Molecular Phylogenetic and Comparative Genomic Analysis of Pleurocordyceps fusiformispora sp. nov. and Perennicordyceps elaphomyceticola in the Family Polycephalomycetaceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Culture and Morphological Observations
2.3. DNA Extraction, Polymerase Chain Reaction (PCR), and Sequencing
2.4. Genome Sequencing and Assembly
2.5. Gene Prediction and Annotation
2.6. Analysis of Secondary Metabolite Biosynthesis Gene Cluster
2.7. Cluster Analysis
3. Results
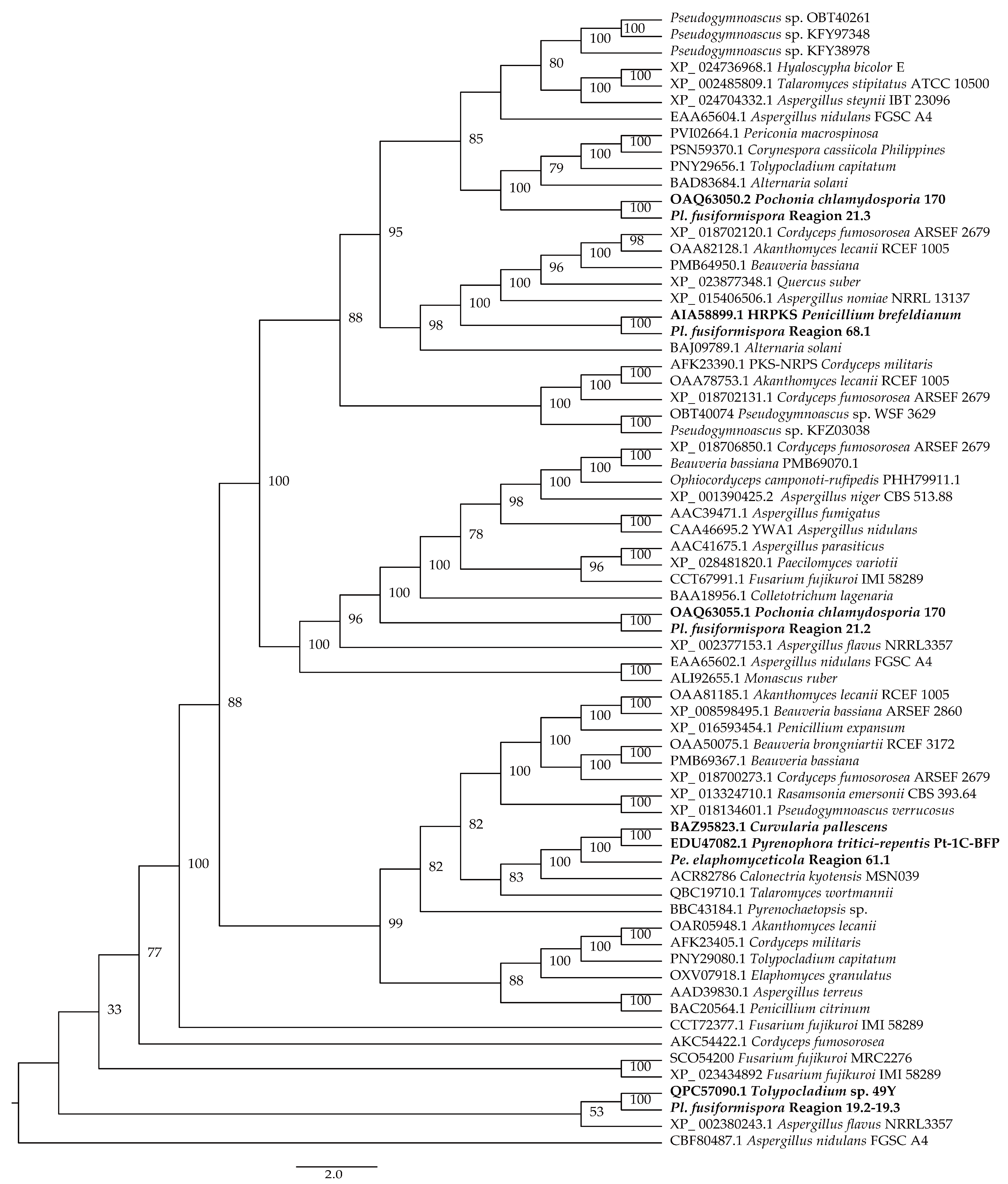
3.1. Phylogenetic Analysis
3.2. Taxonomy
3.2.1. Pleurocordyceps fusiformispora Hong Yu bis and Z.H. Liu, D.X. Tang, Y.L. Lu, sp. nov. Figure 2
MycoBank: MB 851478
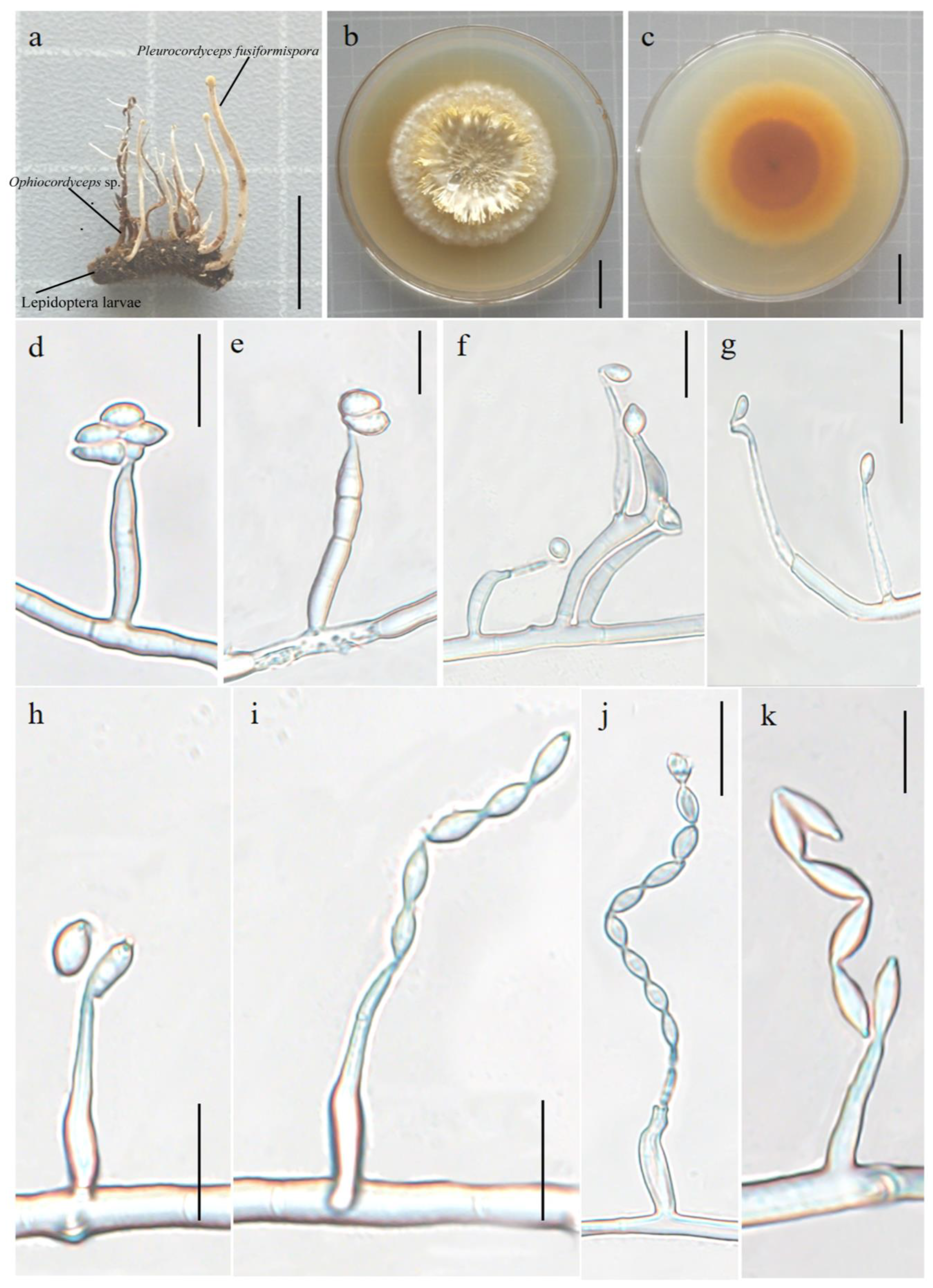
3.2.2. Perennicordyceps elaphomyceticola (WY Chuang, Ariyawansa, Jiuein Yang and Stadler) Y.P. Xiao and K.D. Hyde, comb. nov [1], Figure 3 and Figure 4
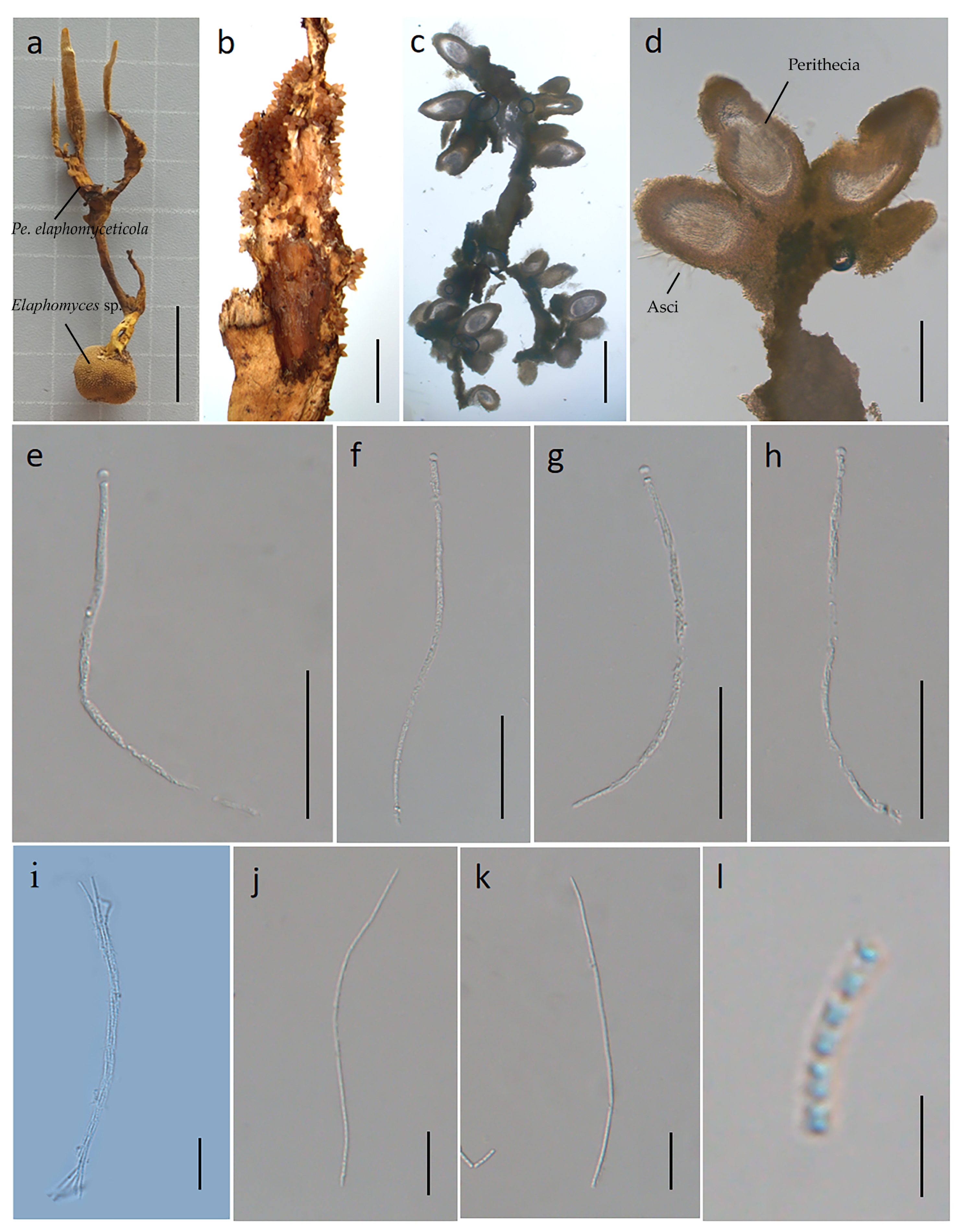
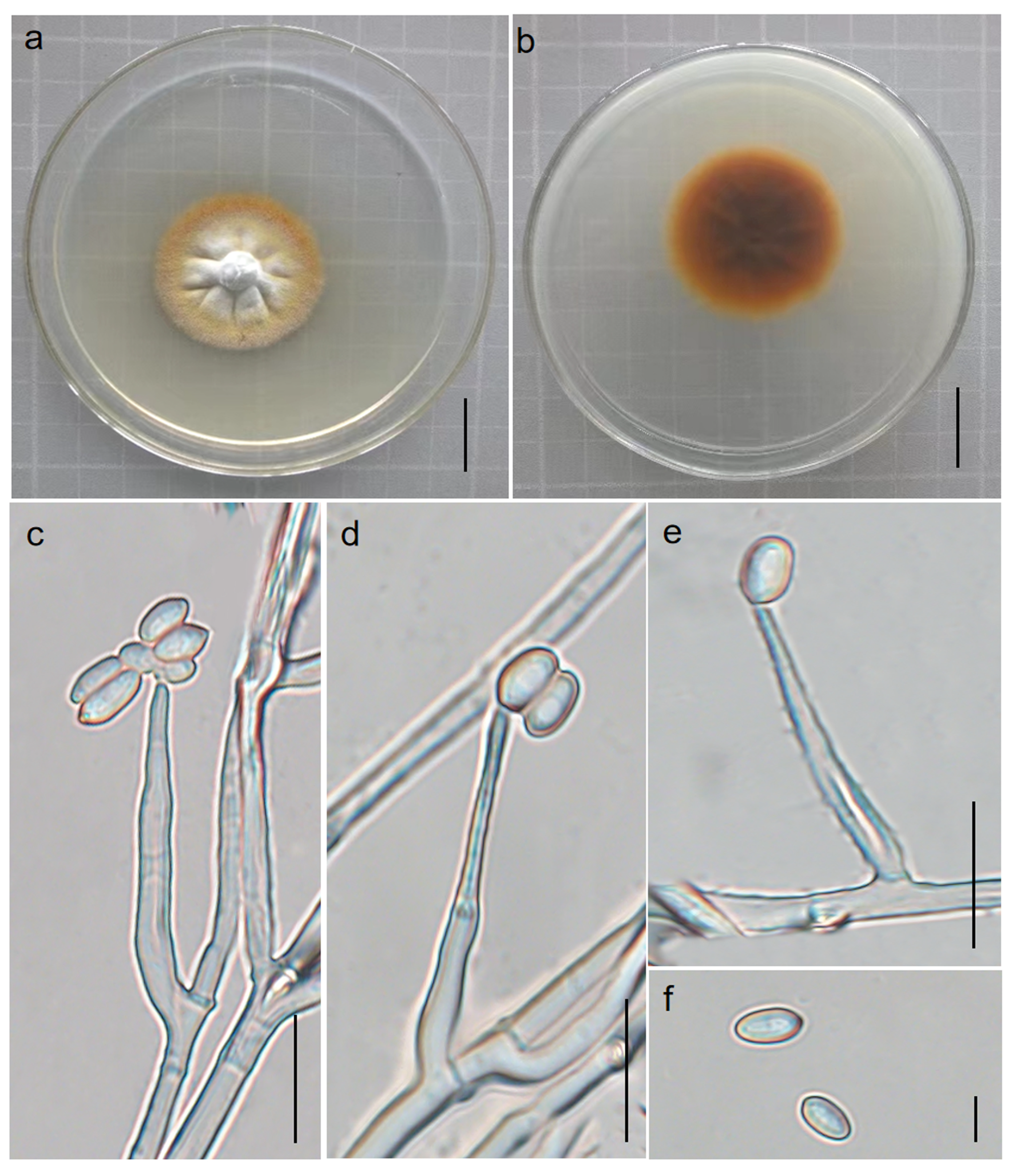
3.3. Basic Genomic Characteristics of Perennicordyceps elaphomyceticola and Pleurocordyceps fusiformispora
3.3.1. Genome Sequencing and Assembly
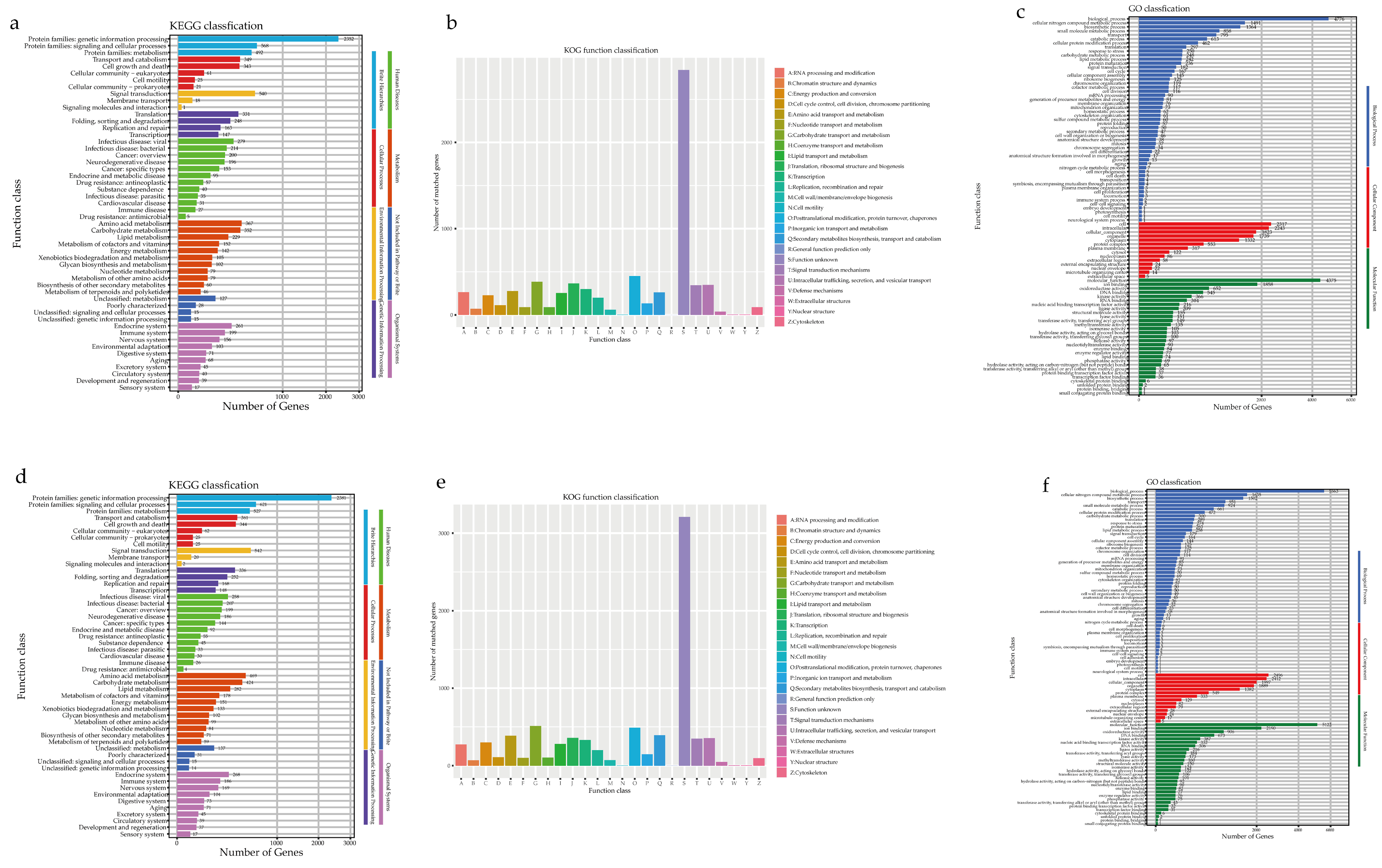
3.3.2. Genome Annotation
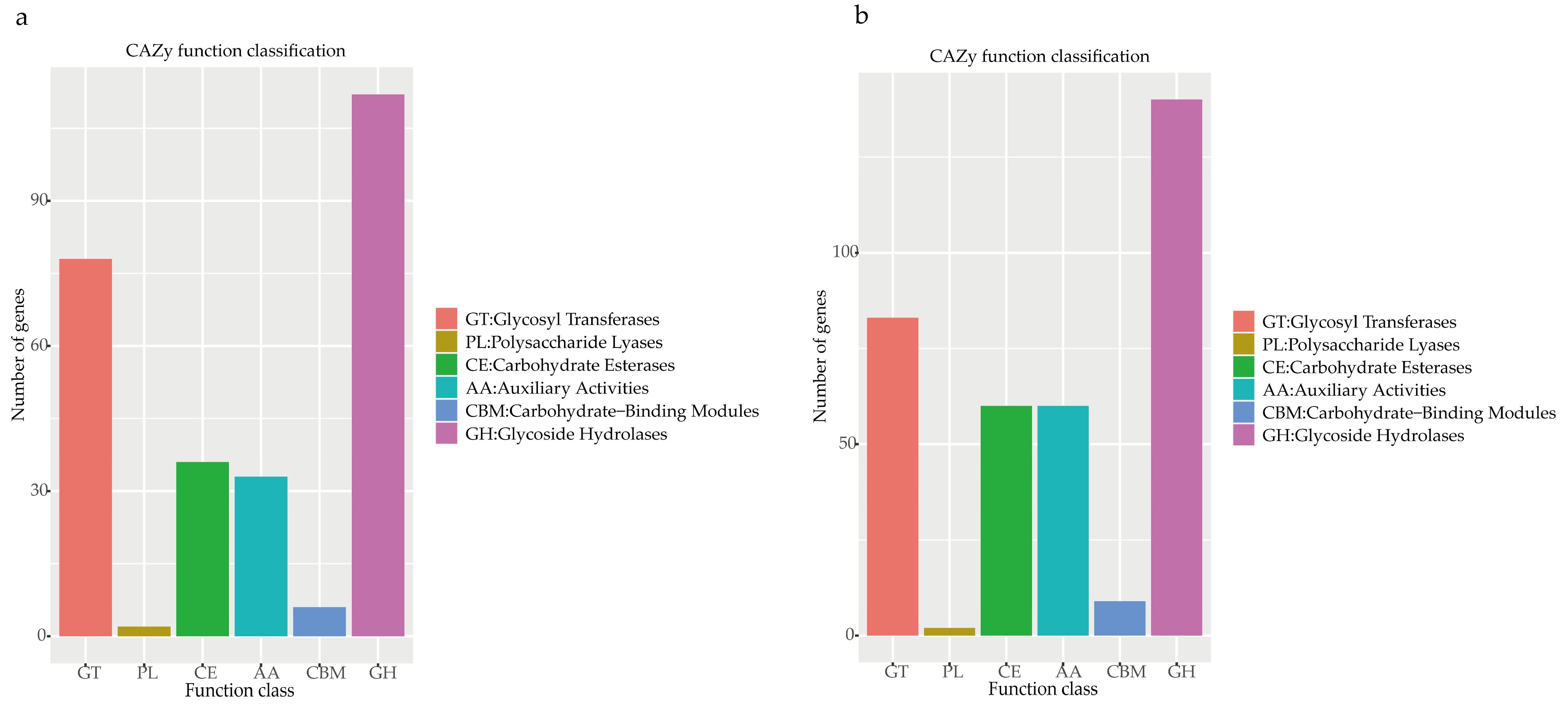
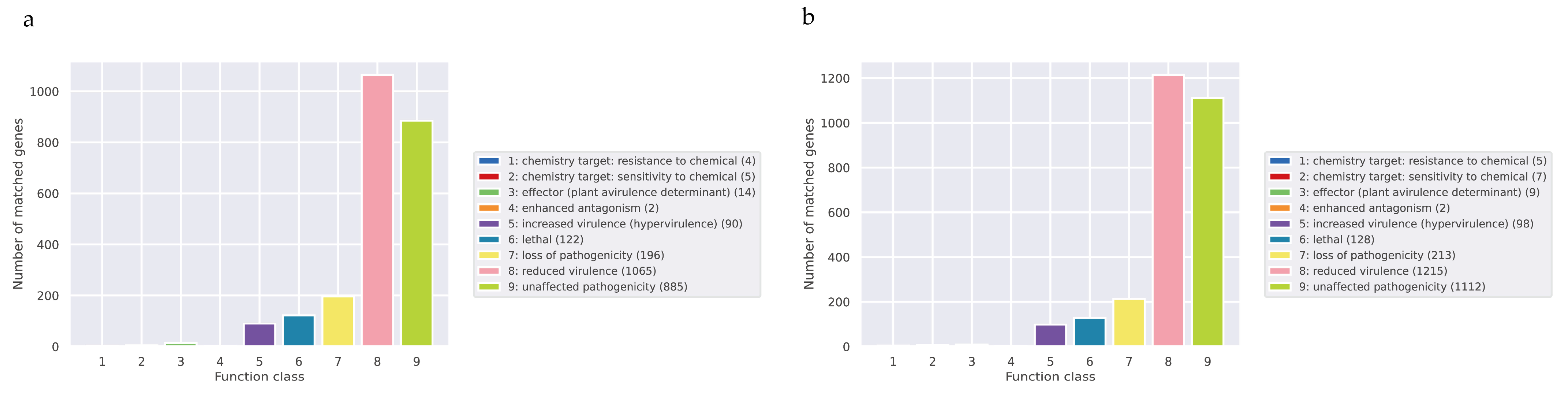
3.3.3. Additional Annotation
Carbohydrate-Active Enzymes (CAZy)
Pathogen–Host Interactions (PHI)
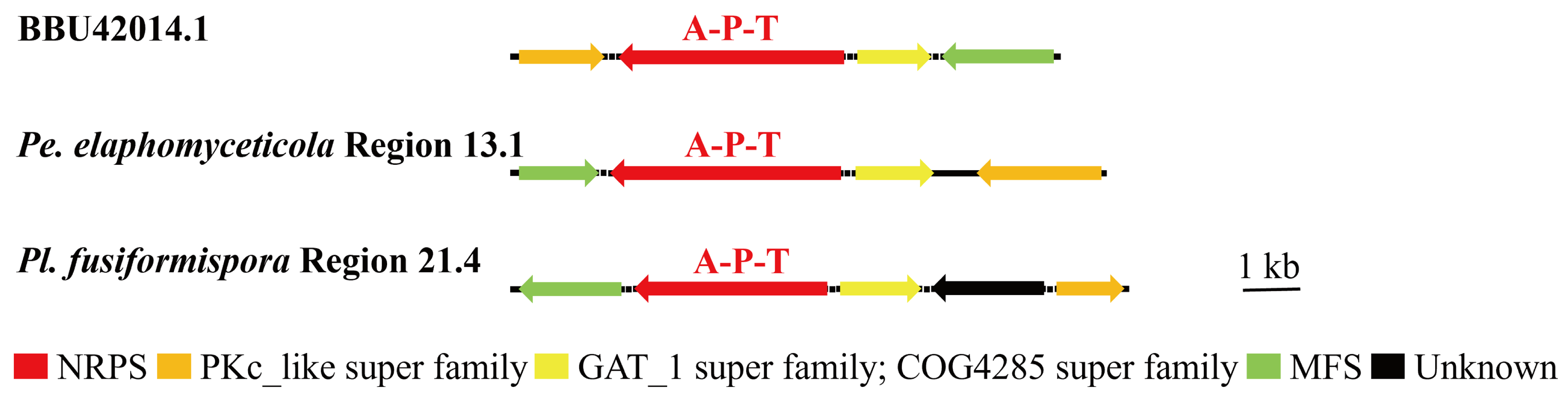
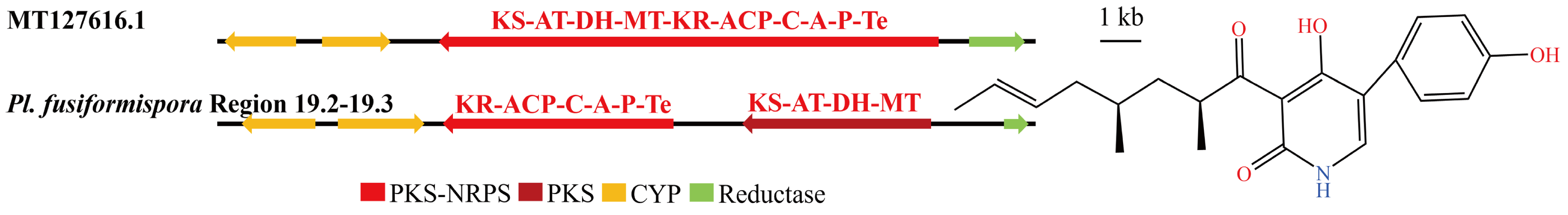
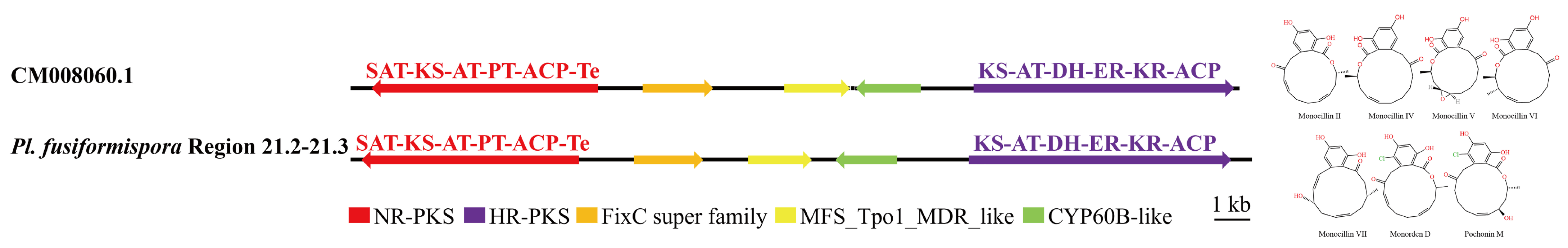
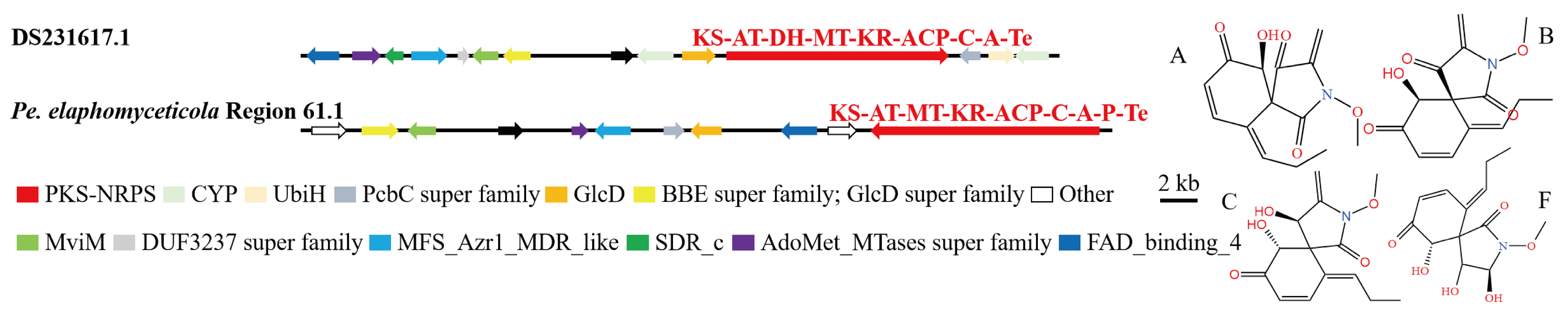
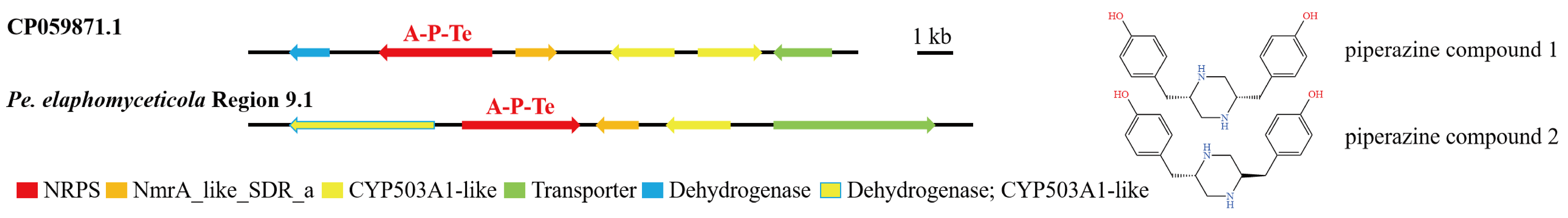
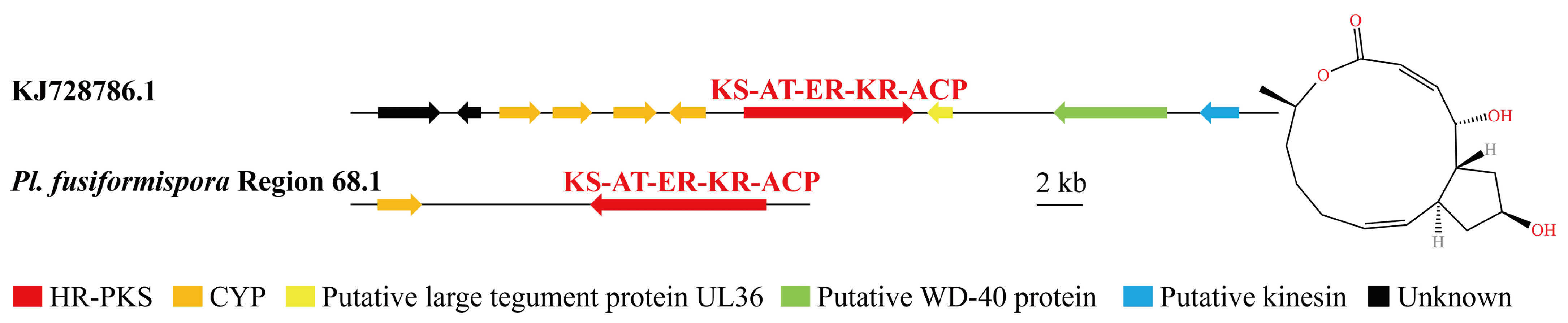
3.4. Analysis of Secondary Metabolite Biosynthesis Gene Cluster
Overview of Twelve Genomic BGCs of Pe. elaphomyceticola and Pl. fusiformispora
3.5. Cluster Analysis
3.6. Synteny Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.-P.; Wang, Y.B.; Hyde, K.D.; Eleni, G.; Sun, J.; Yang, Y.; Meng, J.; Yu, H.; Wen, T.-C. Polycephalomycetaceae, a New Family of Clavicipitoid Fungi Segregates from Ophiocordycipitaceae. Fungal. Diver. 2023, 120, 1–76. [Google Scholar] [CrossRef]
- Xiao, Y.-P.; Yang, Y.; Jayawardena, R.S.; Gentekaki, E.; Peng, X.-C.; Luo, Z.-L.; Lu, Y.-Z. Four Novel Pleurocordyceps (Polycephalomycetaceae) Species from China. Front. Microbiol. 2024, 14, 1256967. [Google Scholar] [CrossRef] [PubMed]
- Thammawat, S.; Sangdee, K.; Sangdee, A. Time-Kill Profiles and Cell-Surface Morphological Effects of Crude Polycephalomyces nipponicus Cod-MK1201 Mycelial Extract against Antibiotic-Sensitive and -Resistant Staphylococcus aureus. Trop. J. Pharm Res 2017, 16, 407. [Google Scholar] [CrossRef]
- Sangdee, K.; Nakbanpote, W.; Sangdee, A. Isolation of the Entomopathogenic Fungal Strain Cod-MK1201 from a Cicada Nymph and Assessment of Its Antibacterial Activities. Int. J. Med. Mushrooms 2015, 17, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Sangdee, K.; Seephonkai, P.; Buranrat, B.; Surapong, N.; Sangdee, A. Effects of Ethyl Acetate Extracts from the Polycephalomyces Nipponicus Isolate Cod-MK1201 (Ascomycetes) against Human Pathogenic Bacteria and a Breast Cancer Cell Line. Int. J. Med. Mushrooms 2016, 18, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Surapong, N.; Sangdee, A.; Chainok, K.; Pyne, S.G.; Seephonkai, P. Production and Antifungal Activity of Cordytropolone and (-)-Leptosphaerone A From the Fungus Polycephalomyces nipponicus. Nat. Prod. Commun. 2019, 14, 1934578X1984412. [Google Scholar] [CrossRef]
- Chu, L.; Huang, J.; Muhammad, M.; Deng, Z.; Gao, J. Genome Mining as a Biotechnological Tool for the Discovery of Novel Marine Natural Products. Crit. Rev. Biotechnol. 2020, 40, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, D.; Shen, Y. Discovery of Novel Bioactive Natural Products Driven by Genome Mining. Drug. Discov. Ther. 2018, 12, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.L.; Zhang, M.L.; Zhang, H.D.; Huang, J.Z.; Li, L. Genome mining and biosynthesis of the Acyl-CoA: Cholesterol acyltransferase inhibitor beauveriolide I and III in Cordyceps militaris. J. Biotechnol. 2020, 309, 85–91. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Wang, Y.; Chen, Y.; Tang, D.; Yu, H. Genomic Comparison of Two Species of Samsoniella with Other Genera in the Family Cordycipitaceae. J. Fungi 2023, 9, 1146. [Google Scholar] [CrossRef]
- Yuan, X.L.; Hua, M.; Chen, J.; Wang, J.; Wang, Y. Identification and cloning of a polyketide synthase III gene in the Lichenized-fungi Nephrmopsis pallescens (Chinese). Acta Bot. Boreal. Occodent. Sin. 2017, 37, 2146–2152. [Google Scholar]
- Sayari, M.; Steenkamp, E.T.; van der Nest, M.A.; Wingfield, B.D. Diversity and evolution of polyketide biosynthesis gene clusters in the Ceratocystidaceae. Fungal. Biol. 2018, 122, 856–866. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Chen, C.; Teng, J.; Wang, C.; Luo, D. Structure and biosynthesis of fumosorinone, a new protein tyrosine phosphatase 1B inhibitor firstly isolated from the entomogenous fungus Isaria fumosorosea. Fungal. Genet. Biol. 2015, 81, 191–200. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Wang, Y.; Fan, Q.; Duan, D.-E.; Zhang, G.-D.; Dai, R.-Q.; Dai, Y.-D.; Zeng, W.-B.; Chen, Z.-H.; Li, D.-D.; et al. Multigene Phylogeny of the Family Cordycipitaceae (Hypocreales): New Taxa and the New Systematic Position of the Chinese Cordycipitoid Fungus Paecilomyces Hepiali. Fungal. Diver. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Yi Liu, Z.; Liang, Z.Q.; Whalley, A.J.S.; Yao, Y.-J.; Liu, A.Y. Cordyceps brittlebankisoides, a New Pathogen of Grubs and Its Anamorph, Metarhizium anisopliae Var. Majus. J. Insect. Pathol. 2001, 78, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Hopple, J.S. Phylogenetic Investigations in the Genus Coprinus Based on Morphological Andmolecular Characters; Duke University: Durham, NC, USA, 1994. [Google Scholar]
- Rehner, S.A.; Buckley, E. A Beauveria Phylogeny Inferred from Nuclear ITS and EF1- Sequences: Evidence for Cryptic Diversification and Links to Cordyceps Teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.-H.; Hyten, A.S.; Spatafora, J.W. Multigene Phylogeny Reveals New Lineage for Stachybotrys chartarum, the Indoor Air Fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef]
- Araújo, J.P.M.; Evans, H.C.; Kepler, R.; Hughes, D.P. Zombie-Ant Fungi across Continents: 15 New Species and New Combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid Species. Stud. Mycol. 2018, 90, 119–160. [Google Scholar] [CrossRef]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. Metarhizium frigidum sp. Nov.: A Cryptic Species of M. anisopliae and a Member of the M. flavoviride Complex. Mycologia 2006, 98, 737–745. [Google Scholar] [CrossRef]
- Schubert, M.; Lindgreen, S.; Orlando, L. AdapterRemoval v2: Rapid Adapter Trimming, Identification, and Read Merging. BMC Res. Notes 2016, 9, 88. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K. Earl AM: Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Dai, Y.-D.; Chen, Z.-H.; Zeng, W.-B.; Yuan, F.; Liang, Z.-Q. Polycephalomyces Yunnanensis (Hypocreales), a New Species of Polycephalomyces Parasitizing Ophiocordyceps Nutans. Phytotaxa 2015, 208, 34. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Yu, H.; Dai, Y.-D.; Wu, C.-K.; Zeng, W.-B.; Yuan, F.; Liang, Z.-Q. Polycephalomyces Agaricus, a New Hyperparasite of Ophiocordyceps Sp. Infecting Melolonthid Larvae in Southwestern China. Mycol. Progress 2015, 14, 70. [Google Scholar] [CrossRef]
- Kepler, R.; Ban, S.; Nakagiri, A.; Bischoff, J.; Hywel-Jones, N.; Owensby, C.A.; Spatafora, J.W. The Phylogenetic Placement of Hypocrealean Insect Pathogens in the Genus Polycephalomyces: An Application of One Fungus One Name. Fungal Biol. 2013, 117, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-P.; Hongsanan, S.; Hyde, K.D.; Brooks, S.; Xie, N.; Long, F.-Y.; Wen, T.-C. Two New Entomopathogenic Species of Ophiocordyceps in Thailand. MycoKeys 2019, 47, 53–74. [Google Scholar] [CrossRef]
- Nikoh, N.; Fukatsu, T. Interkingdom Host Jumping Underground: Phylogenetic Analysis of Entomoparasitic Fungi of the Genus Cordyceps. Mol. Biol. Evol. 2000, 17, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Wang, L.; Tao, M.H.; Chen, Y.Q.; Qu, L.H. Two species of Cordyceps simultaneously parasitic on a larva of Lepidoptera. Mycosystema 2007, 26, 7–21. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Carnegie, A.J.; Hardy, G.E.S.J.; Smith, D.; Summerell, B.A.; Cano-Lira, J.F.; Guarro, J.; Houbraken, J.; et al. Fungal Planet Description Sheets: 625–715. Persoonia 2017, 39, 270–467. [Google Scholar] [CrossRef]
- Chaverri, P.; Bischoff, J.F.; Evans, H.C.; Hodge, K.T. Regiocrella, a New Entomopathogenic Genus with a Pycnidial anamorph and Its Phylogenetic Placement in the Clavicipitaceae. Mycologia 2005, 97, 1225–1237. [Google Scholar] [CrossRef]
- Wang, W.-J.; Wang, X.-L.; Li, Y.; Xiao, S.-R.; Kepler, R.M.; Yao, Y.-J. Molecular and Morphological Studies of Paecilomyces Sinensis Reveal a New Clade in Clavicipitaceous Fungi and Its New Systematic Position. Syst. Biodivers. 2012, 10, 221–232. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, L.; Yao, Y.; Zhou, Y. Maturational Alteration of Differential Expressions of GC:AT-biased Genotypes of Cordyceps sinensis Fungi and Paecilomyces hepiali in Cordyceps sinensis. FASEB J. 2010, 24, 877.4. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Ban, S.; Sakane, T.; Nakagiri, A. Three New Species of Ophiocordyceps and Overview of Anamorph Types in the Genus and the Family Ophiocordyceptaceae. Mycol. Progress 2015, 14, 1017. [Google Scholar] [CrossRef]
- Garron, M.-L.; Henrissat, B. The Continuing Expansion of CAZymes and Their Families. Curr. Opin. Chem. Biol. 2019, 53, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The Carbohydrate-Active Enzyme Database: Functions and Literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Urban, M.; Cuzick, A.; Seager, J.; Wood, V.; Rutherford, K.; Venkatesh, S.Y.; Sahu, J.; Iyer, S.V.; Khamari, L.; De Silva, N.; et al. PHI-Base in 2022: A Multi-Species Phenotype Database for Pathogen–Host Interactions. Nucleic Acids Res. 2022, 50, D837–D847. [Google Scholar] [CrossRef] [PubMed]
- Purev, E.; Kondo, T.; Takemoto, D.; Niones, J.T.; Ojika, M. Identification of ε-Poly-L-Lysine as an Antimicrobial Product from an Epichloë Endophyte and Isolation of Fungal ε-PL Synthetase Gene. Molecules 2020, 25, 1032. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Zhong, Y.; Yu, Y.; Shi, D.-F.; Huang, H.-Y.; Tang, X.-L.; Wang, Y.-H.; Chen, G.-D.; Zhang, H.-P.; Liu, C.-L.; et al. 4-Hydroxy Pyridones from Heterologous Expression and Cultivation of the Native Host. J. Nat. Prod. 2020, 83, 3338–3346. [Google Scholar] [CrossRef]
- Qin, F.; Li, Y.; Lin, R.; Zhang, X.; Mao, Z.; Ling, J.; Yang, Y.; Zhuang, X.; Du, S.; Cheng, X.; et al. Antibacterial Radicicol Analogues from Pochonia chlamydosporia and Their Biosynthetic Gene Cluster. J. Agric. Food Chem. 2019, 67, 7266–7273. [Google Scholar] [CrossRef]
- Xiao, Y.-P.; Wen, T.-C.; Hongsanan, S.; Jeewon, R.; Luangsa-ard, J.J.; Brooks, S.; Wanasinghe, D.N.; Long, F.-Y.; Hyde, K.D. Multigene Phylogenetics of Polycephalomyces (Ophiocordycipitaceae, Hypocreales), with Two New Species from Thailand. Sci. Rep. 2018, 8, 18087. [Google Scholar] [CrossRef]
- Goos, R.D.; Seifert, K.A. A Monograph of Stilbella and Some Allied Hyphomycetes. Mycologia 1986, 78, 980. [Google Scholar] [CrossRef]
- Bischoff, J.F.; Sullivan, R.F.; Struwe, L.; Hywel-Jones, N.L.; White, J.F. Resurrection of Blistum tomentosum and its exclusion from Polycephalomyces (Hyphomycetes, Deuteromycota) based on 28S rDNA sequence data. Mycotaxon 2003, 86, 433–444. [Google Scholar]
- Yuan, X.L.; Li, Y.Q.; Yi, W. The Diversity of Polyketide Synthase (PKS) Gene in Cordyceps militaris. J. West China For. Sci. 2019, 48, 97–103+113. [Google Scholar]
- Sugawara, F.; Takahashi, N.; Strobel, G.A.; Strobel, S.A.; Lu, H.S.M.; Clardy, J. ChemInform Abstract: Triticones A and B, Novel Phytotoxins from the Plant Pathogenic Fungus Drechslera Tritici-repentis. ChemInform 1988, 19, chin.198839319. [Google Scholar] [CrossRef]
- Sandmeier, P.; Tamm, C. New Spirostaphyiotrichins from the Mutant Strain P 84 of Staphylotrichum Coccosporum. Helv. Chim. Acta 1989, 72, 1107–1120. [Google Scholar] [CrossRef]
- Sandmeier, P.; Tamm, C. New Spirostaphylotrichins from the Mutant Strain P 649 of Staphylotrichum Coccosporum: The Biogenetic Interrelationship of the Known Spirostaphylotrichins. Helv. Chim. Acta 1990, 73, 975–984. [Google Scholar] [CrossRef]
- Hallock, Y.F.; Lu, H.S.M.; Clardy, J.; Strobel, G.A.; Sugawara, F.; Samsoedin, R.; Yoshida, S. Triticones, Spirocyclic Lactams from the Fungal Plant Pathogen Drechslera Tritici-Repentis. J. Nat. Prod. 1993, 56, 747–754. [Google Scholar] [CrossRef]
- Abraham, W.-R.; Hanssen, H.-P.; Arfmann, H.-A. Spirostaphylotrichins U and V from Curvularia Pallescens. Phytochemistry 1995, 38, 843–845. [Google Scholar] [CrossRef]
- De Almeida, T.T.; Ribeiro, M.A.D.S.; Polonio, J.C.; Garcia, F.P.; Nakamura, C.V.; Meurer, E.C.; Sarragiotto, M.H.; Baldoqui, D.C.; Azevedo, J.L.; Pamphile, J.A. Curvulin and Spirostaphylotrichins R and U from Extracts Produced by Two Endophytic Bipolaris Sp. Associated to Aquatic Macrophytes with Antileishmanial Activity. Nat. Prod. Res. 2018, 32, 2783–2790. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Liu, Y.; Liu, Y.; Li, K.; Yang, X.; Liu, S.; Zhou, X.; Yang, J. Spirostaphylotrichin X from a Marine-Derived Fungus as an Anti-Influenza Agent Targeting RNA Polymerase PB2. J. Nat. Prod. 2018, 81, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, C.; See, P.T.; Moolhuijzen, P.; Li, H.; Moffat, C.S.; Chooi, Y.; Oliver, R.P. The Identification and Deletion of the Polyketide Synthase-nonribosomal Peptide Synthase Gene Responsible for the Production of the Phytotoxic Triticone A/B in the Wheat Fungal Pathogen Pyrenophora Tritici-repentis. Environ. Microbiol. 2019, 21, 4875–4886. [Google Scholar] [CrossRef]
- Forseth, R.R.; Amaike, S.; Schwenk, D.; Affeldt, K.J.; Hoffmeister, D.; Schroeder, F.C.; Keller, N.P. Homologous NRPS-like Gene Clusters Mediate Redundant Small-Molecule Biosynthesis in Aspergillus Flavus. Angew. Chem. Int. Ed. 2013, 52, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Härri, E.; Loeffler, W.; Sigg, H.P.; Stähelin, H.; Tamm, C. Über Die Isolierung Neuer Stoffwechselprodukte Aus Penicillium Brefeldianum DODGE. Helv. Chim. Acta 1963, 46, 1235–1243. [Google Scholar] [CrossRef]
- Betina, V.; Drobnica, L.; Nemec, P.; Zemanova, M.J. Study of the antifungal activity of the antibiotic, cyanein. J. Antibrot. 1964, 17, 93–95. [Google Scholar]
- Tamura, G.; Ando, K.; Suzuki, S.; Takatsuki, A.; Arima, K.J. Antiviral Activity of Brefeldin A and Verrucarin A. J. Antibrot. 1968, 21, 160–161. [Google Scholar] [CrossRef]
- Zabala, A.O.; Chooi, Y.-H.; Choi, M.S.; Lin, H.-C.; Tang, Y. Fungal Polyketide Synthase Product Chain-Length Control by Partnering Thiohydrolase. ACS Chem. Biol. 2014, 9, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.-H.; Chen, Y.-Q.; Zhang, W.-M.; Qu, L.-H. Polycephalomyces lianzhouensis sp. Nov., a New Species, Co-Occurs with Ophiocordyceps crinalis. Mycol. Progress 2014, 13, 996. [Google Scholar] [CrossRef]
- Chen, Q.T.; Xiao, S.R.; Shi, Z.Y. Paecilomyces sinensis sp. nov. and its connection with Cordyceps sinensis. Acta Mycol. Sin. 1984, 3, 24–28. [Google Scholar]
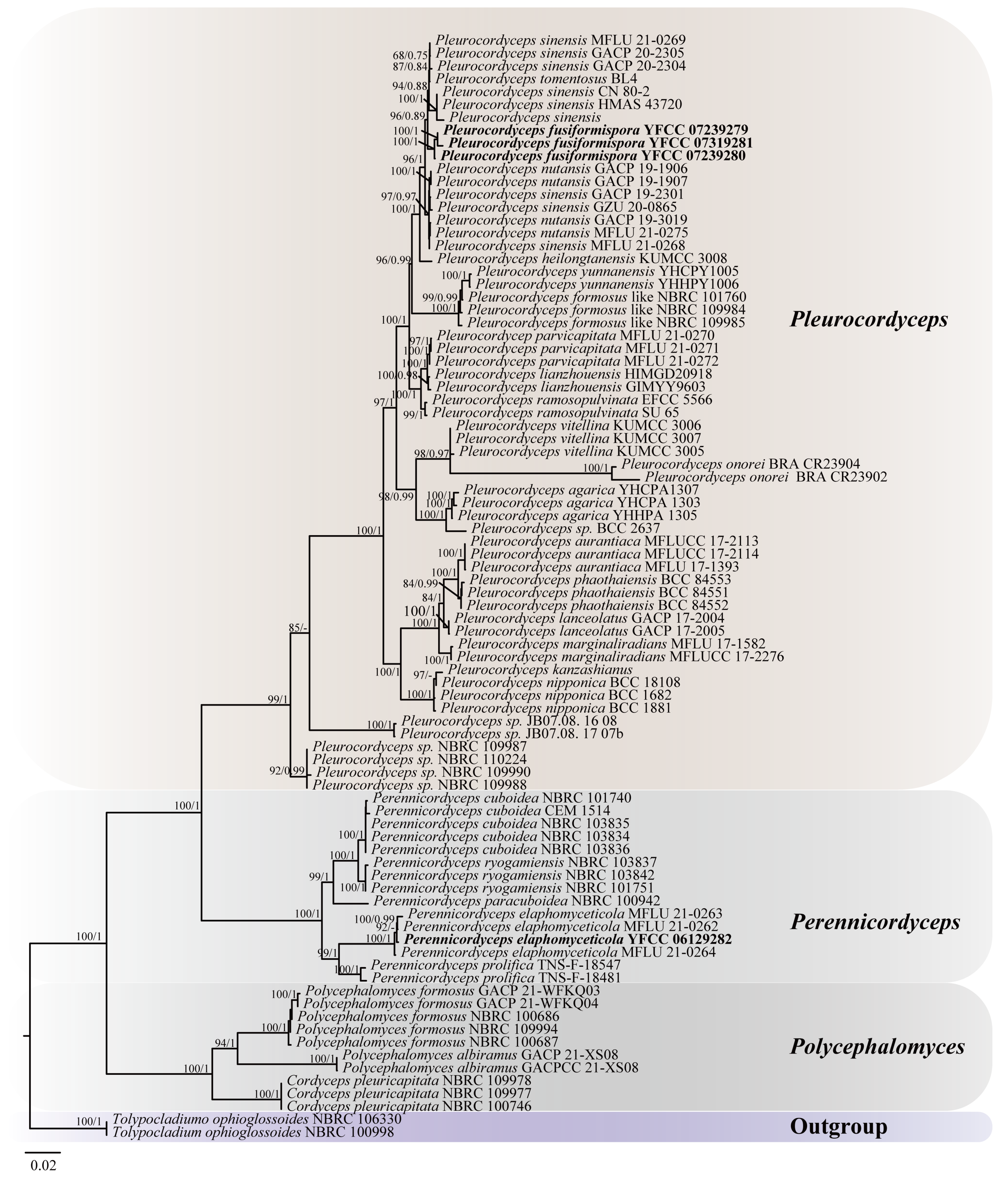


| Species Name | Voucher | ITS | SSU | LSU | TEF-1α | RPB1 | RPB2 | References |
|---|---|---|---|---|---|---|---|---|
| Cordyceps pleuricapitata | NBRC 109978 | AB925940 | AB925977 | Unpublished | ||||
| Cordyceps pleuricapitata | NBRC 109977 | AB925939 | AB925976 | Unpublished | ||||
| Cordyceps pleuricapitata | NBRC 100746 | JN943306 | JN941749 | JN941392 | KF049680 | JN992483 | KF049668 | [26] |
| Pleurocordyceps parvicapitata | MFLU 21-0270 | OQ172082 | OQ172105 | OQ172054 | OQ459722 | OQ459751 | OQ459796 | [1] |
| Pleurocordyceps sinensis | MFLU 21-0269 | OQ172080 | OQ172122 | OQ172050 | OQ459742 | OQ459768 | [1] | |
| Pleurocordyceps sinensis | GACP 20-2305 | OQ172075 | OQ172108 | OQ172045 | OQ459725 | OQ459753 | OQ459799 | [1] |
| Pleurocordyceps sinensis | GACP 19-2301 | OQ172078 | OQ172124 | OQ172053 | OQ459744 | OQ459816 | [1] | |
| Pleurocordyceps sinensis | GACP 20-2304 | OQ172074 | OQ172107 | OQ172044 | OQ459724 | OQ459798 | [1] | |
| Pleurocordyceps sinensis | GZU 20-0865 | OQ172071 | OQ172096 | OQ172043 | OQ459713 | [1] | ||
| Pleurocordyceps sinensis | MFLU 21-0268 | OQ172070 | OQ172123 | OQ172052 | OQ459743 | OQ459815 | [1] | |
| Pleurocordyceps fusiformispora | YFCC 07239279 | PP002030 | PP410610 | PP254877 | PP581807 | PP581824 | This study | |
| Pleurocordyceps fusiformispora | YFCC 07239280 | PP002031 | PP410611 | PP254878 | PP581808 | PP581825 | This study | |
| Pleurocordyceps fusiformispora | YFCC 07319281 | PP254879 | PP581809 | PP581826 | This study | |||
| Pleurocordyceps vitellina | KUMCC 3006 | OQ172089 | OQ172061 | OQ459729 | OQ459757 | OQ459803 | [1] | |
| Pleurocordyceps vitellina | KUMCC 3007 | OQ172090 | OQ172062 | OQ459730 | OQ459758 | OQ459804 | [1] | |
| Pleurocordyceps agarica | YHHPA 1305T | KP276651 | KP276655 | KP276659 | KP276663 | KP276667 | [25] | |
| Pleurocordyceps agarica | YHCPA1307 | KP276654 | KP276658 | KP276662 | KP276666 | KP276670 | [25] | |
| Pleurocordyceps agarica | YHCPA 1303 | KP276653 | KP276657 | KP276661 | KP276665 | KP276669 | [25] | |
| Pleurocordyceps aurantiaca | MFLUCC 17-2113T | MG136916 | MG136904 | MG136910 | MG136875 | MG136866 | MG136870 | [27] |
| Pleurocordyceps aurantiaca | MFLUCC 17-2114 | MG136917 | MG136905 | MG136911 | MG136874 | MG136871 | [27] | |
| Pleurocordyceps aurantiaca | MFLU 17-1393T | MG136907 | MG136913 | MG136877 | MG136868 | MG136873 | [27] | |
| Pleurocordyceps formosus like | NBRC 101760 | MN586827 | MN586818 | MN586836 | MN598051 | MN598042 | MN598060 | [14] |
| Pleurocordyceps formosus like | NBRC 109984 | MN586828 | MN586819 | MN586837 | MN598052 | MN598043 | [14] | |
| Pleurocordyceps formosus like | NBRC 109985 | MN586829 | MN586820 | MN586838 | MN598053 | MN598044 | [14] | |
| Pleurocordyceps heilongtanensis | KUMCC 3008 | OQ172091 | OQ172111 | OQ172063 | OQ459731 | OQ459759 | OQ459805 | [1] |
| Pleurocordyceps kanzashianus | AB027371 | AB027325 | AB027371 | [28] | ||||
| Pleurocordyceps lanceolatus | GACP 17-2004T | OQ172076 | OQ172110 | OQ172046 | OQ459726 | OQ459754 | OQ459800 | [1] |
| Pleurocordyceps lanceolatus | GACP 17-2005T | OQ172109 | OQ172047 | OQ459727 | OQ459755 | OQ459801 | [1] | |
| Pleurocordyceps lianzhouensis | HIMGD20918T | EU149921 | KF226245 | KF226246 | KF226248 | KF226247 | [29] | |
| Pleurocordyceps lianzhouensis | GIMYY9603 | EU149922 | KF226249 | KF226250 | KF226252 | KF226251 | [29] | |
| Pleurocordyceps marginaliradians | MFLU 17-1582T | MG136920 | MG136908 | MG136914 | MG136878 | MG136869 | MG271931 | [27] |
| Pleurocordyceps marginaliradians | MFLUCC 17-2276T | MG136921 | MG136909 | MG136915 | MG136879 | MG271930 | [27] | |
| Pleurocordycepsnipponica | BCC 1682 | KF049664 | KF049620 | KF049638 | KF049694 | [26] | ||
| Pleurocordycepsnipponica | BCC 18108 | KF049657 | MF416624 | MF416569 | MF416517 | MF416676 | MF416462 | [26] |
| Pleurocordyceps nipponica | BCC 1881 | KF049618 | KF049636 | KF049692 | KF049674 | [26] | ||
| Pleurocordyceps nutansis | GACP 19-1906 | OQ172079 | OQ172117 | OQ172049 | OQ459737 | OQ459763 | OQ459809 | [1] |
| Pleurocordyceps nutansis | GACP 19-1907 | OQ172087 | OQ172118 | OQ172059 | OQ459738 | OQ459764 | OQ459810 | [1] |
| Pleurocordyceps nutansis | GACP 19-3019T | OQ172086 | OQ172120 | OQ172058 | OQ459740 | OQ459766 | OQ459812 | [1] |
| Pleurocordyceps nutansis | MFLU 21-0275T | OQ172073 | OQ172119 | OQ172048 | OQ459739 | OQ459765 | OQ459811 | [1] |
| Pleurocordyceps onorei | BRA CR23904 | KU898843 | [30] | |||||
| Pleurocordyceps onorei | BRA CR23902T | KU898841 | [30] | |||||
| Pleurocordyceps parvicapitata | MFLU 21-0271T | OQ172083 | OQ172106 | OQ172055 | OQ459723 | OQ459752 | OQ459797 | [27] |
| Pleurocordyceps parvicapitata | MFLU 21-0272 | OQ172084 | OQ172099 | OQ172056 | OQ459716 | OQ459745 | OQ459790 | [1] |
| Pleurocordyceps phaothaiensis | BCC84553T | MF959733 | MF959737 | MF959742 | MF959745 | [30] | ||
| Pleurocordyceps phaothaiensis | BCC84552 | MF959732 | MF959736 | MF959740 | MF959744 | [30] | ||
| Pleurocordyceps phaothaiensis | BCC84551 | MF959731 | MF959735 | MF959739 | MF959743 | [30] | ||
| Pleurocordyceps ramosopulvinata | EFCC 5566 | KF049627 | KF049682 | KF049645 | [26] | |||
| Pleurocordyceps ramosopulvinata | SU 65 | DQ118742 | DQ118753 | DQ127244 | [31] | |||
| Pleurocordyceps sinensis | CN 80-2T | HQ832884 | HQ832887 | HQ832886 | HQ832890 | HQ832888 | HQ832889 | [32] |
| Pleurocordyceps sinensis | HQ918290 | [33] | ||||||
| Pleurocordyceps sinensis | HMAS 43720T | NR_119928 | NG_042573 | [32] | ||||
| Pleurocordyceps sp. | BCC 2637 | KF049663 | KF049637 | KF049693 | KF049675 | [26] | ||
| Pleurocordyceps sp. | JB07.08. 16_08 | KF049662 | KF049616 | KF049635 | KF049690 | KF049652 | KF049672 | [26] |
| Pleurocordyceps sp. | JB07.08. 17_07b | KF049617 | KF049691 | KF049653 | KF049673 | [26] | ||
| Pleurocordyceps sp. | NBRC 109987 | AB925983 | [14] | |||||
| Pleurocordyceps sp. | NBRC 109988 | AB925984 | [14] | |||||
| Pleurocordyceps sp. | NBRC 109990 | AB925968 | [14] | |||||
| Pleurocordyceps sp. | NBRC 110224 | AB925969 | [14] | |||||
| Pleurocordyceps tomentosus | BL4 | KF049666 | KF049623 | KF049641 | KF049697 | KF049656 | KF049678 | [26] |
| Pleurocordyceps vitellina | KUMCC 3005 | OQ172088 | OQ172060 | OQ459728 | OQ459756 | OQ459802 | [1] | |
| Pleurocordyceps yunnanensis | YHCPY1005 | KF977848 | KF977850 | KF977852 | KF977854 | [24] | ||
| Pleurocordyceps yunnanensis | YHHPY1006T | KF977849 | KF977851 | KF977853 | KF977855 | [24] | ||
| Perennicordyceps elaphomyceticola | MFLU 21-0262 | OQ172064 | OQ172101 | OQ172032 | OQ459718 | OQ459747 | OQ459792 | [1] |
| Perennicordyceps cuboidea | NBRC 103836 | JN943332 | JN941721 | JN941420 | AB972951 | JN992455 | AB972955 | [34] |
| Perennicordyceps cuboidea | NBRC 103834 | JN943330 | JN941723 | JN941418 | JN992457 | [34] | ||
| Perennicordyceps cuboidea | NBRC 103835 | JN943333 | JN941722 | JN941419 | JN992456 | [34] | ||
| Perennicordyceps cuboidea | NBRC 101740 | JN943331 | JN941724 | JN941417 | KF049684 | JN992458 | [34] | |
| Perennicordyceps cuboidea | CEM 1514 | KF049609 | KF049628 | KF049683 | [26] | |||
| Perennicordyceps elaphomyceticola | MFLU 21-0264 | OQ172067 | OQ172103 | OQ172035 | OQ459720 | OQ459749 | OQ459794 | [1] |
| Perennicordyceps elaphomyceticola | MFLU 21-0263 | OQ172065 | OQ172102 | OQ172033 | OQ459719 | OQ459748 | OQ459793 | [1] |
| Perennicordyceps elaphomyceticola | YFCC 06129282 | PP002336 | PP024253 | PP035749 | PP581810 | PP581823 | This study | |
| Perennicordyceps paracuboidea | NBRC 100942 | JN943337 | JN941711 | JN941430 | JN992445 | AB972958 | [34] | |
| Perennicordyceps prolifica | TNS-F-18547 | KF049660 | KF049613 | KF049632 | KF049687 | KF049649 | KF049670 | [26] |
| Perennicordyceps prolifica | TNS-F-18481 | KF049659 | KF049612 | KF049631 | KF049686 | KF049648 | [26] | |
| Perennicordyceps ryogamiensis | NBRC 101751 | JN943343 | JN941703 | JN941438 | KF049688 | JN992437 | [34] | |
| Perennicordyceps ryogamiensis | NBRC 103837 | JN943346 | JN941702 | JN941439 | JN992436 | [34] | ||
| Perennicordyceps ryogamiensis | NBRC 103842 | JN943345 | JN941701 | JN941440 | JN992435 | [34] | ||
| Polycephalomyces formosus | GACP 21-WFKQ03 | OQ172094 | OQ172113 | OQ172039 | OQ459733 | [1] | ||
| Polycephalomyces formosus | GACP 21-WFKQ04 | OQ172095 | OQ172114 | OQ172040 | OQ459734 | [1] | ||
| Polycephalomyces albiramus | GACP 21-XS08T | OQ172092 | OQ172115 | OQ172037 | OQ459735 | OQ459761 | OQ459807 | [1] |
| Polycephalomyces albiramus | GACPCC 21-XS08T | OQ172093 | OQ172116 | OQ172038 | OQ459736 | OQ459762 | OQ459808 | [1] |
| Polycephalomycesformosus | NBRC 100686 | MN586830 | MN586821 | MN586839 | MN598054 | MN598045 | MN598061 | [14] |
| Polycephalomycesformosus | NBRC 100687 | MN586831 | MN586822 | MN586840 | MN598055 | MN598046 | MN598062 | [14] |
| Polycephalomycesformosus | NBRC 109994 | MN586834 | MN586825 | MN586843 | MN598058 | MN598049 | MN598065 | [14] |
| Tolypocladium ophioglossoides | NBRC 100998 | JN943319 | JN941735 | JN941406 | AB968602 | JN992469 | AB968563 | [35] |
| Tolypocladium ophioglossoides | NBRC 106330 | JN943321 | JN941734 | JN941407 | AB968603 | JN992468 | AB968564 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lu, Y.; Tang, D.; Zhu, J.; Luo, L.; Chen, Y.; Yu, H. Molecular Phylogenetic and Comparative Genomic Analysis of Pleurocordyceps fusiformispora sp. nov. and Perennicordyceps elaphomyceticola in the Family Polycephalomycetaceae. J. Fungi 2024, 10, 297. https://doi.org/10.3390/jof10040297
Liu Z, Lu Y, Tang D, Zhu J, Luo L, Chen Y, Yu H. Molecular Phylogenetic and Comparative Genomic Analysis of Pleurocordyceps fusiformispora sp. nov. and Perennicordyceps elaphomyceticola in the Family Polycephalomycetaceae. Journal of Fungi. 2024; 10(4):297. https://doi.org/10.3390/jof10040297
Chicago/Turabian StyleLiu, Zuoheng, Yingling Lu, Dexiang Tang, Juye Zhu, Lijun Luo, Yue Chen, and Hong Yu. 2024. "Molecular Phylogenetic and Comparative Genomic Analysis of Pleurocordyceps fusiformispora sp. nov. and Perennicordyceps elaphomyceticola in the Family Polycephalomycetaceae" Journal of Fungi 10, no. 4: 297. https://doi.org/10.3390/jof10040297
APA StyleLiu, Z., Lu, Y., Tang, D., Zhu, J., Luo, L., Chen, Y., & Yu, H. (2024). Molecular Phylogenetic and Comparative Genomic Analysis of Pleurocordyceps fusiformispora sp. nov. and Perennicordyceps elaphomyceticola in the Family Polycephalomycetaceae. Journal of Fungi, 10(4), 297. https://doi.org/10.3390/jof10040297






