Differential Strategies of Two Arbuscular Mycorrhizal Fungi Varieties in the Protection of Lycium ruthenicum under Saline–Alkaline Stress
Abstract
1. Introduction
2. Materials and Methods
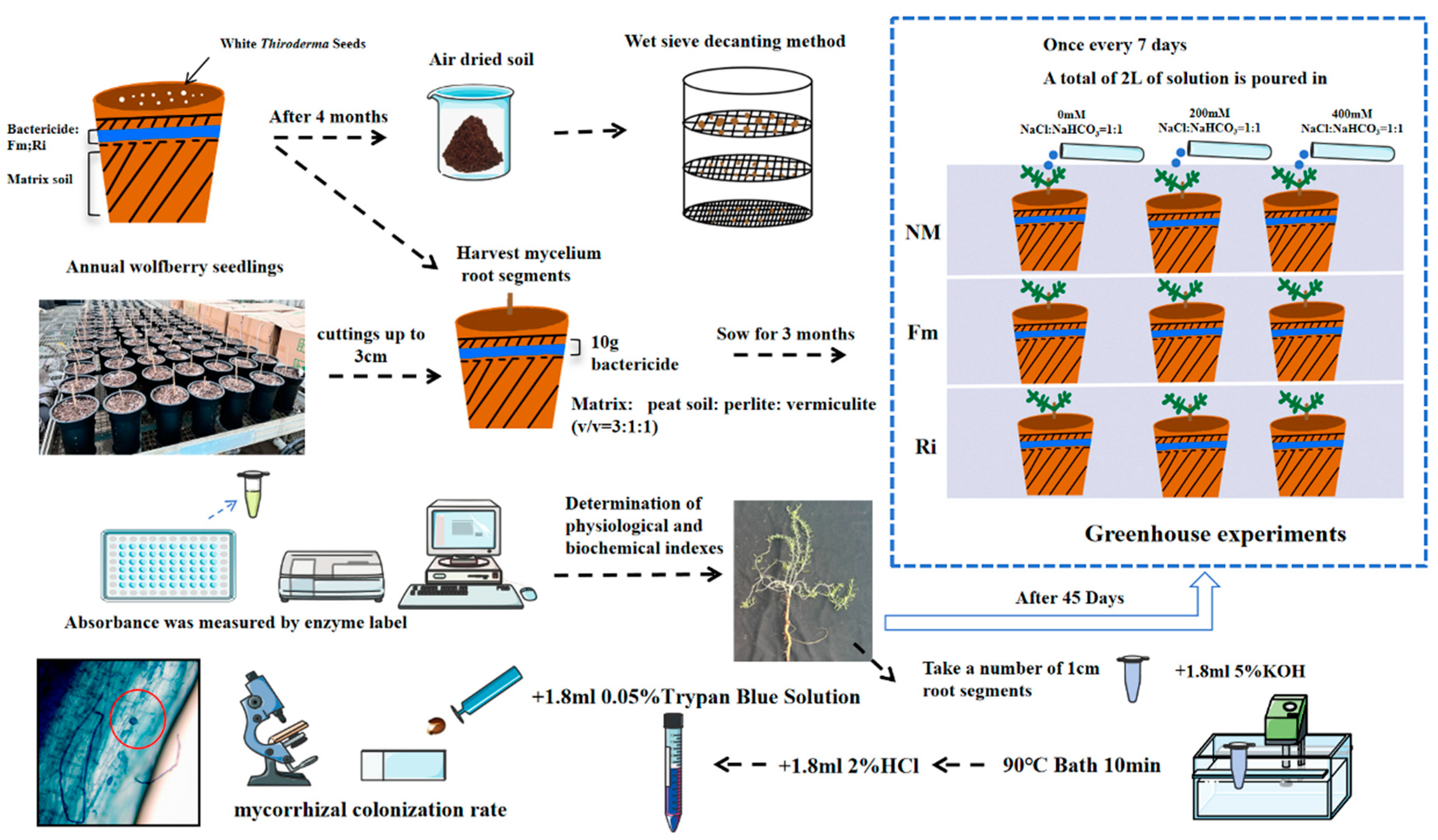
2.1. Experimental Design
2.1.1. Culture of Fungal Species
2.1.2. Material and Treatment
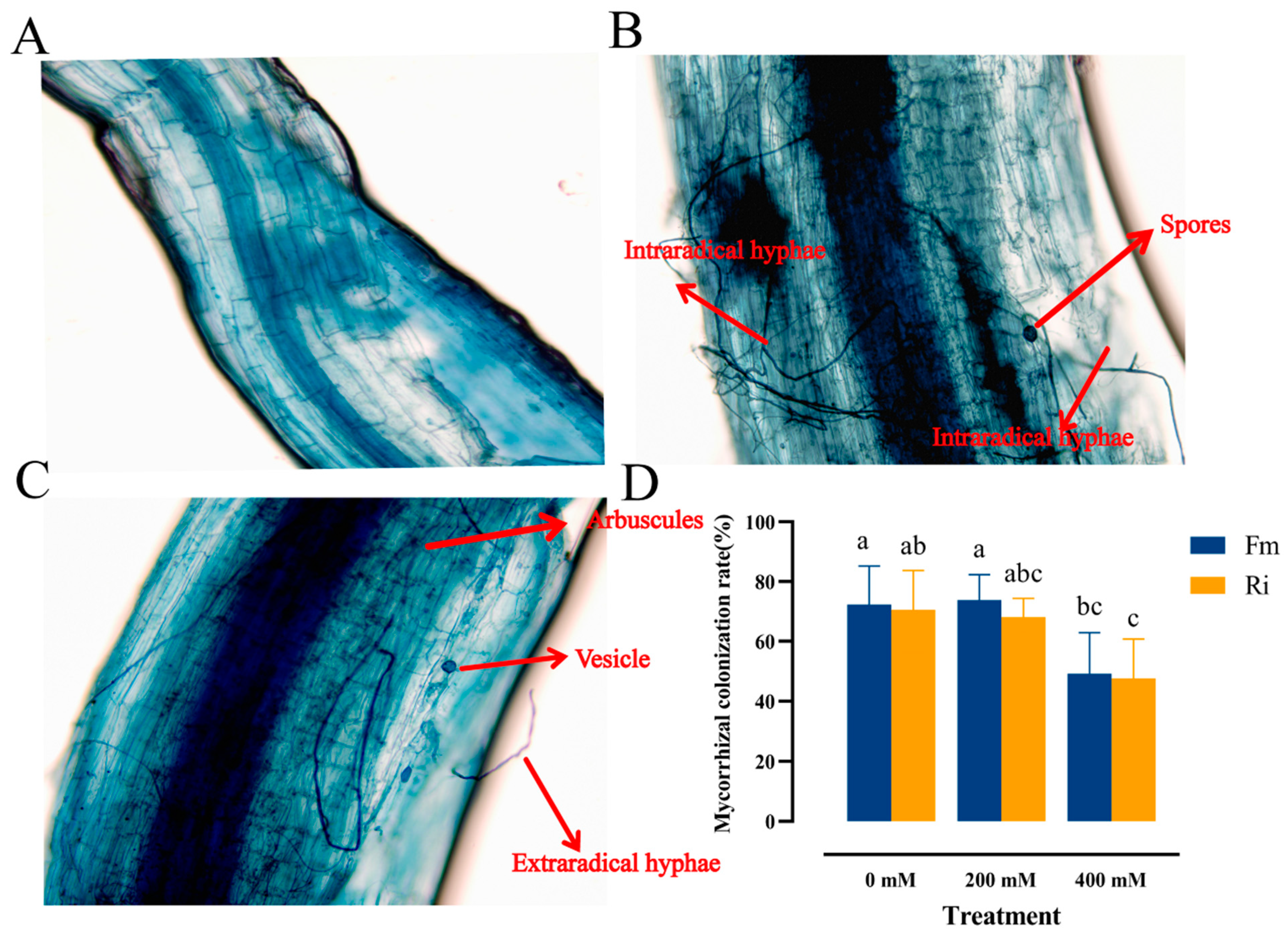
2.1.3. Mycorrhizal Colonization Rate Determination
2.2. Experimental Methods
2.3. Data Statistics and Analysis
3. Results and Analysis
3.1. Effect of Saline–Alkaline Stress on AMF Colonization in the Roots of Black Wolfberry
3.2. Effects of Diverse AMF on the Growth of Lycium ruthenicum under Saline–Alkaline Stress
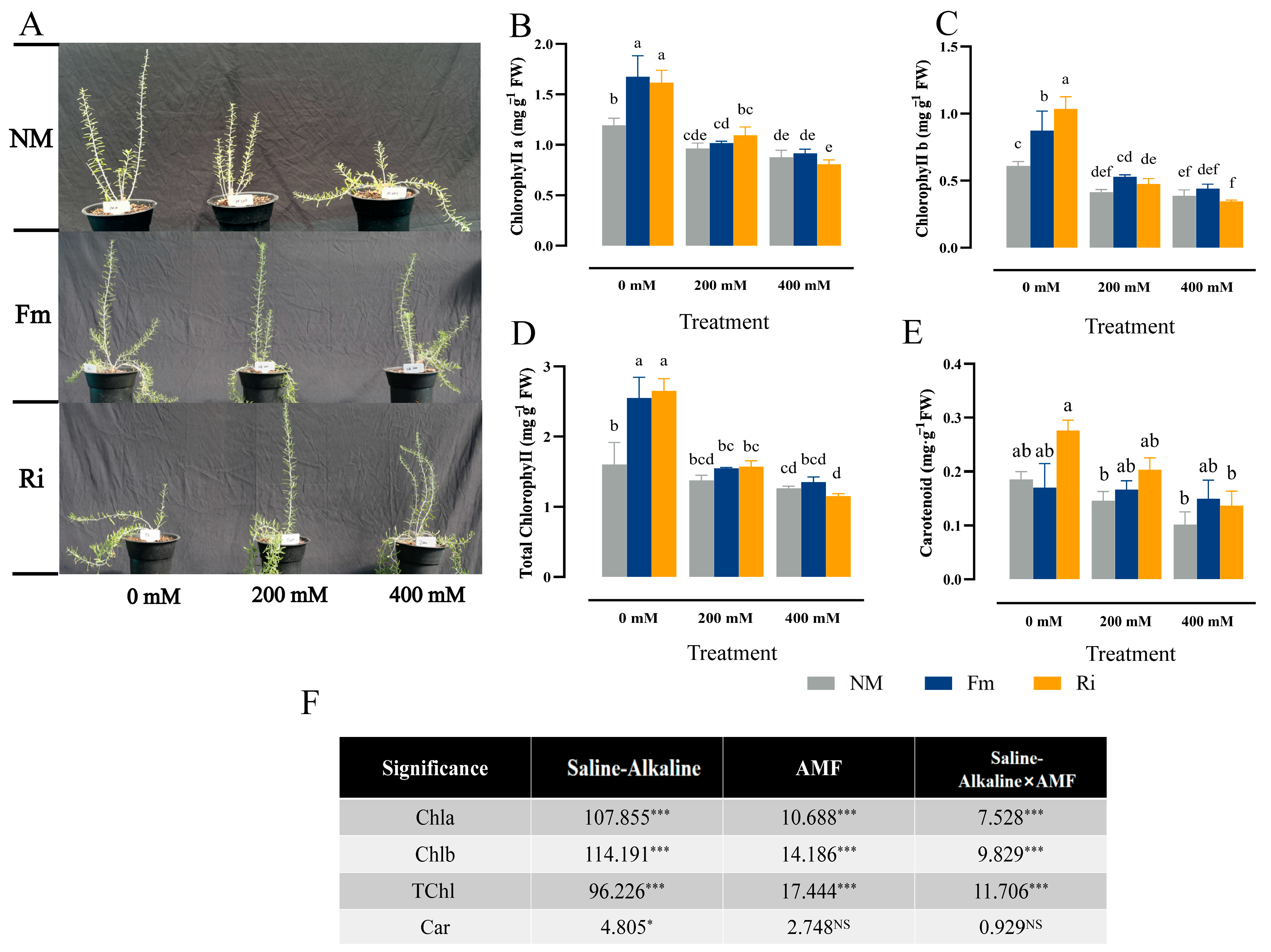
3.3. Effect of Diverse AMF on Photosynthetic Pigment Accumulation of Lycium ruthenicum under Saline–Alkaline Stress
3.4. Effect of Diverse AMF on Membrane Permeability of Lycium ruthenicum under Saline–Alkaline Stress
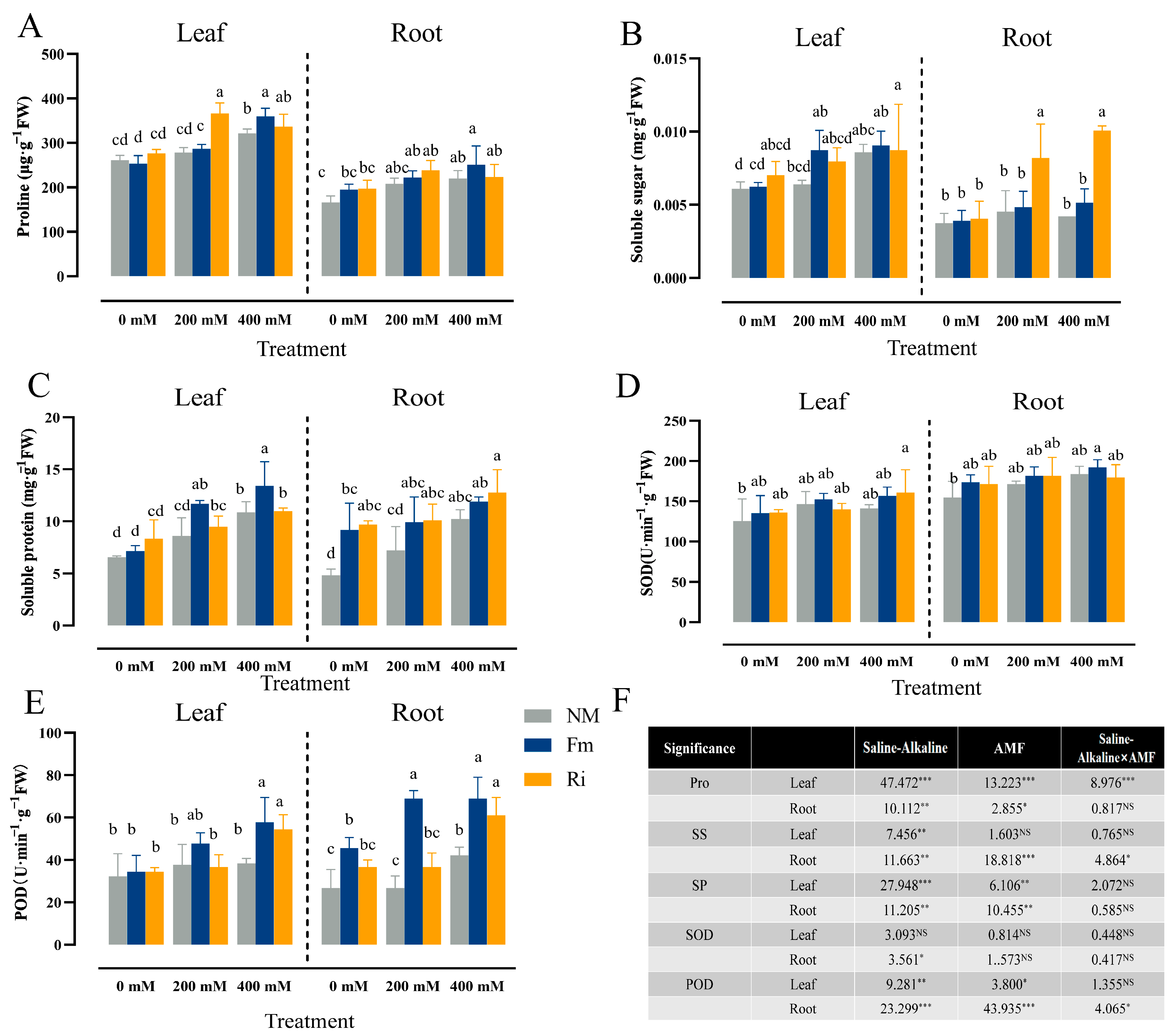
3.5. Effects of Diverse AMF on Osmotic Regulation and Antioxidant Activity of Lycium ruthenicum under Saline–Alkaline Stress
3.6. Effect of Diverse AMF on Endogenous Hormones of Lycium ruthenicum under Saline–Alkaline Stress
3.7. Effect of Diverse AMF under Salt and Alkali Stress on Mineral Element Content of Root of Lycium ruthenicum under Saline–Alkaline Stress
3.7.1. Effect of Diverse AMF under Salt and Alkali Stress on the Content of Macroelements in the Root of Lycium ruthenicum
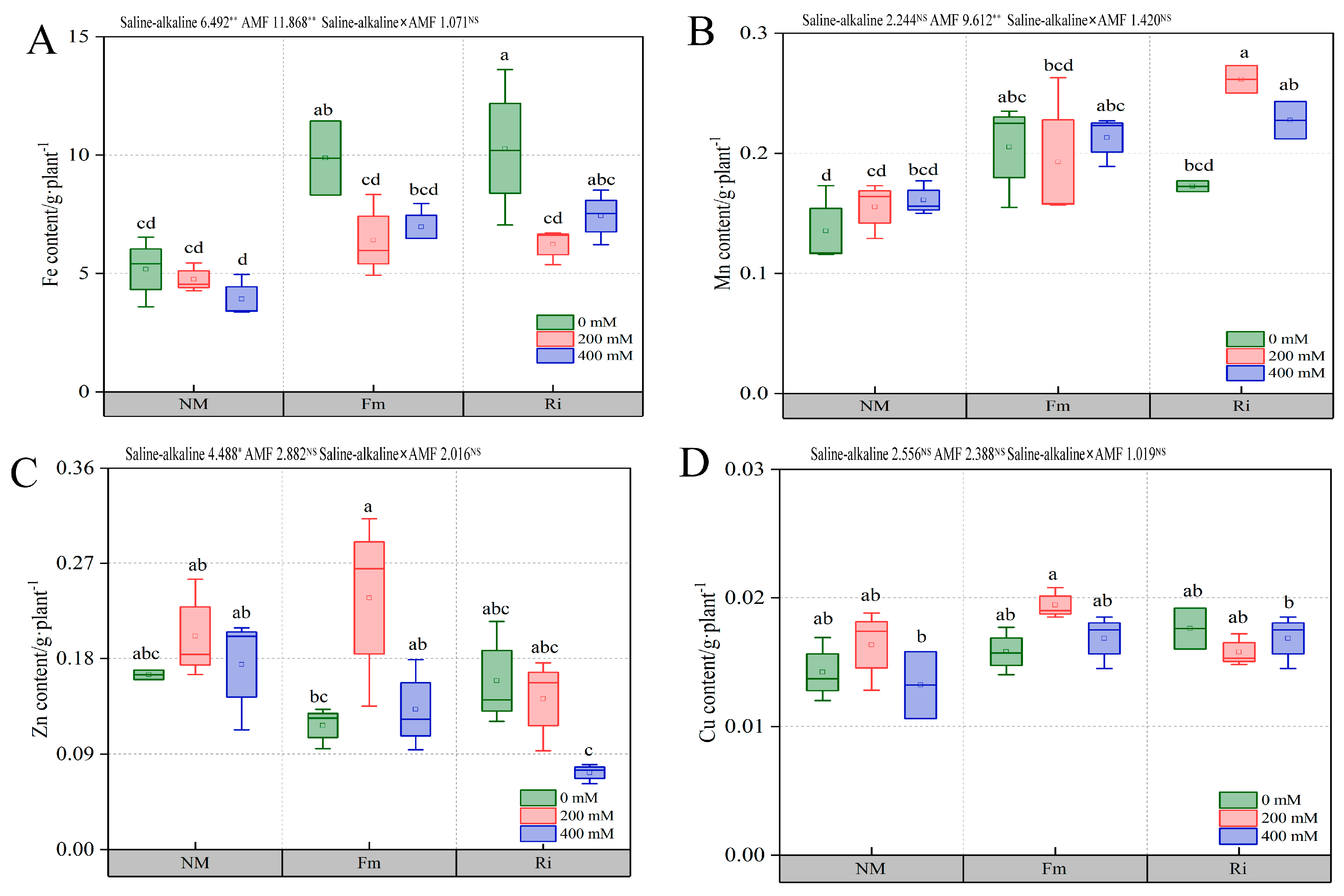
3.7.2. Effect of Diverse AMF under Salt and Alkali Stress on Trace Element Content of Root System of Lycium ruthenicum under Saline–Alkaline Stress
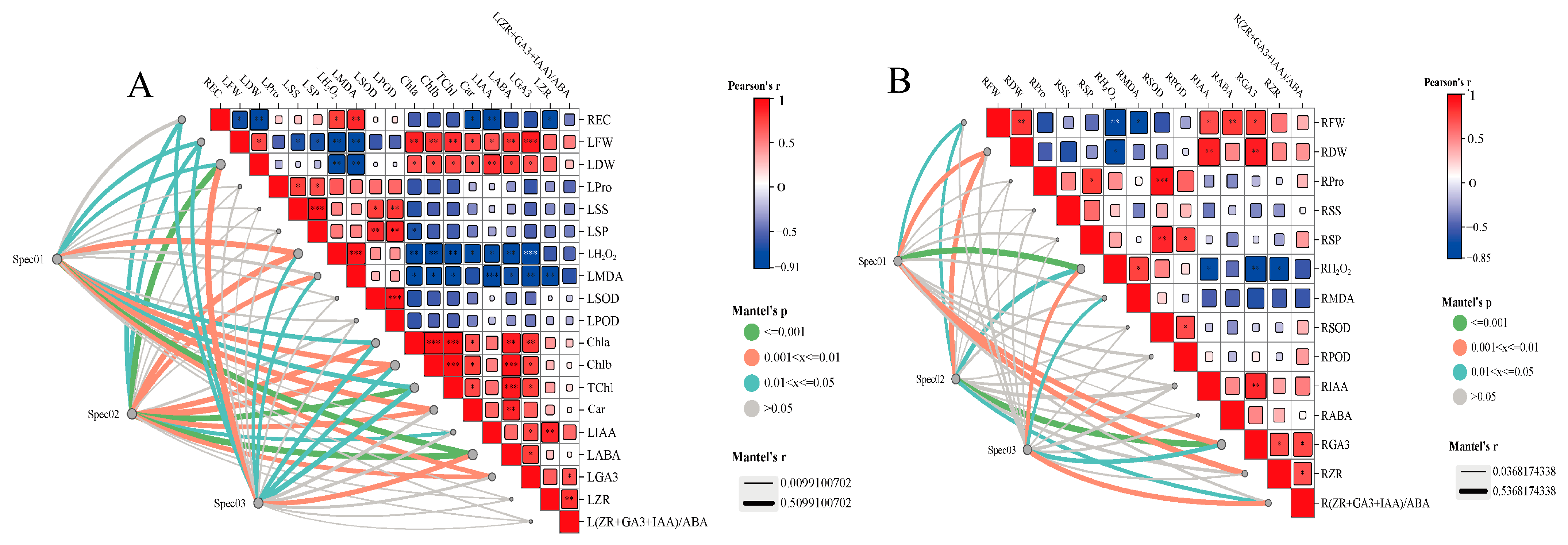
3.8. Evaluation of Correlation between Mineral Elements and Physiological Indices
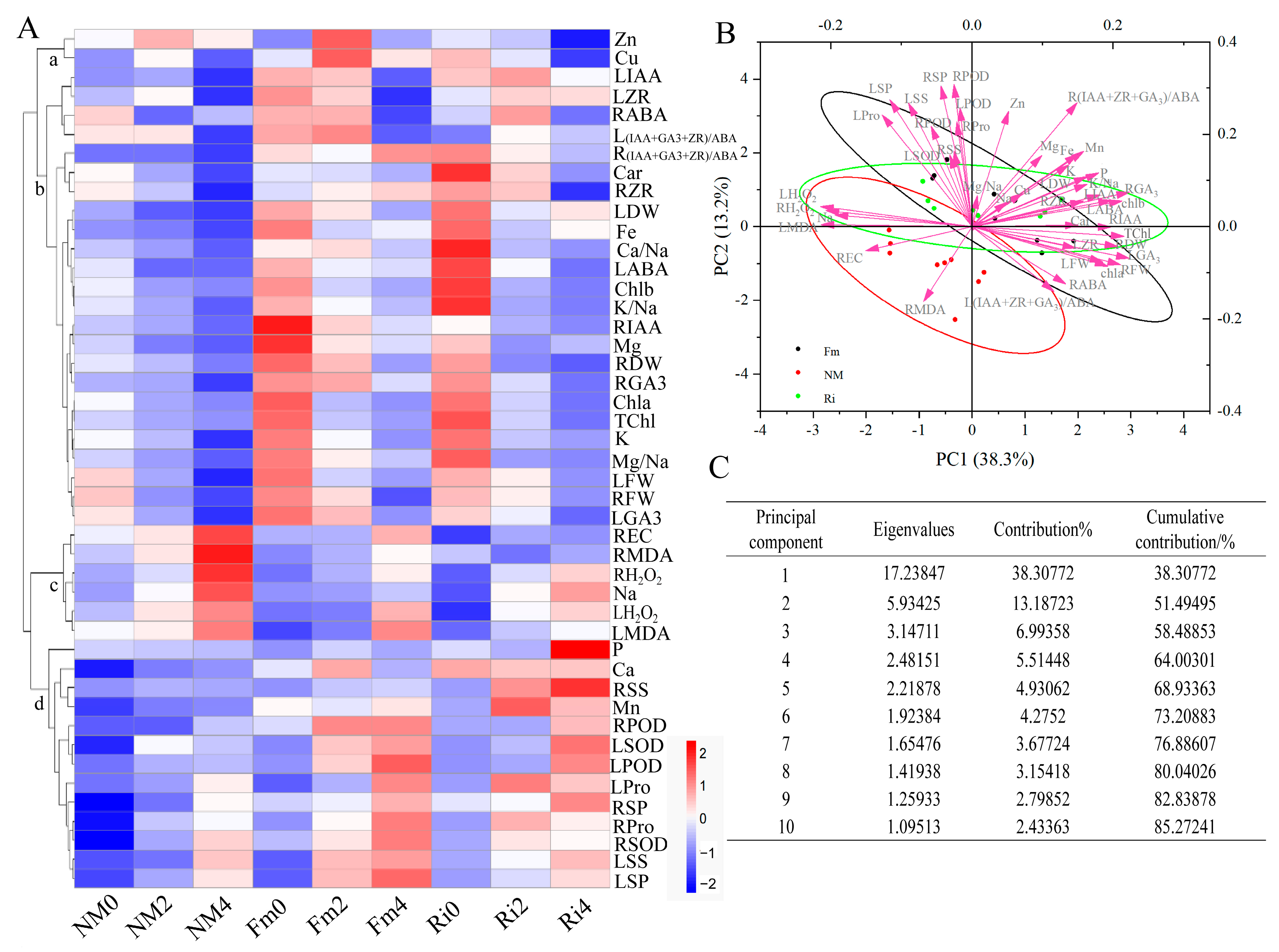
3.9. Comprehensive Evaluation of Lycium ruthenicum under Saline–Alkaline Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashraf, M.; Iram, A. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Sarathambal, C.; Sivaranjani, R.; Srinivasan, V.; Alagupalamuthirsolai, M.; Subila, K.P.; Anamika, B. Effect of arbuscular mycorrhizal inoculation on growth, mineral nutrient uptake, photosynthesis and antioxidant activities of black pepper cuttings. J. Plant Nutr. 2023, 46, 2508–2524. [Google Scholar] [CrossRef]
- Kang, Y. Physiological and Molecular Mechanisms of Potato Response to Alkaline Salt Stress. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Wang, Y.; Wang, J.; Guo, D.; Zhang, H.; Che, Y.; Li, Y.; Tian, B.; Wang, Z.; Sun, G.; Zhang, H. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant Physiol. Biochem. 2021, 167, 140–152. [Google Scholar] [CrossRef]
- Ci, D.; Qin, F.; Tang, Z.; Zhang, G.; Zhang, J.; Si, T.; Yang, J.; Xu, Y.; Yu, T.; Xu, M.; et al. Arbuscular Mycorrhizal Fungi Restored the Saline–Alkali Soil and Promoted the Growth of Peanut Roots. Plants 2023, 12, 3426. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Q.; Park, S.C.; Wang, X.; Liu, Y.J.; Zhang, Y.G.; Tang, W.; Kou, M.; Ma, D.F. Overexpression of CuZnSOD and APX enhance salt stress tolerance in sweet potato. Plant Physiol. Biochem. 2016, 109, 20–27. [Google Scholar] [CrossRef]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef]
- Lv, S.; Yu, D.; Sun, Q.; Jiang, J. Activation of gibberellin 20-oxidase 2 undermines auxin-dependent root and root hair growth in NaCl-stressed Arabidopsis seedlings. Plant Growth Regul. 2018, 84, 225–236. [Google Scholar] [CrossRef]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 2020, 11, 588550. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Wang, X.; Takano, T.; Liu, S. A peroxisomal APX from Puccinellia tenuiflora improves the abiotic stress tolerance of transgenic Arabidopsis thaliana through decreasing of H2O2 accumulation. J. Plant Physiol. 2015, 175, 183–191. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Z.; Zhang, H.; Min, W.; Hou, Z. Comparative Effects of Salt and Alkali Stress on Antioxidant System in Cotton (Gossypium Hirsutum L.) Leaves. Open Chem. 2019, 17, 1352–1360. [Google Scholar] [CrossRef]
- Gunes, H.; Demir, S.; Erdinc, C.; Furan, M.A. Erratum to: Effects of Arbuscular Mycorrhizal Fungi (AMF) and Biochar On the Growth of Pepper (Capsicum annuum L.) Under Salt Stress. Gesunde Pflanz. 2023, 75, 2683. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Zhen, L. Sex-specific photosynthetic capacity and Na+ homeostasis in Populus euphratica exposed to NaCl stress and AMF inoculation. Front. Plant Sci. 2022, 13, 1066954. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Zhang, N.L.; Zhang, L.L.; Zhang, X.L.; Wu, A.P.; Huang, J.Y.; Yu, S.Q.; Wang, Y.H. Mediation of arbuscular mycorrhizal fungi on growth and biochemical parameters of Ligustrum vicaryi in response to salinity. Physiol. Mol. Plant Pathol. 2020, 112, 101522. [Google Scholar] [CrossRef]
- Hadian-Deljou, M.; Esna-Ashari, M.; Mirzaie-Asl, A. Alleviation of salt stress and expression of stress-responsive gene through the symbiosis of arbuscular mycorrhizal fungi with sour orange seedlings. Sci. Hortic. 2020, 268, 109373. [Google Scholar] [CrossRef]
- Ji, X.; Tang, J.; Zheng, X.; Li, A.; Zhang, J. The regulating mechanism of salt tolerance of black walnut seedlings was revealed by the physiological and biochemical integration analysis. Plant Physiol. Biochem. PPB 2024, 210, 108548. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Huang, C.H.; Peng, F.; Xue, X.; Wang, T. Effect of salt stress on photosynthesis and related physiological characteristics of Lycium ruthenicum Murr. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 680–692. [Google Scholar] [CrossRef]
- Cui, Y.X.; Fang, M.L.; Li, Z.W.; Lian, M.G. Changes in the morphology traits, anatomical structure of the leaves and transcriptome in Lycium barbarum L. under salt stress. Front. Plant Sci. 2023, 14, 1090366. [Google Scholar]
- Tao, G.; Gai, J.; Ruan, S.; Lou, X.; Zhao, X. Potential effects of endosymbiotic bacteria on the growth promotion of host arbuscular mycorrhizal fungi in capsicum. North. Hortic. 2024, 1, 1–8. [Google Scholar]
- Li, Z.; Han, X.; Son, X. Overexpressing the Sedum alfredii Cu/Zn Superoxide Dismutase Increased Resistance to Oxidative Stress in Transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1010. [Google Scholar] [CrossRef]
- Zhu, S.; Nong, J.; Luo, G.; Li, Q.; Wang, F.; Jiang, D.; Zhao, X. Varied tolerance and different responses of five citrus rootstocks to acid stress by principle component analysis and orthogonal analysis. Sci. Hortic. 2020, 278, 109853. [Google Scholar] [CrossRef]
- Yi, L.; Yao, L.; Li, S.; Huang, A. Structural changes in‘Newhall’navel oranges infected with Candiatus Liberibacter Asiaticus. J. Fruit Sci. 2018, 35, 853–858. [Google Scholar]
- Liu, N.; Gao, Y.; Jia, C. Changes in POD Activity, Free Proline Content and Cytomembrane Permeability of Lolium multiflorum Leaves Under Different Levels of Osmotic Stress. Plant Physiol. Commun. 2000, 36, 11–13. [Google Scholar]
- Li, X.Y. Physiological and Ecological Responses of Three Aquatic Plants to Na+ Salt and K+ Salt Stresses. Master’s Thesis, Wuhan University, Wuhan, China, 2022. [Google Scholar]
- Wu, M.; Zhang, W.; Zhang, J. Effects of drought stress on growth, physiological and biochemical parameters in fine roots of Quercus variabilis Bl.seedlings. Acta Ecol. Sin. 2014, 34, 4223–4233. [Google Scholar]
- Wang, Z. Study on Mechanism of Salt-Tolerant Response of Piriformospora indica to Ryegrass (Lolium perenne). Master’s Thesis, Shandong Jianzhu University, Jinan, Chian, 2023. [Google Scholar]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Liu, S. Study on the Response Mechanism of Lycium Barbarum Quality to Rhizosphere Microbial Diversity. Master’s Thesis, Shihezi University, Shihezi, China, 2020. [Google Scholar]
- Xiao, L. Study on Microbial Community Diversity in Rhizosphere Soil of Lycium Barbarum and Its Effect on Salt Tolerance of Arbuscular Mycorrhizal Fungi. Master’s Thesis, Northwest A&F University, Xi’an, China, 2018. [Google Scholar]
- Wu, Y. Characteristics of Mangrove Infestation and Soil Spore Density in Different Regions of China. Master’s Thesis, Sun Yat-Sen University, Guangzhuo, China, 2013. [Google Scholar]
- Carvalho, L.M.; Correia, P.M.; Caçador, I.; Martins-Loução, M.A. Effects of salinity and flooding on the infectivity of salt marsh arbuscular mycorrhizal fungi in Aster tripolium L. Biol. Fertil. Soils 2003, 38, 137–143. [Google Scholar] [CrossRef]
- Ahmad, W.; Ayyub, C.M.; Shehzad, M.A.; Ziaf, K.; Ijaz, M.; Sher, A.; Abbas, T.; Shafi, J. Supplemental potassium mediates antioxidant metabolism, physiological processes, and osmoregulation to confer salt stress tolerance in cabbage (Brassica oleracea L.). Hortic. Environ. Biotechnol. 2019, 60, 853–869. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Ren, W.; Zhang, H.; Tang, M. Arbuscular Mycorrhizal Fungi Improve Lycium barbarum Potassium Uptake by Activating the Expression ofLbHAK. Plants 2024, 13, 1244. [Google Scholar] [CrossRef]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef]
- Qin, W.; Yan, H.; Zou, B.; Guo, R.; Ci, D.; Tang, Z.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; et al. Arbuscular mycorrhizal fungi alleviate salinity stress in peanut: Evidence from pot-grown and field experiments. Food Energy Secur. 2021, 10, e314. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, C.; Wang, Q.; Bie, S. Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress. Plants 2024, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Dong, F. Mechanism of Arbuscular Mycorrhizal Fungi Enhancing Salt-Alkali Tolerance of Little Black Poplar. Ph.D. Thesis, College of Forestry, Northwest A&F University, Xi’an, China, 2023. [Google Scholar]
- Zhang, X.; Li, S.; Tang, T.; Liu, Y.; Tahir, M.M.; Wang, C.; Meng, Z.; Niu, J.; Yang, W.; Ma, J.; et al. Comparison of morphological, physiological, and related-gene expression responses to saline-alkali stress in eight apple rootstock genotypes. Sci. Hortic. 2022, 306, 111455. [Google Scholar] [CrossRef]
- Kong, L.; Xiaowen, G.; Xiaolin, Z.; Wenze, Z.; Jin, S.; Bolang, C. Effects of arbuscular mycorrhizal fungi on photosynthesis, ion balance of tomato plants under saline-alkali soil condition. J. Plant Nutr. 2020, 43, 682–698. [Google Scholar] [CrossRef]
- Yang, C. Effects of Melatonin on Arbuscular Mycorrhizal Symbiosis and Drought Tolerance of Kiwifruit. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2023. [Google Scholar]
- Wang, H.; Li, J.; Tao, W. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, R.; Liu, C.; Wang, Y.; Li, P.; Yuan, Y. Effects of AM fungi and salicylic acid on salt tolerance of strawberry. Sci. Agric. Sin. 2009, 42, 1590–1594. [Google Scholar]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, Y.; Lin, J.; Shi, K. Effects of arbuscular mycorrhizal fungi on root growth and hormones of Machilus machilus under salt stress. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 119–124. [Google Scholar]
- Wang, Y. Arbuscular Mycorrhizal Fungi and Potassium Regulate the Salt Tolerance and Potassium Absorption Mechanism of Lycium barbarum in Ningxia. Master’s Thesis, Northwest A&F University, Xi’an, China, 2020. [Google Scholar]
- Li, A.; Wu, C.; Zheng, X. Physiological and biochemical responses of arbuscular mycorrhizal fungi in symbiosis with Juglans nigra L. seedlings to alleviate salt stress. Rhizosphere 2024, 31, 100928. [Google Scholar] [CrossRef]
- Errickson, W.; Huang, B. Rhizobacteria-enhanced drought tolerance and post-drought recovery of creeping bentgrass involving differential modulation of leaf and root metabolism. Physiol. Plant. 2023, 175, e14004. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. Effects of Arbuscular Mycorrhizae and Zinc on the Growth and Development of Citrus Seedlings. Master’s Thesis, Southwest University, Chongqing, China, 2023. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, F.; Chen, H.; Xu, T.; Tang, M. Effects of Arbuscular Mycorrhizal Fungus on Sodium and Chloride Ion Channels of Casuarina glauca under Salt Stress. Int. J. Mol. Sci. 2023, 24, 3680. [Google Scholar] [CrossRef]
- Alam, M.Z.; Choudhury, T.R.; Mridha, M.A.U. Arbuscular Mycorrhizal Fungi Enhance Biomass Growth, Mineral Content, and Antioxidant Activity in Tomato Plants under Drought Stress. J. Food Qual. 2023, 2023, 2581608. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y. Alleviating Effect of Arbuscular Mycorrhizal Fungi on Saline-Alkali Stress of Watermelon and Its Regulatory Mechanism. Ph.D. Thesis, Northwest A&F University, Xi’an, China, 2019. [Google Scholar]
- Lian, X.; Wang, Y.; Liang, X.; Yang, J.; Wang, Z. Effects of different fertilization levels on the absorption of trace elements in plant tomato. Jiangsu Agric. Sci. 2020, 48, 197–200. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined Inoculation with Multiple Arbuscular Mycorrhizal Fungi Improves Growth, Nutrient Uptake and Photosynthesis in Cucumber Seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Sun, Y.; Zhang, G.; Han, J. Absorption and distribution of mineral elements in tomato under salt stress. North. Hortic. 2024, 11, 40–49. [Google Scholar]
- Qu, L.; Guo, X.; Jia, Z.; Zhang, Y.; Yang, Z.; Ma, H.; Liu, Y. Research progress of arbuscular mycorrhizal fungi improving plant abiotic stress adaptability. Agrostology 2024, 4–9. [Google Scholar] [CrossRef]
- Maryam, A.; Nematollah, E.; Ali, N. The arbuscular mycorrhizal symbiosis alleviating long-term salt stress through the modulation of nutrient elements, osmolytes, and antioxidant capacity in rosemary. Biologia 2022, 78, 993–1010. [Google Scholar]



| Index | pH | Dry Matter (g/L) | EC Value | Nitrate Nitrogen Content (g·kg−1) | Ammonium Nitrogen Content (g·kg−1) | Phosphorus Content (g·kg−1) |
|---|---|---|---|---|---|---|
| Matrix soil | 6.0 | 55–75 | 2.0–4.0 | 70 | 50 | 140 |
| AMF | Saline–Alkaline (mM) | SFW (g) | RFW (g) | SDW (g) | RDW (g) |
|---|---|---|---|---|---|
| NM | 0 | 12.39 ± 2.35 ab | 17.14 ± 2.08 b | 2.23 ± 0.96 bc | 4.30 ± 0.26 bc |
| 200 | 8.67 ± 1.39 bc | 10.11 ± 1.83 cd | 1.60 ± 0.50 c | 3.49 ± 0.59 cd | |
| 400 | 5.20 ± 1.30 c | 7.11 ± 2.89 d | 1.31 ± 0.61 c | 2.36 ± 1.00 cd | |
| Fm | 0 | 14.76 ± 4.83 a | 19.76 ± 2.93 a | 3.87 ± 0.38 a | 7.49 ± 2.26 a |
| 200 | 9.70 ± 1.71 abc | 16.31 ± 2.70 b | 3.27 ± 1.25 ab | 5.90 ± 1.01 ab | |
| 400 | 8.28 ± 1.61 bc | 7.53 ± 3.48 d | 2.24 ± 0.01 bc | 2.96 ± 1.58 cd | |
| Ri | 0 | 13.19 ± 2.03 ab | 17.58 ± 1.92 b | 4.32 ± 0.70 a | 6.52 ± 1.29 a |
| 200 | 11.49 ± 1.335 ab | 15.10 ± 1.04 bc | 2.84 ± 1.02 abc | 2.57 ± 0.57 cd | |
| 400 | 8.20 ± 4.66 bc | 9.87 ± 2.03 cd | 3.24 ± 1.26 ab | 1.79 ± 0.26 d | |
| Significance | |||||
| Saline–alkaline | 12.011 *** | 51.605 *** | 5.010 ** | 23.455 *** | |
| AMF | 1.976 NS | 21.941 *** | 10.845 *** | 8.550 *** | |
| Saline–alkaline×AMF | 0.835 NS | 9.936 *** | 0.635 NS | 0.088 NS | |
| AMF | Saline–Alkaline (mM) | μ(1) | μ(2) | μ(3) | μ(4) | D Value | Average | Order |
|---|---|---|---|---|---|---|---|---|
| NM | 0 | 0.61 | 0.00 | 0.37 | 0.39 | 0.47 | 0.34 | 3 |
| 200 | 0.35 | 0.25 | 0.48 | 0.59 | 0.39 | |||
| 400 | 0.00 | 0.32 | 0.72 | 0.28 | 0.18 | |||
| Fm | 0 | 0.98 | 0.59 | 0.41 | 0.37 | 0.85 | 0.71 | 1 |
| 200 | 0.65 | 0.85 | 0.63 | 1.00 | 0.77 | |||
| 400 | 0.25 | 0.95 | 1.00 | 0.34 | 0.52 | |||
| Ri | 0 | 1.00 | 0.70 | 0.75 | 0.00 | 0.90 | 0.62 | 2 |
| 200 | 0.50 | 0.81 | 0.23 | 0.57 | 0.59 | |||
| 400 | 0.21 | 1.00 | 0.00 | 0.14 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Li, A.; Nie, R.; Wu, C.; Ji, X.; Tang, J.; Zhang, J. Differential Strategies of Two Arbuscular Mycorrhizal Fungi Varieties in the Protection of Lycium ruthenicum under Saline–Alkaline Stress. J. Fungi 2024, 10, 554. https://doi.org/10.3390/jof10080554
Zheng X, Li A, Nie R, Wu C, Ji X, Tang J, Zhang J. Differential Strategies of Two Arbuscular Mycorrhizal Fungi Varieties in the Protection of Lycium ruthenicum under Saline–Alkaline Stress. Journal of Fungi. 2024; 10(8):554. https://doi.org/10.3390/jof10080554
Chicago/Turabian StyleZheng, Xu, Ao Li, Ruining Nie, Chengxu Wu, Xinying Ji, Jiali Tang, and Junpei Zhang. 2024. "Differential Strategies of Two Arbuscular Mycorrhizal Fungi Varieties in the Protection of Lycium ruthenicum under Saline–Alkaline Stress" Journal of Fungi 10, no. 8: 554. https://doi.org/10.3390/jof10080554
APA StyleZheng, X., Li, A., Nie, R., Wu, C., Ji, X., Tang, J., & Zhang, J. (2024). Differential Strategies of Two Arbuscular Mycorrhizal Fungi Varieties in the Protection of Lycium ruthenicum under Saline–Alkaline Stress. Journal of Fungi, 10(8), 554. https://doi.org/10.3390/jof10080554





