Prospective Roles of Extremophilic Fungi in Climate Change Mitigation Strategies
Abstract
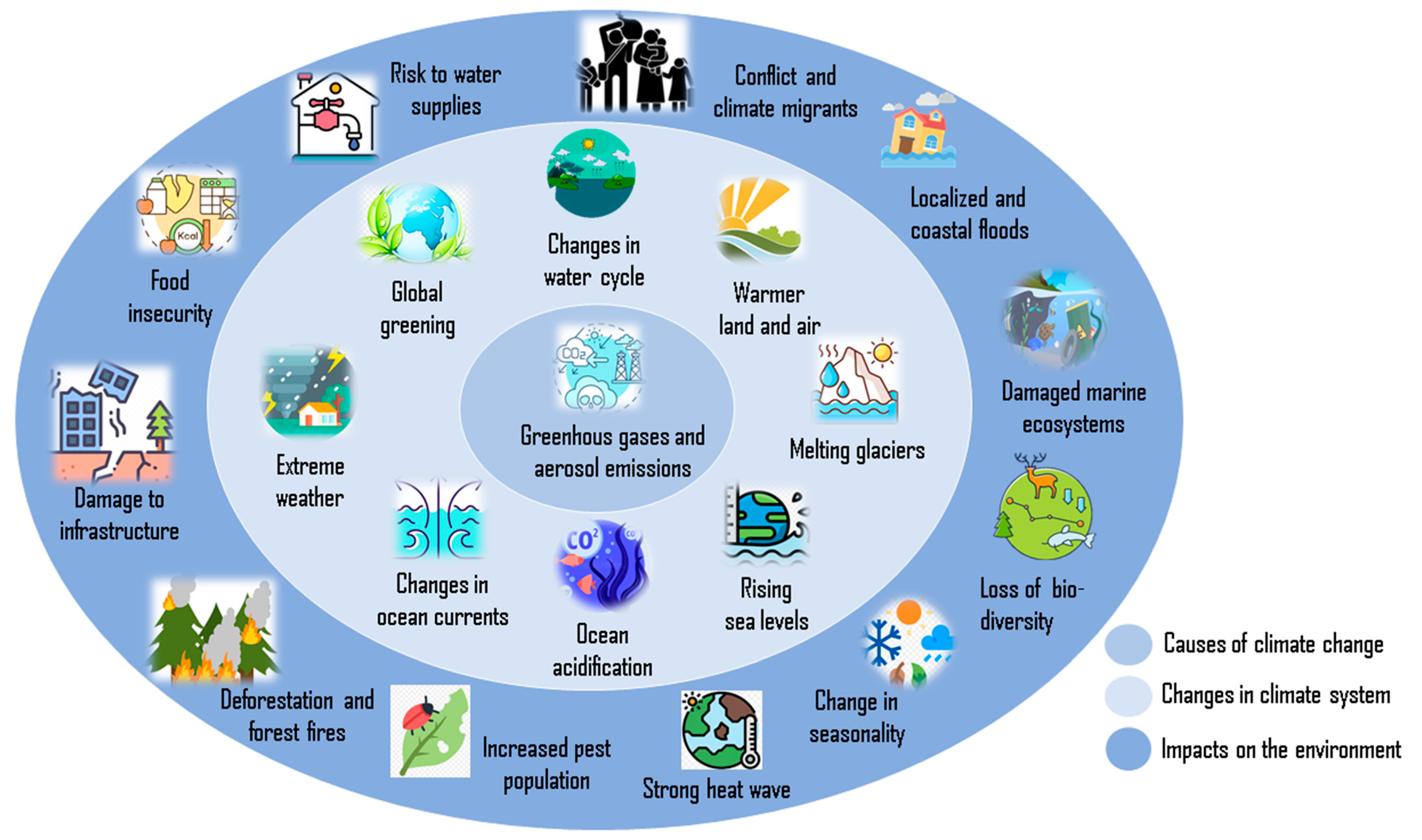
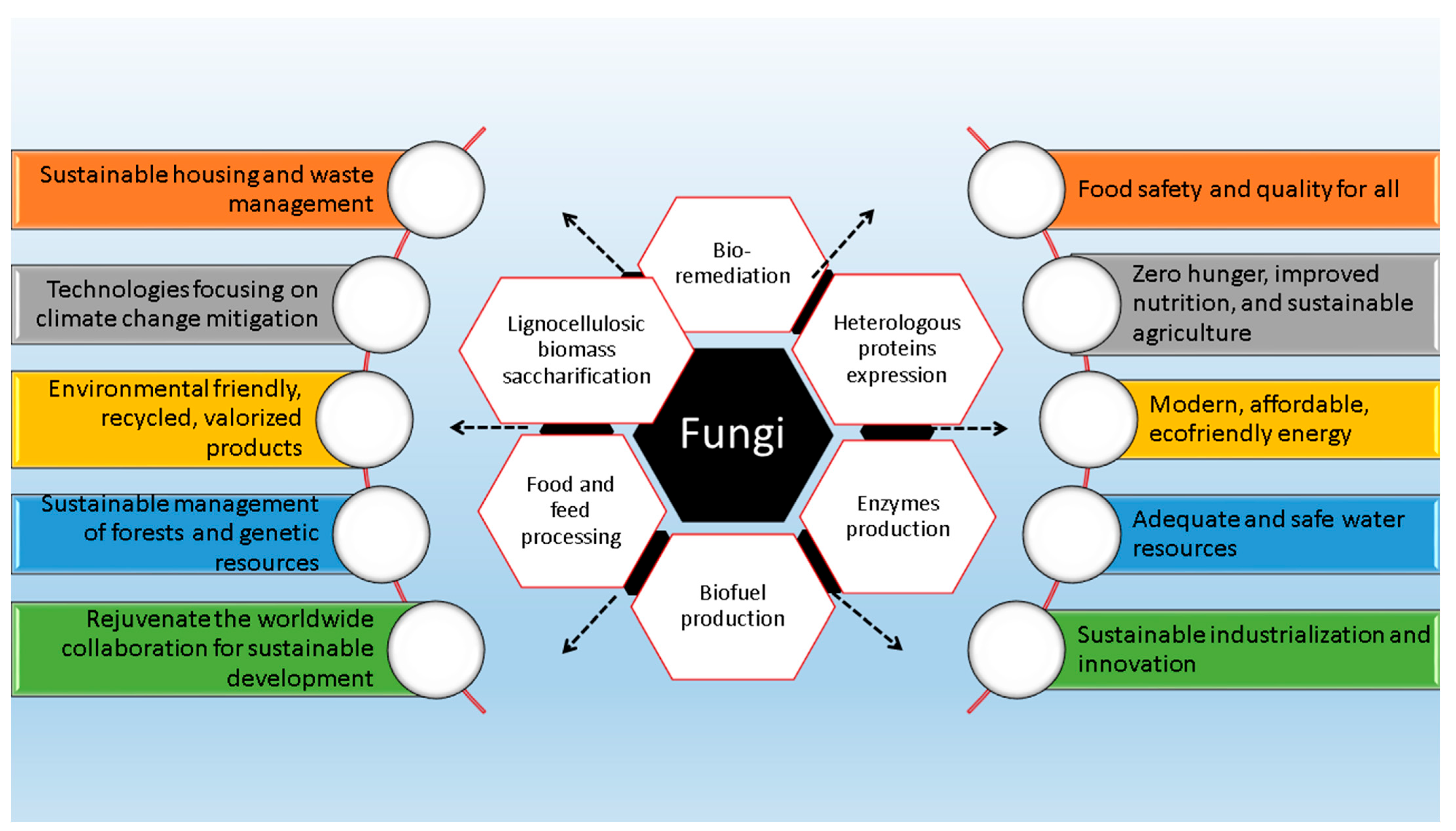
1. Introduction
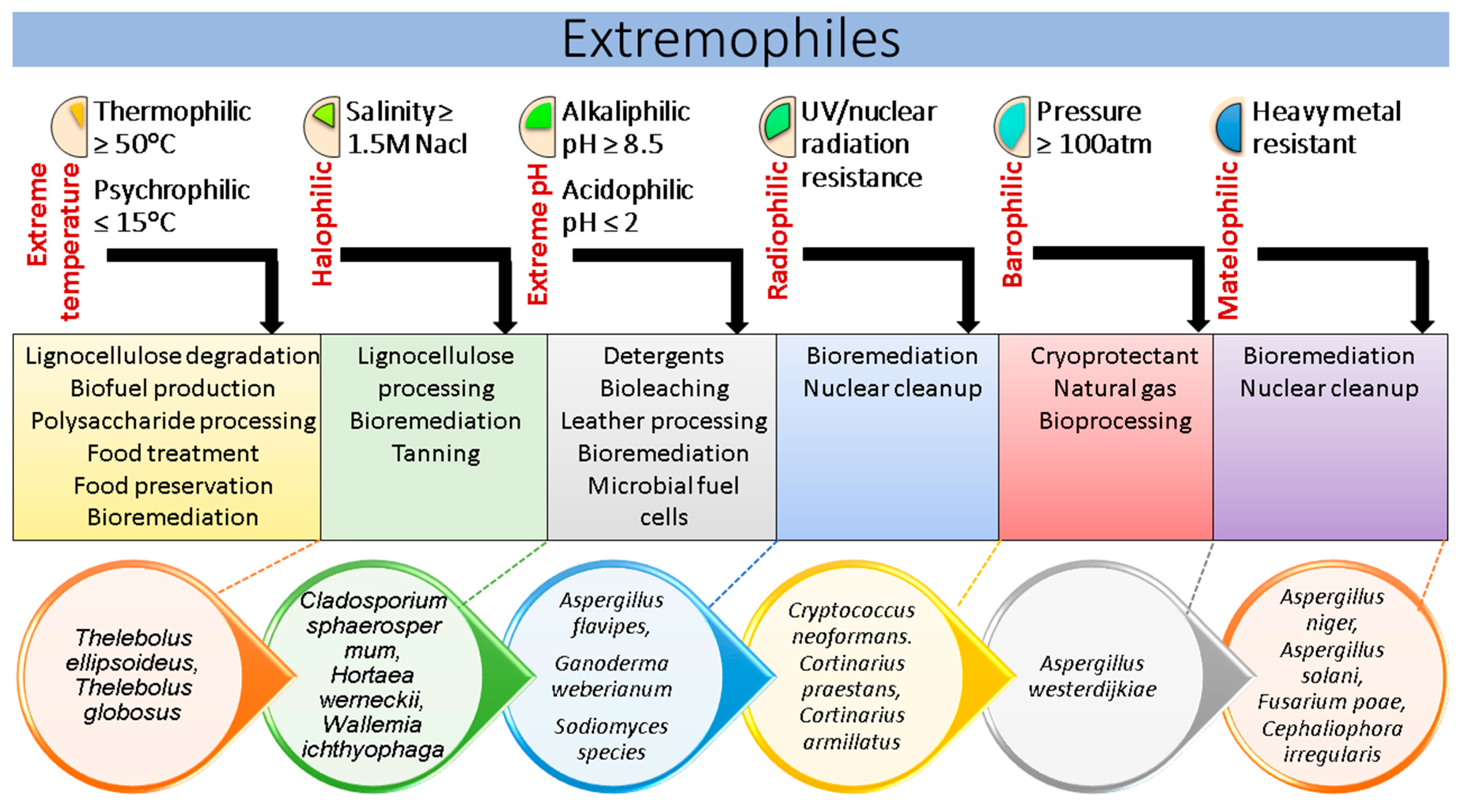
Extremophiles at the Interface of Climate Change
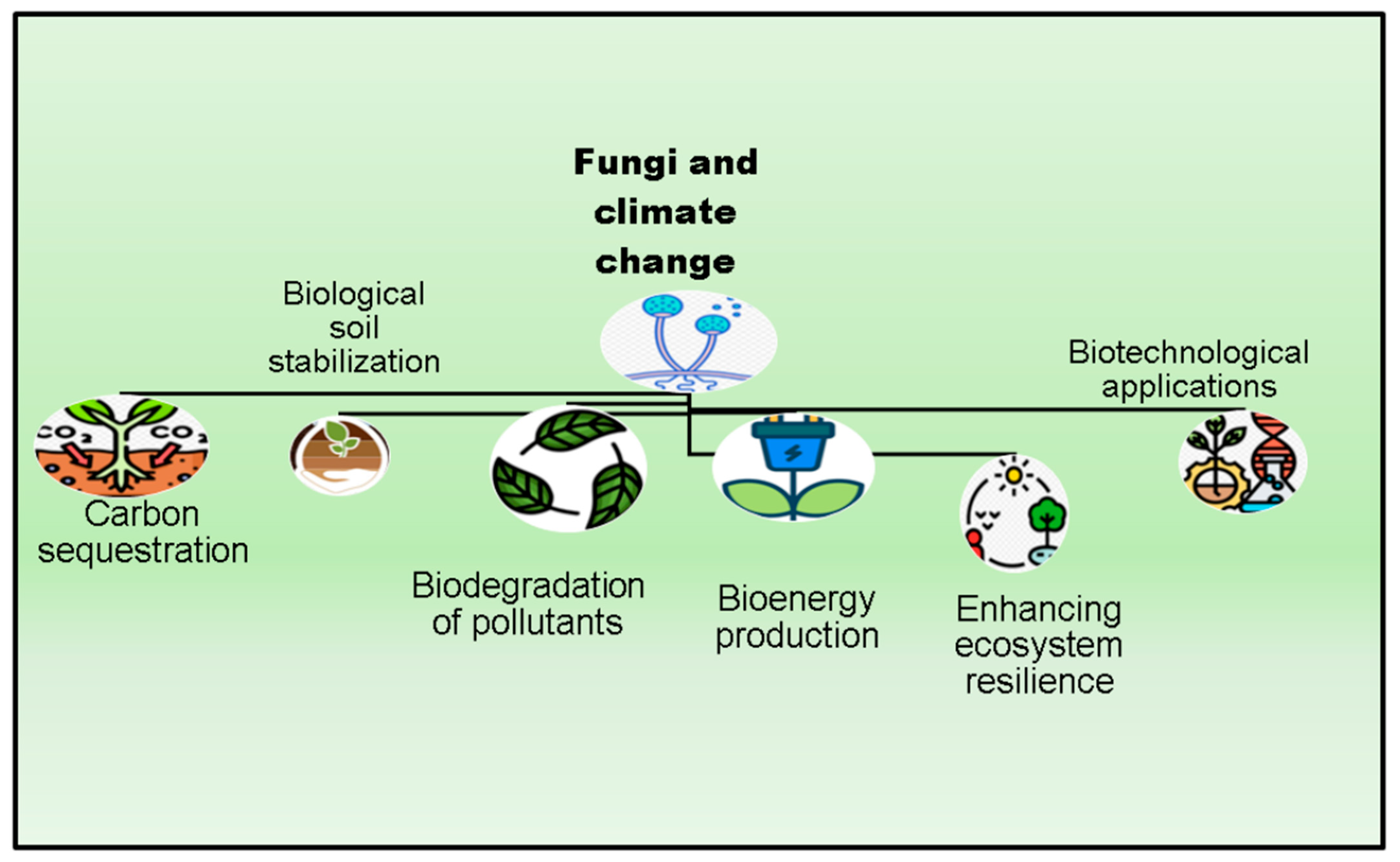
2. Biological Variability in Fungi and Its Significance
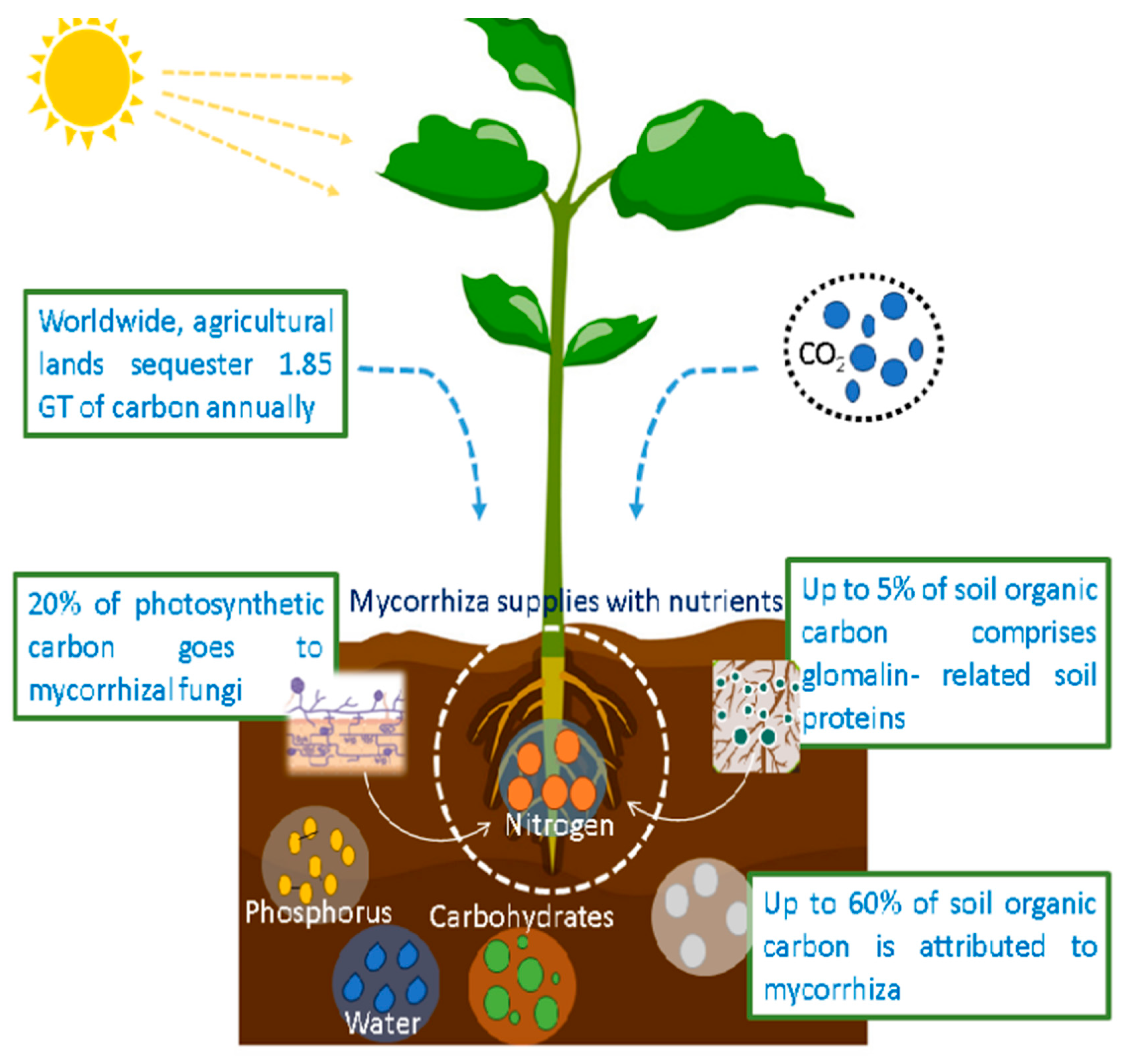
2.1. Carbon Sequestration by Fungi
2.2. Biological Soil Stabilization by Fungi
2.3. Extremophilic Fungal Endophytes for Improved Crop Performance
2.4. Biological Interventions Based on Fungi for Sustainable Environments
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malyan, S.K.; Kumar, A.; Baram, S.; Kumar, J.; Singh, S.; Kumar, S.S.; Yadav, A.N. Role of Fungi in Climate Change Abatement through Carbon Sequestration; Yadav, A.N., Singh, S., Mishra, S., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 283–295. [Google Scholar]
- Rastogi, M.; Singh, S.; Pathak, H. Emission of carbon dioxide from soil. Curr. Sci. 2002, 82, 510–517. [Google Scholar]
- Bhattacharyya, R.; Bhatia, A.; Das, T.; Lata, S.; Kumar, A.; Tomer, R.; Singh, G.; Kumar, S.; Biswas, A. Aggregate-associated N and global warming potential of conservation agriculture-based cropping of maize-wheat system in the north-western Indo-Gangetic Plains. Soil Tillage Res. 2018, 182, 66–77. [Google Scholar] [CrossRef]
- Kumar, S.; Malyan, S. Nitrification inhibitors: A perspective tool to mitigate greenhouse gas emission from rice soils. Curr. World Environ. 2016, 11, 423–428. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Ballesteros, G.I.; Newsham, K.K.; Acuña-Rodríguez, I.S.; Atala, C.; Torres-Díaz, C.; Molina-Montenegro, M.A. Extreme environments as sources of fungal endophytes mitigating climate change impacts on crops in Mediterranean-type ecosystems. Plants People Planet 2024, 6, 148–161. [Google Scholar] [CrossRef]
- Ali, F.S.; Akbar, A.; Prasongsuk, S.; Permpornsakul, P.; Yanwisetpakdee, B.; Lotrakul, P.; Punnapayak, H.; Asrar, M.; Ali, I. Penicillium imranianum, a new species from the man-made solar saltern of Phetchaburi province, Thailand. Pak. J. Bot. 2018, 50, 2055–2058. [Google Scholar]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar]
- Jörgensen, K.; Granath, G.; Strengbom, J.; Lindahl, B.D. Links between boreal forest management, soil fungal communities and below-ground carbon sequestration. Funct. Ecol. 2022, 36, 392–405. [Google Scholar] [CrossRef]
- Oberti, I.; Paciello, A. Bioplastic as a Substitute for Plastic in Construction Industry. Encyclopedia 2022, 2, 1408–1420. [Google Scholar] [CrossRef]
- Saye, L.M.; Navaratna, T.A.; Chong, J.P.; O’Malley, M.A.; Theodorou, M.K.; Reilly, M. The anaerobic fungi: Challenges and opportunities for industrial lignocellulosic biofuel production. Microorganisms 2021, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Sangamesh, M.; Jambagi, S.; Vasanthakumari, M.; Shetty, N.J.; Kolte, H.; Ravikanth, G.; Nataraja, K.N.; Uma Shaanker, R. Thermotolerance of fungal endophytes isolated from plants adapted to the Thar Desert, India. Symbiosis 2018, 75, 135–147. [Google Scholar] [CrossRef]
- Zhou, W.-N.; White, J.F.; Soares, M.A.; Torres, M.S.; Zhou, Z.-P.; Li, H.-Y. Diversity of fungi associated with plants growing in geothermal ecosystems and evaluation of their capacities to enhance thermotolerance of host plants. J. Plant Interact. 2015, 10, 305–314. [Google Scholar] [CrossRef]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Ali, N.; Jan, G.; Hamayun, M.; Jan, F.G.; Iqbal, A.; Hussain, A.; Lee, I.-J. Salt stress alleviation in Triticum aestivum through primary and secondary metabolites modulation by Aspergillus terreus BTK-1. Front. Plant Sci. 2022, 13, 779623. [Google Scholar] [CrossRef]
- Bibi, N.; Jan, G.; Jan, F.G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Rehman, H.; Tawab, A.; Khushdil, F. Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant Physiol. Biochem. 2019, 139, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E.A.; Maharachchikumbura, S.S.; Al-Sadi, A.M.; Rashid, U.; Kang, S.-M.; Lee, I.-J. Actinomucor elegans and podospora bulbillosa positively improves endurance to water deficit and Salinity Stresses in tomato plants. J. Fungi 2022, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Redman, R.S.; Kim, Y.O.; Cho, S.; Mercer, M.; Rienstra, M.; Manglona, R.; Biaggi, T.; Zhou, X.-G.; Chilvers, M.; Gray, Z. A symbiotic approach to generating stress tolerant crops. Microorganisms 2021, 9, 920. [Google Scholar] [CrossRef]
- Sampangi-Ramaiah, M.H.; Jagadheesh Dey, P.; Jambagi, S.; Vasantha Kumari, M.; Oelmueller, R.; Nataraja, K.N.; Venkataramana Ravishankar, K.; Ravikanth, G.; Uma Shaanker, R. An endophyte from salt-adapted Pokkali rice confers salt-tolerance to a salt-sensitive rice variety and targets a unique pattern of genes in its new host. Sci. Rep. 2020, 10, 3237. [Google Scholar] [CrossRef] [PubMed]
- Manasa, K.; Vasanthakumari, M.; Nataraja, K.; Shaanker, R.U. Endophytic fungi of salt adapted Ipomea pes-caprae LR Br. Curr. Sci. 2020, 118, 1448–1453. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.-B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Galeano, R.M.S.; Silva, S.M.; Yonekawa, M.K.A.; de Alencar Guimarães, N.C.; Giannesi, G.C.; Masui, D.C.; Corrêa, B.O.; da Silva Brasil, M.; Zanoelo, F.F. Penicillium chrysogenum strain 34-P promotes plant growth and improves initial development of maize under saline conditions. Rhizosphere 2023, 26, 100710. [Google Scholar] [CrossRef]
- Tarroum, M.; Romdhane, W.B.; Al-Qurainy, F.; Ali, A.A.M.; Al-Doss, A.; Fki, L.; Hassairi, A. A novel PGPF Penicillium olsonii isolated from the rhizosphere of Aeluropus littoralis promotes plant growth, enhances salt stress tolerance, and reduces chemical fertilizers inputs in hydroponic system. Front. Microbiol. 2022, 13, 996054. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Parekh, V.; Patel, K.; Jha, S. Plant growth-promoting activities of Penicillium sp. NAUSF2 ameliorate Vigna radiata salinity stress in phosphate-deficient saline soil. Appl. Biochem. Microbiol. 2021, 57, 500–507. [Google Scholar] [CrossRef]
- Moghaddam, M.S.H.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, N.; Pang, Q.; Khan, R.A.A.; Xu, Q.; Wu, C.; Liu, T. A Salt-Tolerant Strain of Trichoderma longibrachiatum HL167 Is Effective in Alleviating Salt Stress, Promoting Plant Growth, and Managing Fusarium Wilt Disease in Cowpea. J. Fungi 2023, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 217856. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Ding, J.; Lin, W.; Li, Q.; Xu, C.; Zheng, Q.; Li, Y. Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil 2019, 439, 373–391. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2011, 6, e14823. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Sherameti, I.; Tripathi, S.; Varma, A.; Oelmüller, R. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress–related genes in leaves. Mol. Plant-Microbe Interact. 2008, 21, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Hamayun, M.; Rauf, M.; Arif, M.; Afzal Khan, S.; Ud-Din, J.; Gul, H.; Hussain, A.; Iqbal, A.; Kim, H.-Y. Molecular mechanism of Cu metal and drought stress resistance triggered by Porostereum spadiceum AGH786 in Solanum lycopersicum L. Front. Plant Sci. 2022, 13, 1029836. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, S.; Eshghi, S.; Banihashemi, Z. Application of candidate endophytic fungi isolated from extreme desert adapted trees to mitigate the adverse effects of drought stress on maize (Zea mays L.). Plant Physiol. Biochem. 2023, 202, 107961. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Černoša, A.; Sun, X.; Fang, C.; Gunde-Cimerman, N.; Song, Z.; Gostinčar, C. From glaciers to refrigerators: The population genomics and biocontrol potential of the black yeast Aureobasidium subglaciale. Microbiol. Spectr. 2022, 10, e01455-22. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Zajc, J.; Gunde-Cimerman, N.; Aprea, E.; Gasperi, F.; Placì, N.; Caruso, F.; Baraldi, E. Bioactivity of volatile organic compounds by Aureobasidium species against gray mold of tomato and table grape. World J. Microbiol. Biotechnol. 2020, 36, 171. [Google Scholar] [CrossRef]
- Treseder, K.K.; Holden, S.R. Fungal carbon sequestration. Science 2013, 339, 1528–1529. [Google Scholar] [CrossRef] [PubMed]
- Fellbaum, C.R.; Mensah, J.A.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. The role of carbon in fungal nutrient uptake and transport: Implications for resource exchange in the arbuscular mycorrhizal symbiosis. Plant Signal Behav. 2012, 7, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Millan, M.; Dignac, M.-F.; Rumpel, C.; Derenne, S. Quantitative and qualitative analysis of cutin in maize and a maize-cropped soil: Comparison of CuO oxidation, transmethylation and saponification methods. Org. Geochem. 2010, 41, 187–191. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Ma, X.; Xu, X.; Geng, Q.; Luo, Y.; Ju, C.; Li, Q.; Zhou, Y. Global arbuscular mycorrhizal fungal diversity and abundance decreases with soil available phosphorus. Glob. Ecol. Biogeogr. 2023, 32, 1423–1434. [Google Scholar] [CrossRef]
- Bahn, M.; Rodeghiero, M.; Anderson-Dunn, M.; Dore, S.; Gimeno, C.; Drösler, M.; Williams, M.; Ammann, C.; Berninger, F.; Flechard, C. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems 2008, 11, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Cox, P.; Betts, R.; Bopp, L.; von Bloh, W.; Brovkin, V.; Cadule, P.; Doney, S.; Eby, M.; Fung, I. Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J. Clim. 2006, 19, 3337–3353. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, L.; Li, X.; Feng, G.; Tian, C.; Christie, P. Diversity of arbuscular mycorrhizal fungi associated with desert ephemerals in plant communities of Junggar Basin, northwest China. Appl. Soil Ecol. 2007, 35, 10–20. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, M.; Wu, J. Temperature sensitivity of soil respiration and its effects on ecosystem carbon budget: Nonlinearity begets surprises. Ecol. Model. 2002, 153, 131–142. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 316246. [Google Scholar] [CrossRef] [PubMed]
- Dhawi, F. How can we stabilize soil using microbial communities and mitigate desertification? Sustainability 2023, 15, 863. [Google Scholar] [CrossRef]
- Cameron, R.E. Cold desert characteristics and problems relevant to other arid lands. Arid Lands Perspect. 2013, 1969, 202–205. [Google Scholar]
- Apple, M.E. Aspects of mycorrhizae in desert plants. In Desert Plants: Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 121–134. [Google Scholar]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Mortensen, B.; Haber, M.; DeJong, J.; Caslake, L.; Nelson, D. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Chittoori, B.C.; Rahman, T.; Burbank, M. Microbial-facilitated calcium carbonate precipitation as a shallow stabilization alternative for expansive soil treatment. Geotechnics 2021, 1, 558–572. [Google Scholar] [CrossRef]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Frąc, M.; Jezierska-Tys, S.; Yaguchi, T. Occurrence, detection, and molecular and metabolic characterization of heat-resistant fungi in soils and plants and their risk to human health. Adv. Agron. 2015, 132, 161–204. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Al-Whaibi, M.H. Desert Plants and Mycorrhizae (A mini-review). J. Pure Appl. Microbiol. 2009, 3, 457–466. [Google Scholar]
- Nasielski, J.; Furze, J.R.; Tan, J.; Bargaz, A.; Thevathasan, N.V.; Isaac, M.E. Agroforestry promotes soybean yield stability and N 2-fixation under water stress. Agron. Sustain. Dev. 2015, 35, 1541–1549. [Google Scholar] [CrossRef]
- Prasad, P.V.; Boote, K.J.; Allen, L.H., Jr.; Thomas, J.M. Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Glob. Chang. Biol. 2002, 8, 710–721. [Google Scholar] [CrossRef]
- Conde, A.; Chaves, M.M.; Gerós, H. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Nat. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic Social Affairs. Available online: https://www.un.org/development/desa/dpad/ (accessed on 15 April 2024).
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Hochman, Z.; Gobbett, D.L.; Horan, H. Climate trends account for stalled wheat yields in Australia since 1990. Glob. Chang. Biol. 2017, 23, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate change impacts and adaptation strategies of agriculture in Mediterranean-climate regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Alvarez-Garreton, C.; Barichivich, J.; Boisier, J.P.; Christie, D.; Galleguillos, M.; LeQuesne, C.; McPhee, J.; Zambrano-Bigiarini, M. The 2010–2015 megadrought in central Chile: Impacts on regional hydroclimate and vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef]
- Williams, C.J. Climate change in Chile: An analysis of state-of-the-art observations, satellite-derived estimates and climate model simulations. J. Earth Sci. Clim. Chang. 2017, 8, 400. [Google Scholar] [CrossRef]
- Maia de Queiroz, G.C.; Francismar de Medeiros, J.; Cesar Constante, D.; Pires de Souza, M.V.; Vieira de Sousa, L.; de Castro Granjeiro, J.C.; Rafael da Silva, R. Soil susceptibility to salinity and sodicity in sorghum areas under abiotic stress. Rev. Bras. Agric. Irrig. 2022, 16, 73–80. [Google Scholar]
- Nda, M.; Adnan, M.S.; Ahmad, K.A.; Usman, N.; Razi, M.A.M.; Daud, Z. A review on the causes, effects and mitigation of climate changes on the environmental aspects. Int. J. Integr. Eng. 2018, 10, 169–175. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Atala, C.; Gundel, P.E.; Molina-Montenegro, M.A. Hardening blueberry plants to face drought and cold events by the application of fungal endophytes. Agronomy 2022, 12, 1000. [Google Scholar] [CrossRef]
- Garnica, S.; Liao, Z.; Hamard, S.; Waller, F.; Parepa, M.; Bossdorf, O. Environmental stress determines the colonization and impact of an endophytic fungus on invasive knotweed. Biol. Invasions 2022, 24, 1785–1795. [Google Scholar] [CrossRef]
- Bertini, L.; Perazzolli, M.; Proietti, S.; Capaldi, G.; Savatin, D.V.; Bigini, V.; Longa, C.M.O.; Basaglia, M.; Favaro, L.; Casella, S. Biodiversity and bioprospecting of fungal endophytes from the antarctic plant Colobanthus quitensis. J. Fungi 2022, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Evolution of endophyte–plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Bae, H. Endophytic fungi: Key insights, emerging prospects, and challenges in natural product drug discovery. Microorganisms 2022, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural diversity and biological activities of the cyclodipeptides from fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ma, H.; Chen, S.; Gu, T.; Gong, J. Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. J. Exp. Bot. 2018, 69, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K.; Boyom, F.F. Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications. 3 Biotech. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A.; Magkourilou, E.; Barker, H.; Barker, A.; Urwin, P.E.; Field, K.J. Arbuscular mycorrhizal fungal-induced tolerance is determined by fungal identity and pathogen density. Plants People Planet 2023, 5, 241–253. [Google Scholar] [CrossRef]
- Rosikiewicz, P.; Bonvin, J.; Sanders, I.R. Cost-efficient production of in vitro Rhizophagus irregularis. Mycorrhiza 2017, 27, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Madouh, T.A.; Quoreshi, A.M. The function of arbuscular mycorrhizal fungi associated with drought stress resistance in native plants of arid desert ecosystems: A review. Diversity 2023, 15, 391. [Google Scholar] [CrossRef]
- Valentin, D.N.; Voyron, S.; Soteras, F.; Iriarte, H.J.; Giovannini, A.; Lumini, E.; Lugo, M.A. Modeling geographic distribution of arbuscular mycorrhizal fungi from molecular evidence in soils of Argentinean Puna using a maximum entropy approach. PeerJ 2023, 11, e14651. [Google Scholar] [CrossRef]
- Cantrell, S.A.; Dianese, J.; Fell, J.; Gunde-Cimerman, N.; Zalar, P. Unusual fungal niches. Mycologia 2011, 103, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Kapulnik, Y. Arbuscular Mycorrhizal Symbiosis under Stress Conditions: Benefits and Costs; Seckbach, G., Ed.; Springer: Dordrecht, The Netherlands, 2010; Volume 17, pp. 339–356. [Google Scholar]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Valderrama, J.R.; Nguyen, H.D.; Aime, M.C. Wallemia peruviensis sp. nov., a new xerophilic fungus from an agricultural setting in South America. Extremophiles 2017, 21, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Le Pioufle, O.; Declerck, S. Reducing water availability impacts the development of the arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833 and its ability to take up and transport phosphorus under in vitro conditions. Front. Microbiol. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Boerstler, B.; Thiery, O.; Sýkorová, Z.; Berner, A.; Redecker, D. Diversity of mitochondrial large subunit rDNA haplotypes of Glomus intraradices in two agricultural field experiments and two semi-natural grasslands. Mol. Ecol. 2010, 19, 1497–1511. [Google Scholar] [CrossRef]
- Picone, C. Diversity and abundance of arbuscular–mycorrhizal fungus spores in tropical forest and pasture. Biotropica 2000, 32, 734–750. [Google Scholar]
- Pathak, H.; Jain, N.; Bhatia, A.; Kumar, A.; Chatterjee, D. Improved nitrogen management: A key to climate change adaptation and mitigation. Indian. J. Fertil. 2016, 12, 151–162. [Google Scholar]
- Sarkhel, R.; Sengupta, S.; Das, P.; Bhowal, A. Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J. Polym. Res. 2020, 27, 16. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Bai, M.; Chen, A.; Peng, L.; Shao, J.; Luo, S.; Deng, Y.; Yan, B.; Peng, C. Valorization of waste biomass through fungal technology: Advances, challenges, and prospects. Ind. Crops Prod. 2022, 188, 115608. [Google Scholar] [CrossRef]
- Molelekoa, T.B.J.; Regnier, T.; da Silva, L.S.; Augustyn, W. Production of pigments by filamentous fungi cultured on agro-industrial by-products using submerged and solid-state fermentation methods. Fermentation 2021, 7, 295. [Google Scholar] [CrossRef]
- Huang, D.-L.; Zeng, G.-M.; Jiang, X.-Y.; Feng, C.-L.; Yu, H.-Y.; Huang, G.-H.; Liu, H.-L. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. J. Hazard. Mater. 2006, 134, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, A.S.; Mori, T.; Kamei, I.; Nishii, T.; Kondo, R. Application of mushroom waste medium from Pleurotus ostreatus for bioremediation of DDT-contaminated soil. Int. Biodeterior. Biodegrad. 2010, 64, 397–402. [Google Scholar] [CrossRef]
- Rigas, F.; Papadopoulou, K.; Philippoussis, A.; Papadopoulou, M.; Chatzipavlidis, J. Bioremediation of lindane contaminated soil by Pleurotus ostreatus in non sterile conditions using multilevel factorial design. Water Air Soil Pollut. 2009, 197, 121–129. [Google Scholar] [CrossRef]
- Aminabhavi, T.; Balundgi, R.; Cassidy, P. A review on biodegradable plastics. Polym. Plast. Technol. Eng. 1990, 29, 235–262. [Google Scholar] [CrossRef]
- Sodre, V.; Vilela, N.; Tramontina, R.; Squina, F.M. Microorganisms as bioabatement agents in biomass to bioproducts applications. Biomass Bioenergy 2021, 151, 106161. [Google Scholar] [CrossRef]
- Pearce, T.R.; Antonelli, A.; Brearley, F.Q.; Couch, C.; Campostrini Forzza, R.; Gonçalves, S.C.; Magassouba, S.; Morim, M.P.; Mueller, G.M.; Nic Lughadha, E. International collaboration between collections-based institutes for halting biodiversity loss and unlocking the useful properties of plants and fungi. Plants People Planet 2020, 2, 515–534. [Google Scholar] [CrossRef]
| Fungal Species | Source Plant | Targeted Plant | References |
|---|---|---|---|
| Heat-Stress Tolerant | |||
| Aspergillus glaucus | Euphorbia indica L. | Glycine max, Helianthus sp. | [12] |
| Chaetomium sp. | Lasiurus scindicus | Oryza sativa | [13] |
| Curvularia crepinii | Hedyotis diffusa, Trifolium repens, Digitaria ischaemum, Silene tenuis, Cynodon dactylon Alternanthera philoxeroides | Oryza sativa | [14] |
| C. protuberata | Dichanthelium lanuginosum | Solanum lycopersicum | [15] |
| C. protuberata | Dichanthelium lanuginosum | Leymus mollis, Dichanthelium lanuginosum, Solanum lycopersicum, Oryza sativa | [16] |
| Salt-Stress Tolerant | |||
| Aspergillus terreus | Chenopodium album L. | Triticum aestivum | [17] |
| Cochliobolus lunatus | Mirabilis jalapa L. | Ablemoschus esculentus L. | [18] |
| Cunninghamella bertholletiae | Solanum lycopersicum | Solanum lycopersicum | [19] |
| C. brachyspora SCb | Oryza sativa L. | Oryza sativa L. | [20] |
| Fusarium sp. V-4J | Pokkali Rice varieties IR-64 and JBT 36/14. | Oryza sativa (Pokkali) | [21] |
| Fusarium oxysporum | pomea pescaprae | Oryza sativa L. variety-IR-64 | [22] |
| Glomus mosseae, G. intraradices, G. etunicatum | Acacia gerrardii | Cucumis sativus L. | [23] |
| Penicillium chrysogenum | Axonopus purpusii | Zea mays | [24] |
| Penicillium olsonii A3 | Aleuropus littoralis | Nicotiana tabacum | [25] |
| Penicillium sp. NAUSF2 | Coastal rhizospheric soil (South Gujarat, India) | Vigna radiata L. cv. Co-4 | [26] |
| Periconia mascrospinosa, Neocamarosporium goegapense, N. chichastianum | Seidlitzia rosmarinus (Boiss.), Zygophyllum eichwaldii (C. A. Mey.), Haloxylon ammodendron (C. A. Mey.) | Cucumis sativus L. | [27] |
| Thermomyces longibrachiatum | Saline-alkali soil | Vigna unguiculata | [28] |
| Trichoderma longibrachiatum T6 | Triticum aestivum roots | Triticum aestivum | [29] |
| Drought Tolerant | |||
| Alternaria alternata | Elymus dahuricus | Triticum aestivum L. | [30] |
| Cunninghamella bertholletiae | Solanum lycopersicum | Solanum lycopersicum | [19] |
| Curvularia brachyspora SCb | Oryza sativa L. | Oryza sativa L. | [20] |
| C. protuberata | Dichantelium lanuginosum | Oryza sativa | [31] |
| Paecilomyces formosus LHL10, Penicillium funiculosum LHL06 | Cucumus sativus L. and Glycine max roots | Glycine max | [32] |
| Periconia mascrospinosa, Neocamarosporium goegapense, N. chichastianum | Seidlitzia rosmarinus (Boiss.), Zygophyllum eichwaldii (C. A. Mey.), Haloxylon ammodendron (C. A. Mey.) | Cucumis sativus L. | [27] |
| Piriformospora indica | Prosopis juliflora (Swartz) DC. and Zizyphus nummularia (Burm. fil.) | Arabidopsis thaliana | [33] |
| Porostereum spadiceum AGH786 | Glycine max roots cv. Hwangkeumkong | Solanum lycopersicum | [34] |
| Thermomyces harzianum ThSM3a (three strain consortium) | Annual grasses (arid región CA, USA) | Corn and cotton | [20] |
| Thermomyces harzianum 81Y1 and Fusarium solani 19 K3 | Roots of desert trees (Iran) | Zea mays L. cv. Simon | [35] |
| Biocontrol Agent | |||
| Aureobasidium subglaciale | Subglacial ice | Golden delicious apples (Malus domestica) | [36] |
| Aureobasidium pullulans, Aureobasidium melanogenum, Aureobasidium subglaciale | Culture collection | Tomatoes and grapes | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Qaiser, H.; Abdullah, R.; Kaleem, A.; Iqtedar, M.; Iqbal, I.; Chen, X. Prospective Roles of Extremophilic Fungi in Climate Change Mitigation Strategies. J. Fungi 2024, 10, 385. https://doi.org/10.3390/jof10060385
Ali I, Qaiser H, Abdullah R, Kaleem A, Iqtedar M, Iqbal I, Chen X. Prospective Roles of Extremophilic Fungi in Climate Change Mitigation Strategies. Journal of Fungi. 2024; 10(6):385. https://doi.org/10.3390/jof10060385
Chicago/Turabian StyleAli, Imran, Hina Qaiser, Roheena Abdullah, Afshan Kaleem, Mehwish Iqtedar, Irfana Iqbal, and Xiaoming Chen. 2024. "Prospective Roles of Extremophilic Fungi in Climate Change Mitigation Strategies" Journal of Fungi 10, no. 6: 385. https://doi.org/10.3390/jof10060385
APA StyleAli, I., Qaiser, H., Abdullah, R., Kaleem, A., Iqtedar, M., Iqbal, I., & Chen, X. (2024). Prospective Roles of Extremophilic Fungi in Climate Change Mitigation Strategies. Journal of Fungi, 10(6), 385. https://doi.org/10.3390/jof10060385






