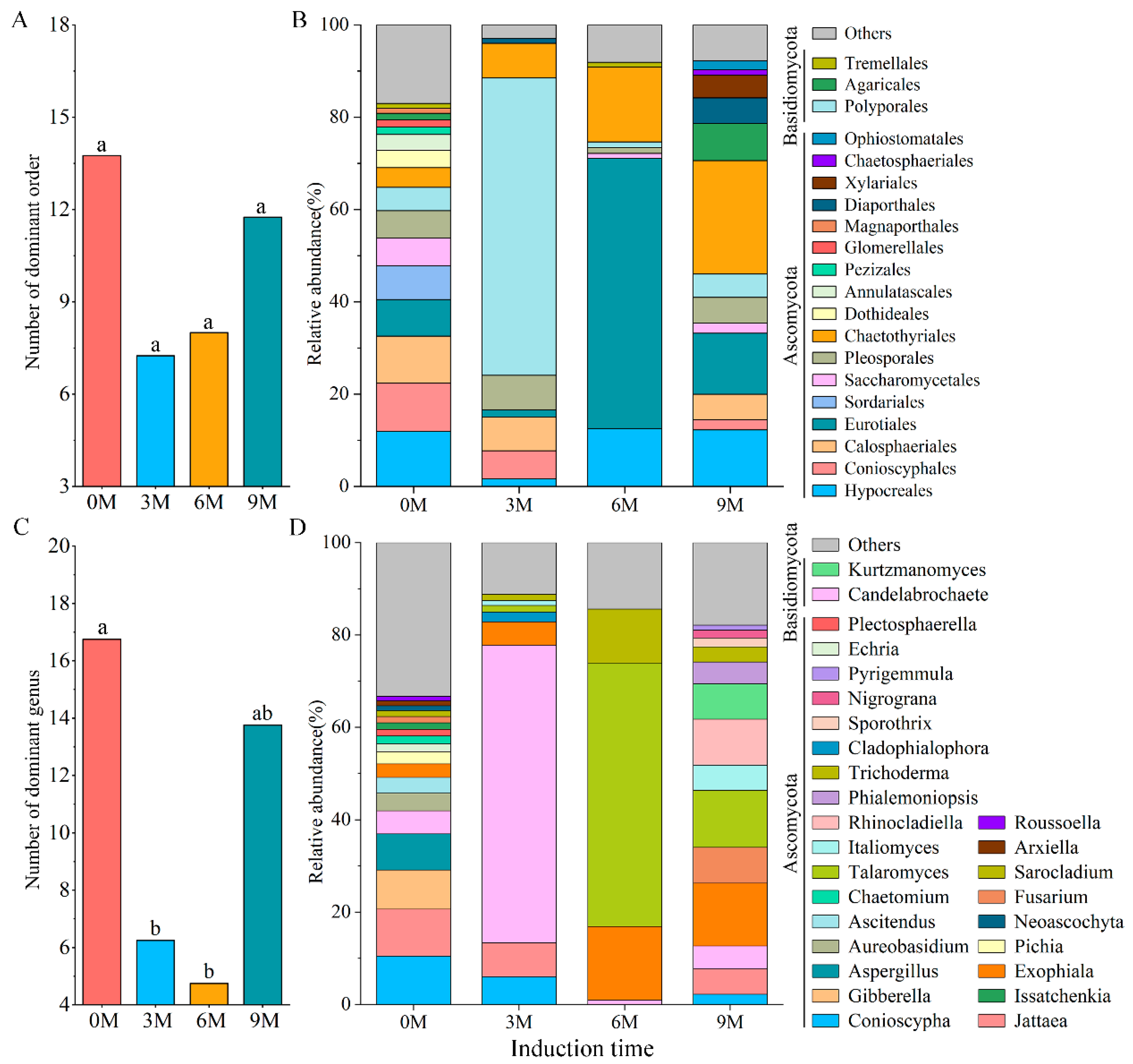
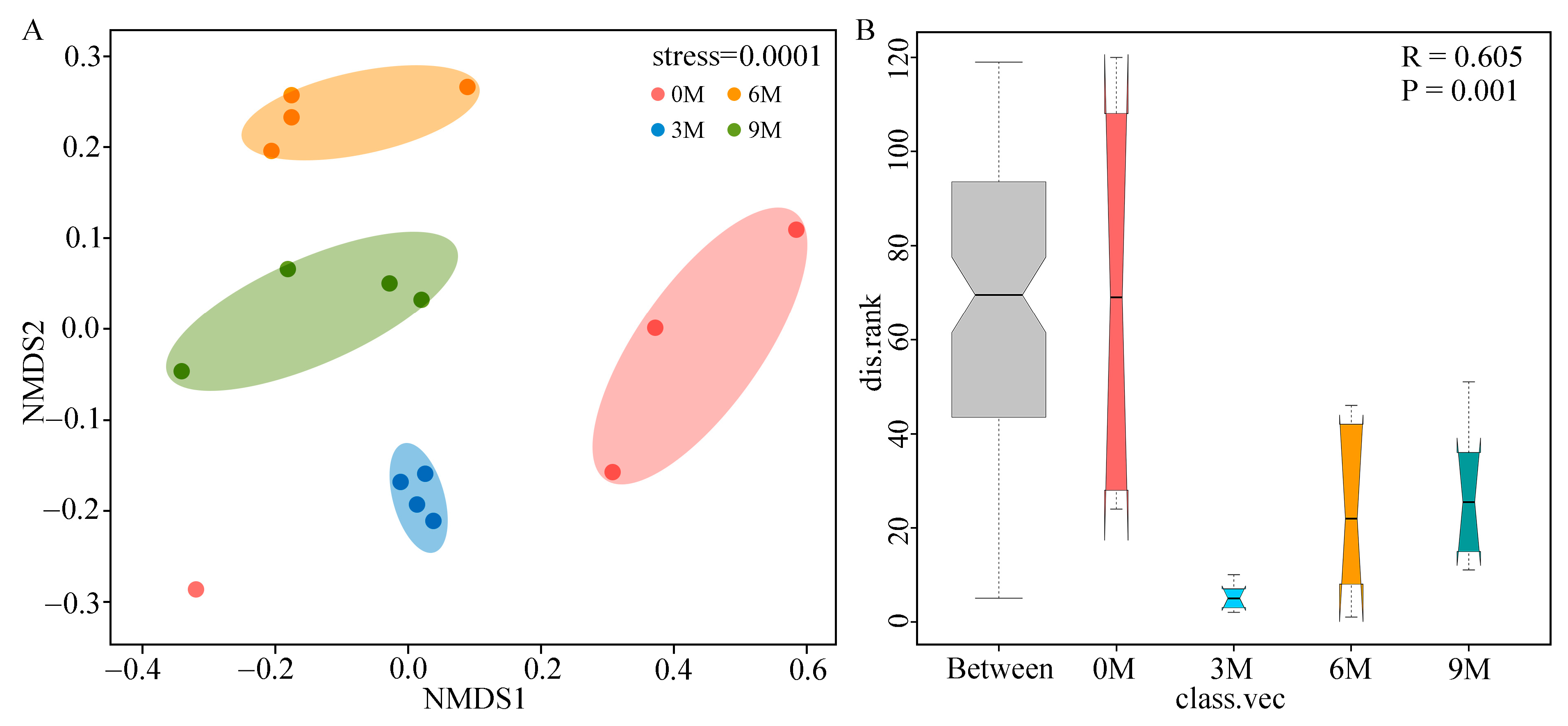
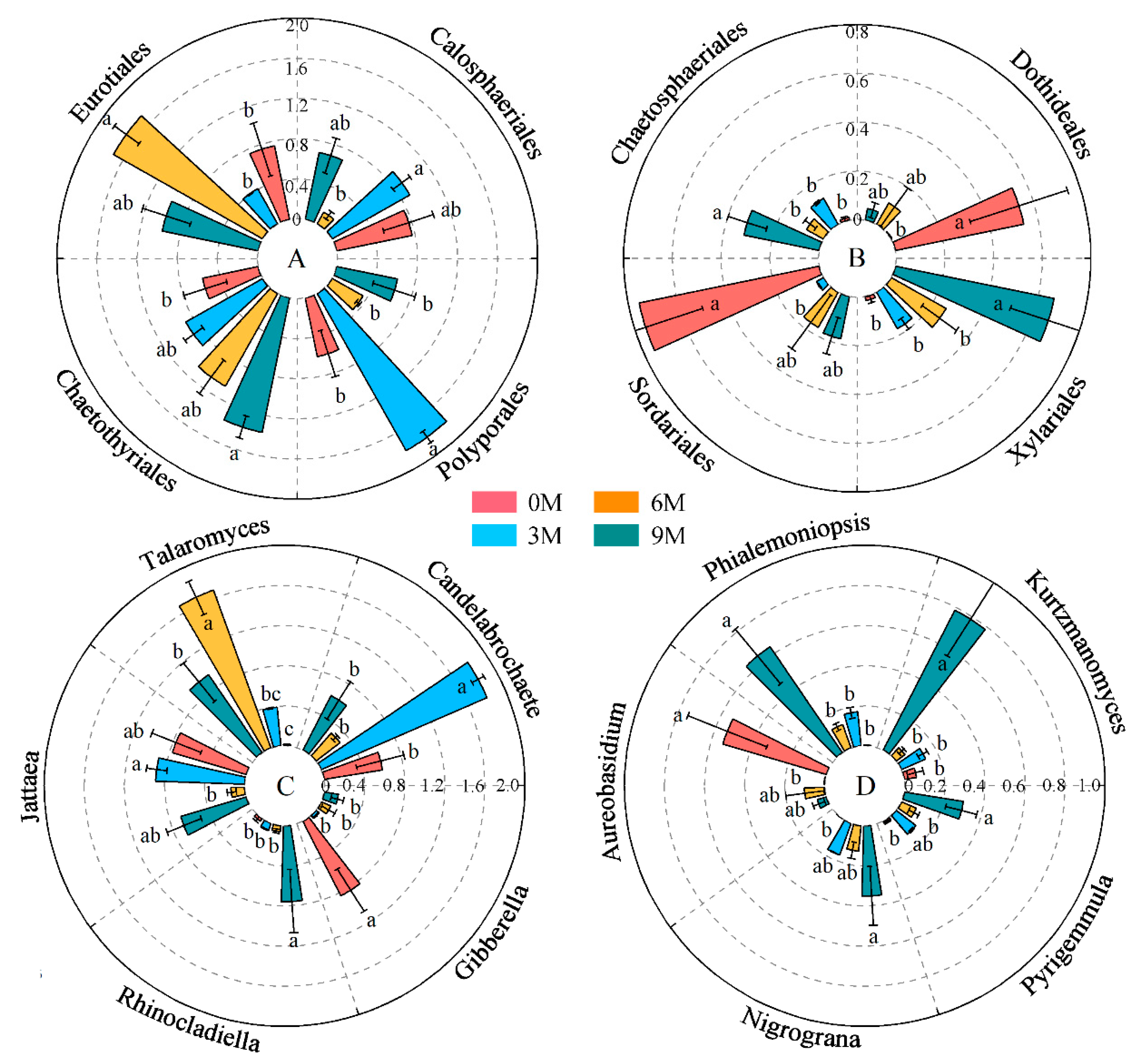
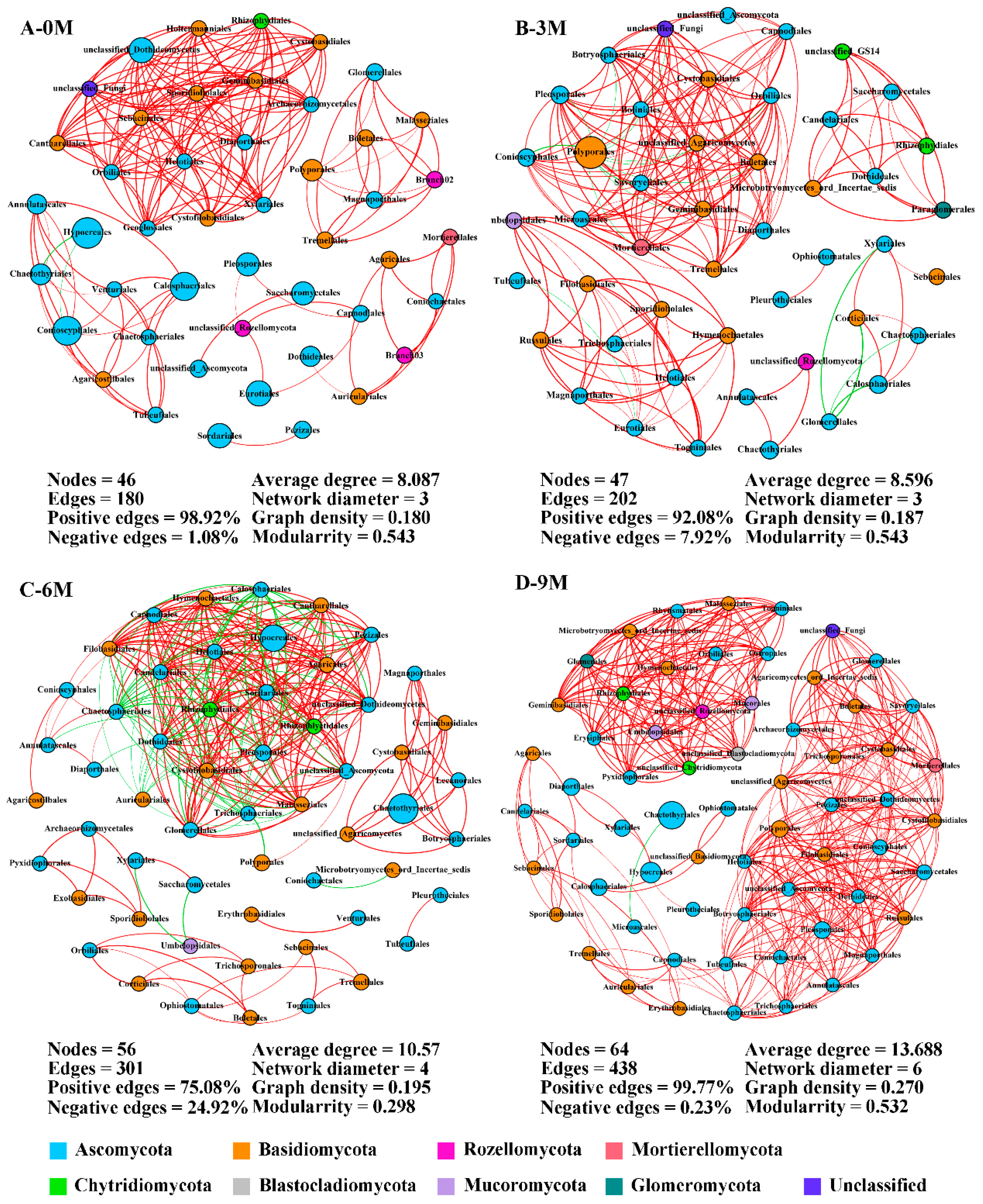
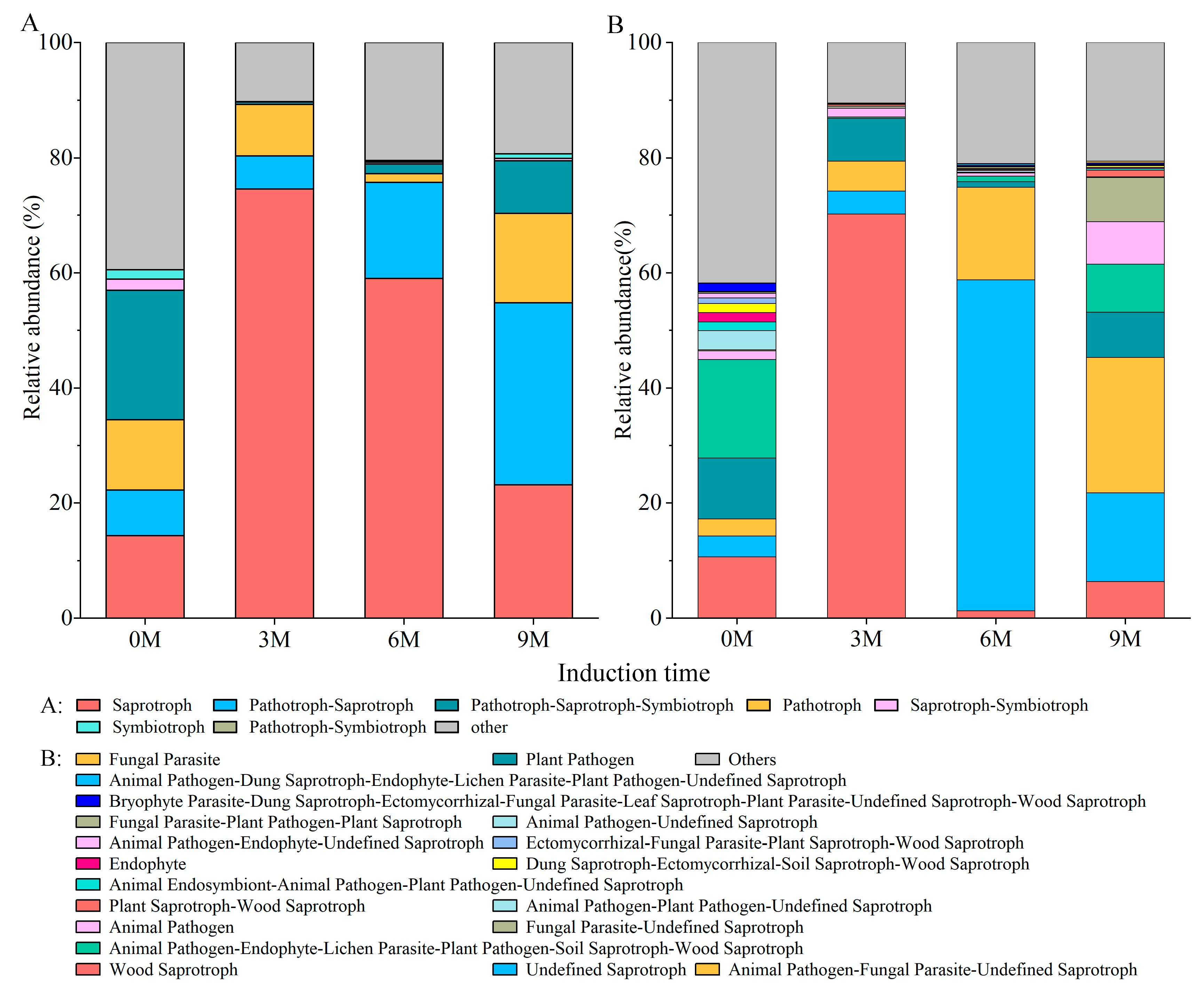
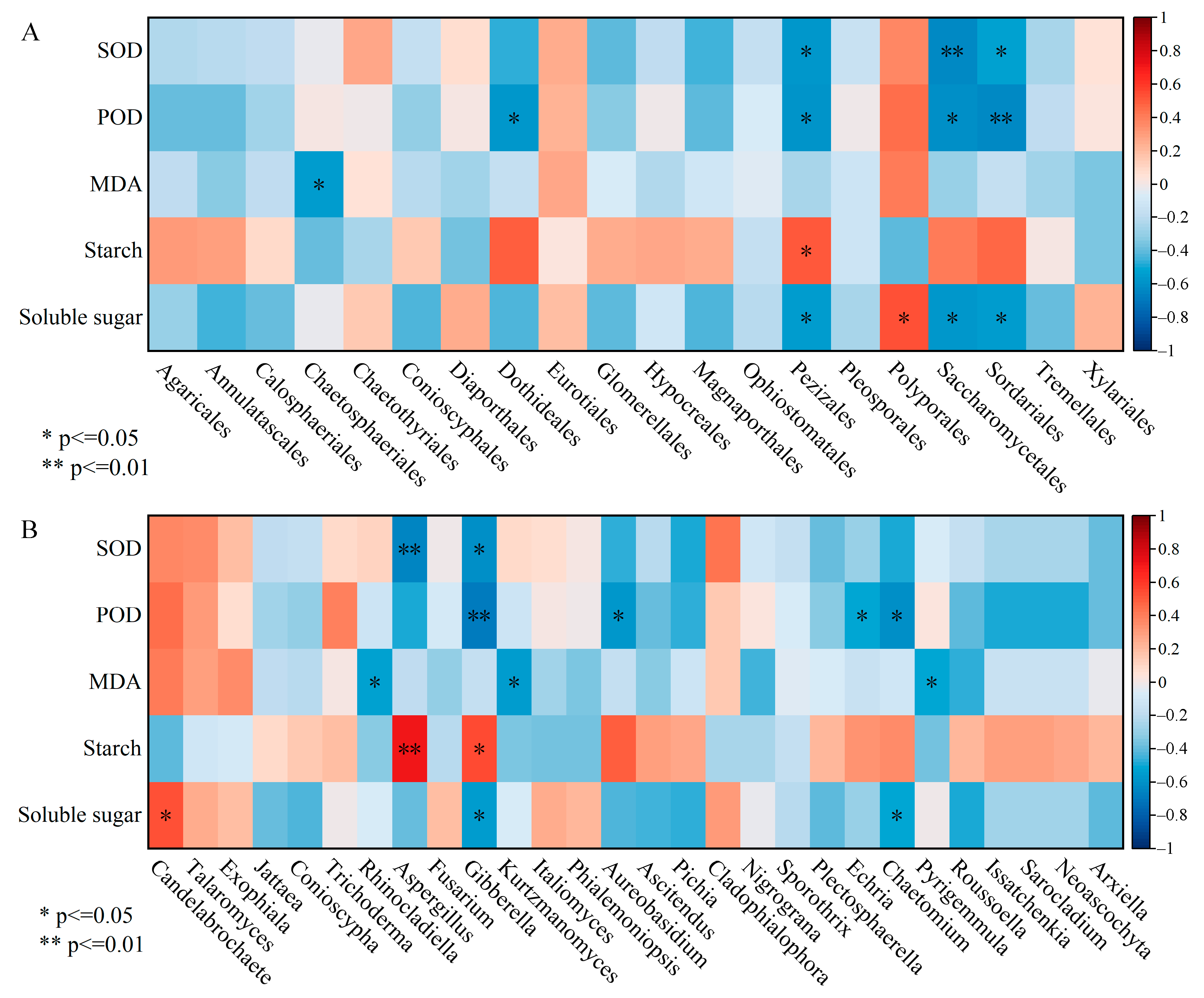
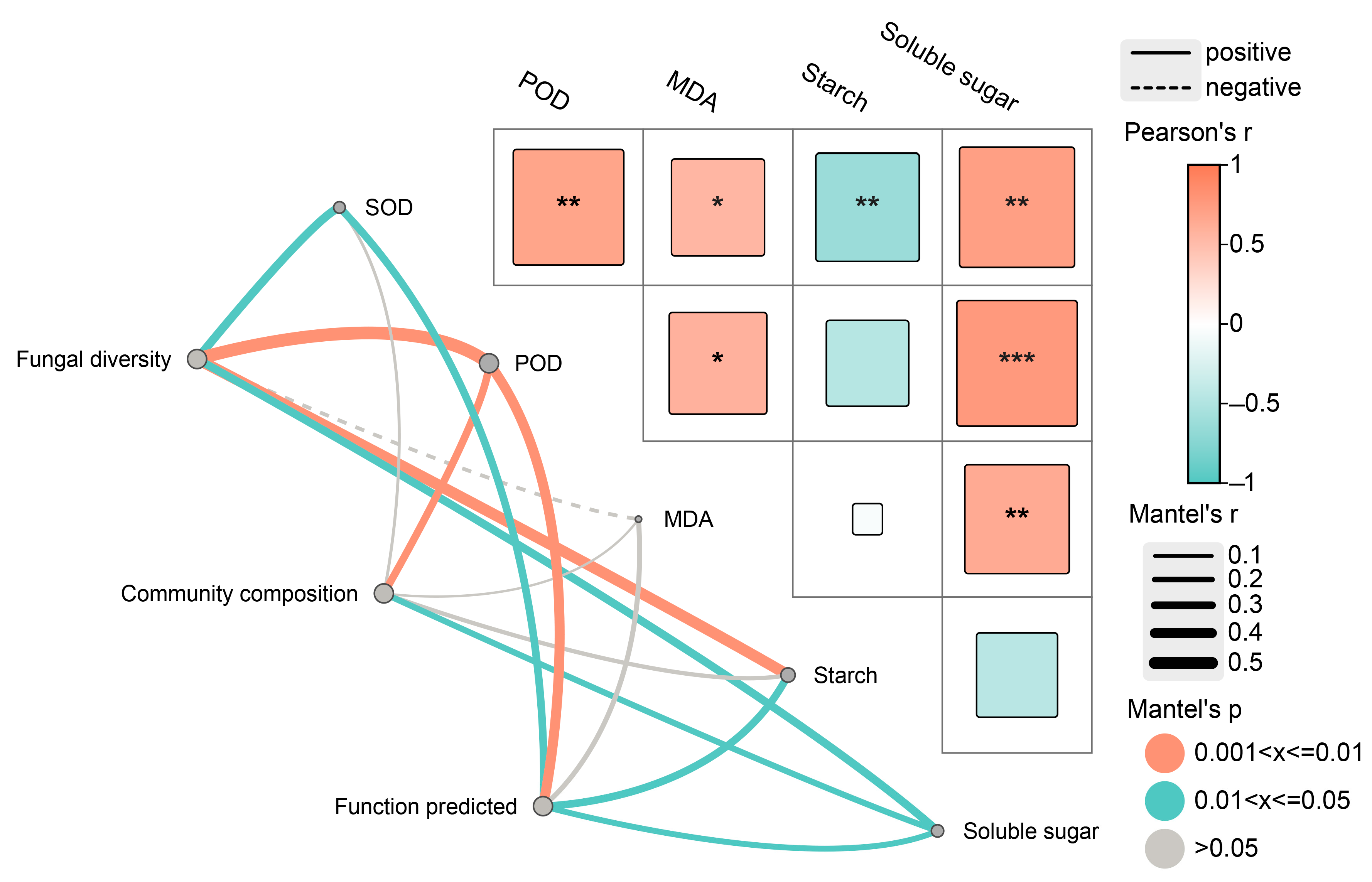
| Induction Time | Physio-Biochemistry Properties | ||||
|---|---|---|---|---|---|
| SOD (U·g−1) | POD (U·mg−1) | MDA (nmol·g−1) | Starch Content (mg·g−1) | Soluble Sugar (mg·g−1) | |
| 0M | 69.52 ± 4.71 b | 8.53 ± 0.33 c | 36.91 ± 1.42 bc | 215.00 ± 4.96 a | 23.53 ± 1.11 b |
| 3M | 106.06 ± 5.19 a | 11.83 ± 0.30 a | 44.57 ± 1.00 a | 182.91 ± 5.31 b | 33.78 ± 1.39 a |
| 6M | 104.74 ± 3.26 a | 11.62 ± 0.36 a | 43.93 ± 2.22 ab | 198.02 ± 5.56 ab | 32.17 ± 1.58 a |
| 9M | 86.20 ± 6.50 ab | 10.10 ± 0.11 b | 33.34 ± 2.01 c | 184.93 ± 2.99 b | 28.11 ± 1.91 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, R.; Lin, Y.; Zhao, W.; Lin, Q.; Ouyang, L.; Pang, S.; Zeng, H. Dynamics of Physiological Properties and Endophytic Fungal Communities in the Xylem of Aquilaria sinensis (Lour.) with Different Induction Times. J. Fungi 2024, 10, 562. https://doi.org/10.3390/jof10080562
Zhang Q, Li R, Lin Y, Zhao W, Lin Q, Ouyang L, Pang S, Zeng H. Dynamics of Physiological Properties and Endophytic Fungal Communities in the Xylem of Aquilaria sinensis (Lour.) with Different Induction Times. Journal of Fungi. 2024; 10(8):562. https://doi.org/10.3390/jof10080562
Chicago/Turabian StyleZhang, Qingqing, Rongrong Li, Yang Lin, Weiwei Zhao, Qiang Lin, Lei Ouyang, Shengjiang Pang, and Huahao Zeng. 2024. "Dynamics of Physiological Properties and Endophytic Fungal Communities in the Xylem of Aquilaria sinensis (Lour.) with Different Induction Times" Journal of Fungi 10, no. 8: 562. https://doi.org/10.3390/jof10080562
APA StyleZhang, Q., Li, R., Lin, Y., Zhao, W., Lin, Q., Ouyang, L., Pang, S., & Zeng, H. (2024). Dynamics of Physiological Properties and Endophytic Fungal Communities in the Xylem of Aquilaria sinensis (Lour.) with Different Induction Times. Journal of Fungi, 10(8), 562. https://doi.org/10.3390/jof10080562






