Abstract
Although nebulized liposomal amphotericin B (NLAB) is being used in invasive pulmonary aspergillosis (IPA) prophylaxis, no clinical trial has shown its efficacy as a therapeutic strategy. NAIFI is the inaugural randomized, controlled clinical trial designed to examine the safety and effectiveness of NLAB (dosage: 25 mg in 6 mL, three times per week for 6 weeks) against a placebo, in the auxiliary treatment of IPA. Throughout the three-year clinical trial, thirteen patients (six NLAB, seven placebo) were included, with 61% being onco-hematological with less than 100 neutrophils/μL. There were no significant differences noted in their pre- and post-nebulization results of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and oxygen saturation between the groups. Neither bronchospasm nor serum amphotericin B levels were reported in any patients given NLAB. 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET-TC) was carried out at the baseline and after 6 weeks. A notable decrease in median SUV (standardized uptake value) was observed in NLAB patients after 6 weeks (−3.6 vs. −0.95, p: 0.039, one tail). Furthermore, a reduction in serum substance galactomannan and beta-D-Glucan was identified within NLAB recipients. NLAB is well tolerated and safe for patients with IPA. Encouraging indirect efficacy data have been derived from image monitoring or biomarkers. However, further studies involving more patients are necessary.
1. Introduction
Despite advancements in treatment, invasive pulmonary aspergillosis (IPA) still results in high mortality rates. The most recent large-scale clinical trial compared the effects of two first-line drugs, isavuconazole and voriconazole, in patients with hemato-oncological IPA. The results showed a 20% mortality rate in both groups [1]. In larger case studies involving routine clinical practice, this mortality rate can rise to over 40% [2].
Liposomal, lipidic, and conventional nebulized amphotericin B are not currently approved for the treatment of IPA. However, various studies have examined their use as a preventative measure against fungal infections in lung transplant recipients [3,4,5], high-risk hematological patients [6,7], and patients with allergic broncho-pulmonary aspergillosis [8]. These studies have shown promising results, demonstrating high concentrations in the distal airway, no systemic toxicity, and good tolerance for the procedure. These factors suggest its potential as an adjunct treatment for IPA, specifically in broncho-pulmonary cases.
Experimental studies have verified the significant advantages of using nebulized amphotericin B for both treating and preventing IPA. A meta-analysis of eight studies examined 839 immunocompromised animals. The results indicated substantial decreases in mortality rates in those animals treated with nebulized amphotericin B compared with untreated animals. This effect was particularly notable when animals were treated prior to exposure to Aspergillus. No notable distinctions were found concerning efficacy and renal toxicity between standard and lipid amphotericin B. However, differences were found in pulmonary surfactant functionality when using conventional amphotericin B [9].
In humans, a recent comprehensive review, which included 13 case reports, 11 observational studies and 3 clinical trials, concluded that administering nebulized liposomal amphotericin B (NLAB) appears to be safe, with no severe side effects [10]. Our team recently detailed our experience using NLAB with 11 patients, which included adjunctive antifungal therapy in 5 patients and secondary prophylaxis in 6. After 3 months, we noted a marginally better clinical outcome in patients treated with NLAB. In a multivariate Cox regression analysis, accounting for any uncontrolled underlying disease revealed that NLAB use was linked to reduced mortality at the 12-month mark [11].
The 18F-fluorodeoxyglucose positron emission tomography FDG/PET/CT method offers an objective and quantifiable assessment of cellular metabolic activity, whether tumoral or infectious. This facilitates the monitoring of therapeutic effects, making it an outstanding surrogate marker for evaluating the therapeutic efficacy of IPA [12,13,14].
Thus far, the majority of the clinical use of NLAB has been centered on prophylaxis. No randomized controlled studies have been conducted to assess the therapeutic effectiveness of nebulized liposomal amphotericin B therapy.
The NAIFI study is a pilot, randomized phase I/IIa clinical trial that compares the safety and efficacy of NLAB (25 mg, three times per week) versus a placebo nebulization as an auxiliary therapy in patients having proved or probable IPA. The main goal is to assess the safety and tolerability of nebulized liposomal amphotericin B in these patients. Additional aims involve evaluating the clinical, radiological, and microbiological effectiveness of nebulized liposomal amphotericin B as a supplementary treatment for IPA.
2. Methods
2.1. Study Design and Inclusion Criteria
We included patients who were over 18 years old, diagnosed with IPA according to the recent EORTC/MSG criteria [15], and had provided informed consent. The diagnosis of probable IPA required either CT scan evidence showing dense, well-circumscribed lesions with or without a halo sign, air crescent sign cavity, or wedge-shaped consolidations, along with an isolation of Aspergillus species in respiratory samples or a positive serum or bronchoalveolar lavage (BAL) galactomannan (GM) test result, or a positive direct test.
For GM testing (both BAL and serum), we relied on the Ag Virclia® Monotest (Vircell S.L., Granada, Spain, cut-off: 0.2). Fungal identification was determined by the microscopic examination of lactophenol cotton-blue stained slides and MALDI-TOF mass spectrometry (Bruker Daltonics, Bremen, Germany) following the manufacturer’s instructions.
All patients were treated with systemic antifungal therapy, including voriconazole, isavuconazole, and liposomal amphotericin B. We randomized patients for an open-label trial involving nebulized therapy. The interventions were nebulized liposomal amphotericin B (25 mg in 6 mL, three times per week for 6 weeks) for the study group or nebulized injection water (6 mL, three times per week, 6 weeks) for the control group. The systemic therapy duration was determined on an individual basis according to medical judgment.
2.2. Study Setting and Institutional Approvals
The prospective study was funded by the Spanish Ministry of Health’s ‘Fondo de Investigaciones Sanitarias’ (Health Research Fund; FIS grant, PI18/00179), and was conducted from October 2019 to October 2022 at a tertiary hospital in Madrid, Spain. The study’s promoter was Hospital Universitario Ramón y Cajal, and its protocol (version 3.0, 4 July 2019; NAIFI01 code, EUDRACT no: 2019-000745-12) was approved by the Institutional Review Board. Patient consent was duly obtained. Additionally, the Spanish Agency of Medicines and Sanitary Products (AEMPS) granted its approval on 13 August 2019; locator PXB29SAA6A.
2.3. Objectives
To ensure the safety of NLAB tolerance, forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) readings were taken each week for the first 6 weeks. Any reductions greater than 20% in FVC and FEV1 after nebulization compared with pre-nebulization values were deemed significant. These readings were made using a micro-spirometer, courtesy of Micro Medical Ltd., based in Rochester, UK.
Additional measurements taken at each visit, pre- and post-nebulization, included oxygen saturation, blood pressure, symptoms such as cough and dyspnea, bronchospasm occurrence, need for bronchodilator treatment, and signs of nausea, vomiting, dysphagia, foul taste, and chest pain.
To support NLAB safety monitoring, serum amphotericin B levels were assessed using high-performance liquid chromatography coupled with ultra-violet detection (HPLC-UV). This involved a heat extraction and protein precipitation technique. For Amphotericin B identification and quantification, we used a photodiode array detector (PDA) to establish the molecule’s specific UV profile under set chromatographic conditions and to determine the peak absorption wavelength (405 nm).
To track the response in both arms, we conducted weekly tests during the first 6 weeks. These tests included the serum Ag Virclia® Monotest (produced by Vircell S.L., Granada, Spain)., with a cut-off value of 0.2), the serum BDGlucan (made by Fujifilm Wako Chemicals Europe GmbH®, Neuss, Germany) with a cut-off of 7 pg/mL), and a customized serum PCR multiplex real-time PCR test to detect A. fumigatus, A. terreus, and A. flavus.
Furthermore, we utilized 18F-Fluorodeoxyglucose Positron Emission Tomography (FDG-PET-CT) at diagnosis and at the sixth week to monitor radiological responses. By measuring the standardized uptake value (SUV) index, which indicates the uptake of 18F-FDG in tissues relative to the administered dose, we could calculate metabolic activity.
The follow-up period ended at week 12. We examined several outcomes: (a) complete response, defined as both the resolution of signs and symptoms and over 90% radiological improvement in the CT scan; (b) partial response, characterized by more than a 50% radiological improvement; (c) stability, indicating a clinical response but less than 50% radiological improvement; and (d) progression or death.
2.4. Nebulization
eFlow® (PARI Pharma Iberia, Madrid, Spain) nebulizers were used for nebulization [16].
2.5. Randomization
Patients were randomized 1:1 at baseline (day 0) using permuted block randomization. The randomization took into account onco-hematologic patients with severe neutropenia conditions (<100 cells/μL). This method ensured a homogeneous distribution across both groups.
2.6. Exclusion Criteria
The following exclusion criteria were applied: inability or refusal of the patient (or legal representative) to give consent; pregnancy or planning to become pregnant during the study; lactation; formal contraindication to administration of nebulized drugs; hypersensitivity to amphotericin B; confirmation at the time of diagnosis of extra-pulmonary aspergillosis; intubated patients or requiring imminent intubation at the time of randomization because some studies have confirmed that NLAB can precipitate in the breathing tubes; participation in another clinical trial in the previous month; and life expectancy < 1 week.
2.7. Concomitant Antifungal Therapy
To prevent bias, the study adhered to standard medical practices and international guidelines when administering systemic treatments to all patients. Therefore, any patient in the study might have received voriconazole, isavuconazole, or liposomal amphotericin B as systemic antifungal treatment. Any other antifungal treatment, if administered in the absence of those mentioned above, was deemed suboptimal.
Patients undergoing nebulized therapies with other antimicrobials, including antifungals, were not eligible for inclusion.
2.8. Compliance and Visits
The Pharmacy Department oversaw the tracking of treatment compliance and medication control for the study. In line with RD 1090/2015, they meticulously recorded the patient’s randomization number, supplied medication, batch number, and expiration date in the case report form (CRF).
The study consisted of nine visits: baseline visit (also known as visit 0 or screening visit), weeks 1–6, week 9, and lastly, week 12 (day 84, which entailed an overall evaluation and marked the conclusion of the follow-up). During the first 6 weeks, spirometric tests and the evaluation of factors relating to nebulization tolerance were conducted before and after nebulization at each visit. Outpatients were required to bring their nebulizers to each visit for on-site usage and monitoring.
2.9. Statistical Analysis
Standard descriptive statistics were used to summarize the study population characteristics. Categorical variables were compared with the χ2 test. The Student’s t-test or Mann–Whitney U test was applied for continuous variables. Repeated measurements across time points were compared using paired parametric or nonparametric tests (Student’s t-test for paired samples or Wilcoxon signed-rank test). The treatment of IPA naturally leads to an improvement in the radiological infiltrate and consequently to a reduction in the SUV index; therefore, for the statistical analysis, a one-tailed p was used to analyze the effect of nebulization with NLAB compared with the placebo. Continuous variables were previously dichotomized at the median values to be entered into the model. Statistical analysis was performed with SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Characteristics of Patients
Over a 3-year period, 13 patients were enrolled (Table 1), including 6 who received NLAB and 7 who were given a placebo. Originally, the plan was to include 30 patients, but due to the SARS-CoV-2 pandemic and the subsequent prohibition of nebulized treatments at the facility, the study was halted for 20 months. Our subjects consisted of 6 males and 7 females, all of whom met the criteria for probable aspergillosis (EORTC 2019) [15].

Table 1.
Baseline clinical characteristics, randomization, and outcome of patients.
Isolation of Aspergillus spp. in BAL registered positive in 61% of cases, with elevated GM in BAL and serum detected in 77% and 69% of cases, respectively. In two instances, fungal hyphae were also found in respiratory samples. Aspergillus fumigatus complex was confirmed in ten participants (77%), Aspergillus terreus in two, and Aspergillus flavus in one patient.
The majority of patients (61%) were undergoing onco-hematological treatments. All of them suffered from severe neutropenia (less than 100 WBC/µL). Two patients had autoimmune hepatitis and were being treated with high doses of corticosteroids. Another two patients had collagen diseases and were also undergoing corticosteroid and immunosuppressive therapy. One patient presented with alcoholism and malnutrition (Table 1).
3.2. Safety Analysis
The primary safety endpoint showed that NLAB was well tolerated. Nebulization was feasible in all patients, including those who received a placebo (nebulized water injection). Table 2 presents FEV1 values before and after nebulization, as well as the percentage change after administration. There were no significant differences in the decreases in FEV1 in patients receiving NLAB or the placebo. Only in three tests of the same patient with NLAB (#1) and in two tests of a placebo patient (#6) were reductions greater than 20% observed, which were predefined as significant. Moreover, the variance in FVC revealed no differences between the two groups, supporting the data observed in FEV1 (Table 3). No differences in oxygen saturation values were noted before or after nebulization.

Table 2.
Percentage of variation in post-nebulization FEV1 with respect to pre-nebulization FEV1.

Table 3.
Percentage of variation in post-nebulization FVC with respect to pre-nebulization FVC.
Tolerance was similar in both groups; all patients experienced a cough during nebulization, but it was well tolerated. No instances of bronchospasm occurred, and three patients (two with NLAB, one placebo) needed prophylactic bronchodilator treatment before nebulization. One NLAB patient reported throat discomfort or irritation, and three reported a metallic taste during nebulization. There were no instances of shortness of breath, nausea, vomiting, or chest pain related to nebulization. Serum amphotericin B tests were performed during patients’ corresponding visits who were receiving NLAB by HPLC-UV. Notably, no serum amphotericin B levels were recorded in any test.
3.3. Clinical Response and Outcome
Table 1 presents data on patients’ clinical responses and outcomes. Patients #7 and #9 could not be evaluated due to their admission to the ICU at weeks +3 and +1, respectively. Nebulization was suspended, and their second FDG-PET-CT scans could not be conducted. Both patients exhibited progression in their underlying conditions. Of the remaining patients, seven displayed partial or complete responses at week 12 (four received NLAB, and three received a placebo). Four other patients did not demonstrate favorable clinical responses (two received a placebo and two received NLAB). Among these, three succumbed to the progression of their illnesses, independent of IPA evolution. Patient #11, who was on the placebo, exhibited a cavitated image and persistent microbiological presence at week 12. As a result, it was necessary to continue antifungal therapy. This patient also needed an increase in immunosuppressive treatment to manage her primary condition (systemic pulmonary sclerosis). Therapeutic drug monitoring was carried out in patients receiving voriconazole and the dose was adjusted to maintain serum levels between 1 and 5 ug/mL. No TDM was performed in patients receiving isavuconazole. Table 1 details the unique characteristics and progress of each patient.
3.4. Radiological Response, FDG-PET/CT Imaging, and Uptake Evaluations
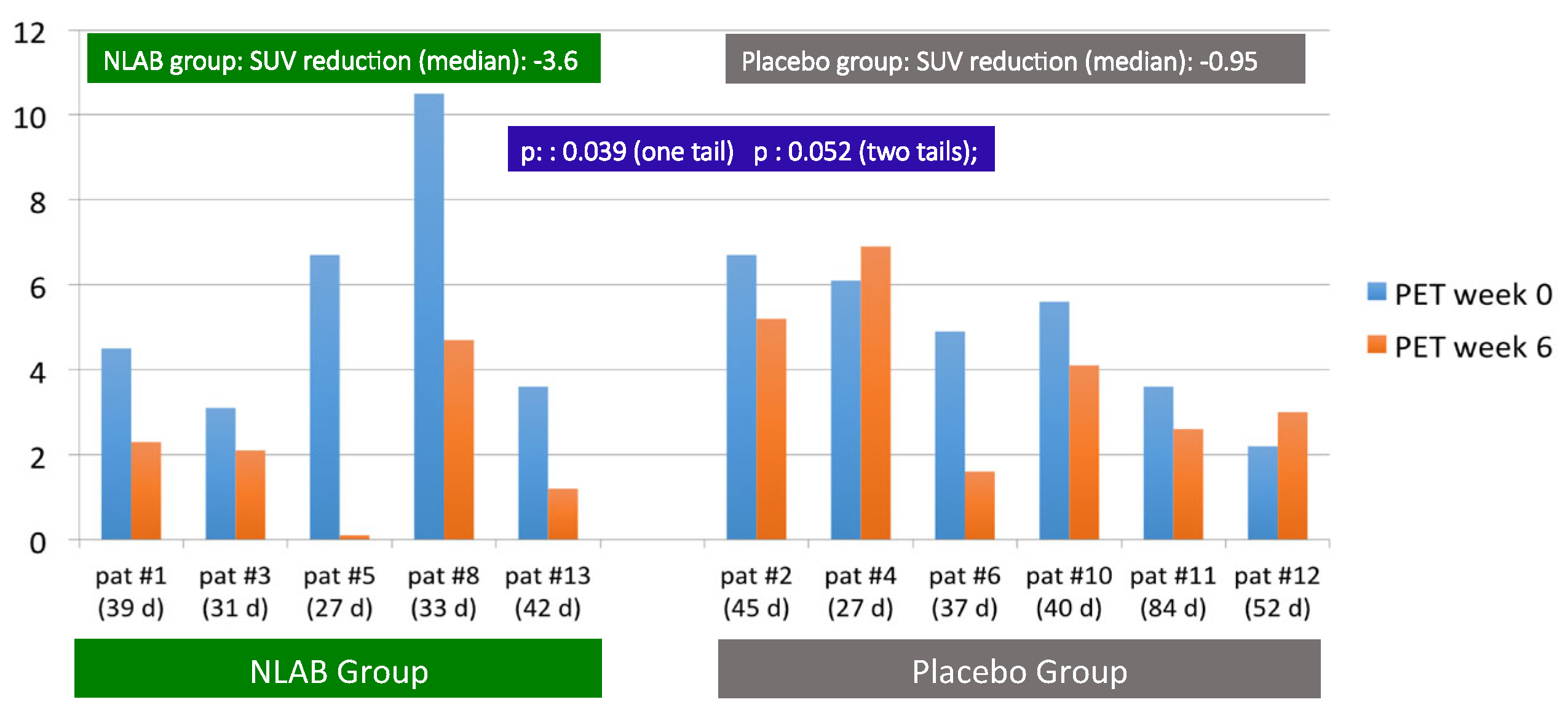
In addition to the standard clinical evaluation at week 12, patients underwent a study using FDG-PET-CT at the start and at week 6. This method produces a quantitative measurement of the metabolic activity of targeted tissues through the SUV index. Figure 1 depicts the SUV indices for each patient’s pulmonary lesion, identified by nuclear medicine experts as suggestive of IPA, both at the start (week 0) and at week 6.
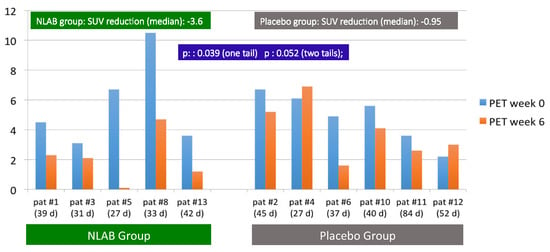
Figure 1.
F2G/PET/CT uptake. SUV at inclusion (week 0) and at the follow-up (week 6). Patients in both groups and in real time (days) between two procedures.
Due to technical constraints, the initial FDG-PET-CT was conducted between days 2 and 7, while the follow-up procedure at week 6 occurred between days 38 and 55. The time between these two tests for each patient is also shown in Figure 1. Overall, we observed a significant decrease in SUV at week 6 in patients treated with NLAB—a median SUV reduction of −3.6 compared with −0.95 in the placebo group [p: 0.039 (one-tailed); p: 0.052 (two-tailed)].
Except for patient #8, who started with a high initial SUV of 10.5, the beginning indices were comparable in both groups, with SUVs between 3.1 and 6.7. Notably, at week 6, except for patient #8 (who experienced a significant reduction to 4.7), four out of five patients treated with NLAB nebulization demonstrated an SUV of less than 2.3, compared with only one out of six in the placebo group. In fact, two placebo patients (patient #4 and patient #12) actually showed an increase in SUV at week 6 compared with their baseline readings.
3.5. Evolution of Serum GM and BDG and PCR
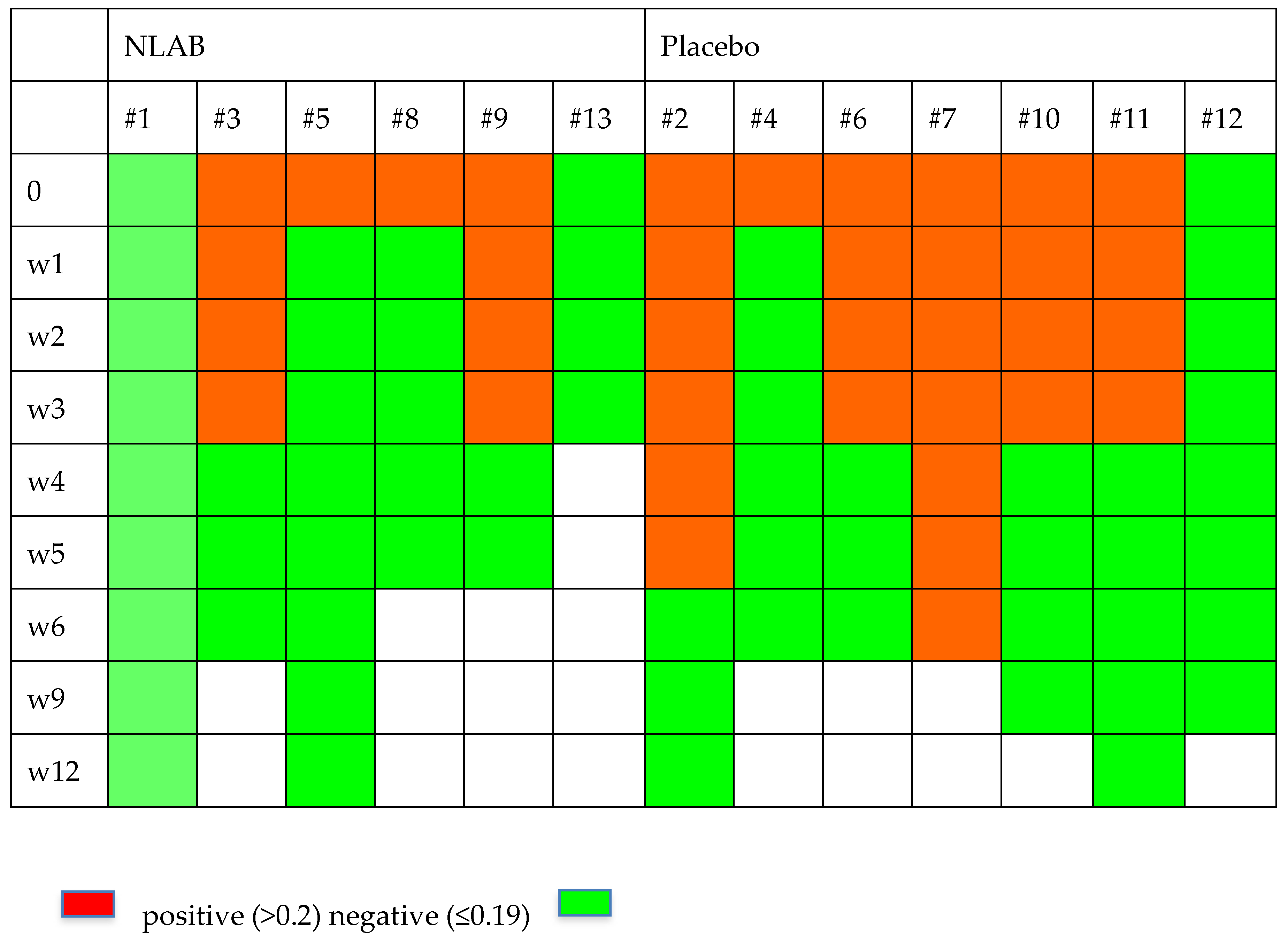
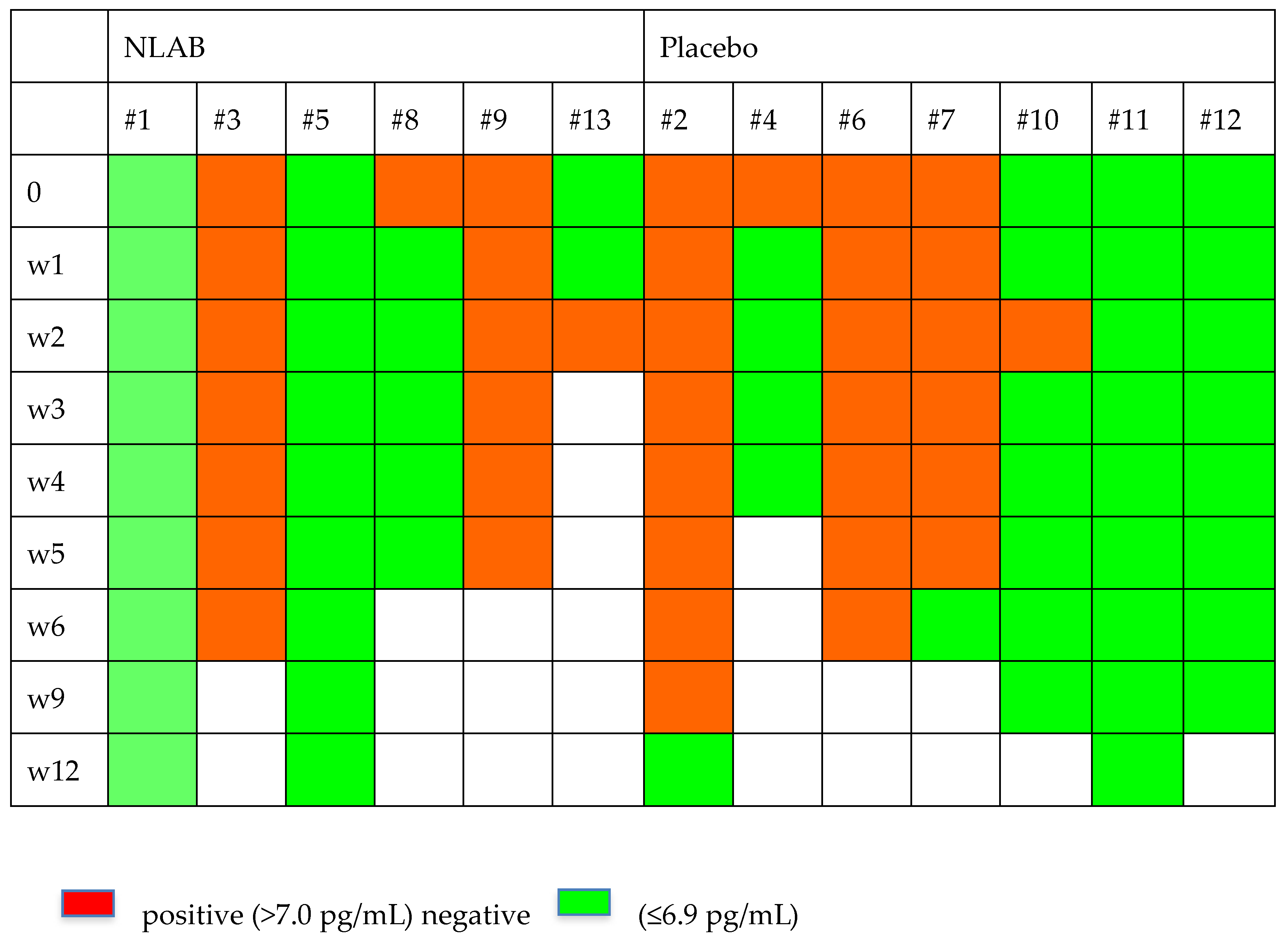
Figure 2 and Figure 3 display the GM and BDG results during the follow-up weeks, including the data from patients 7 and 9, who were not included in the clinical evaluation analysis. Despite the limited patient sample, Figure 2 exhibits a higher clearance rate among those who received NLAB. By week 3, five out of seven patients in the placebo group still had positive serum GM (>0.2, Virclia), compared with only two out of six patients in the NLAB group. The BDG results also imply a lower clearance rate in the placebo group, although the low specificity of this test constrains the reliability of this observation. The determination of PCR (multiplex real-time) in serum did not show positive results in any of the patients.
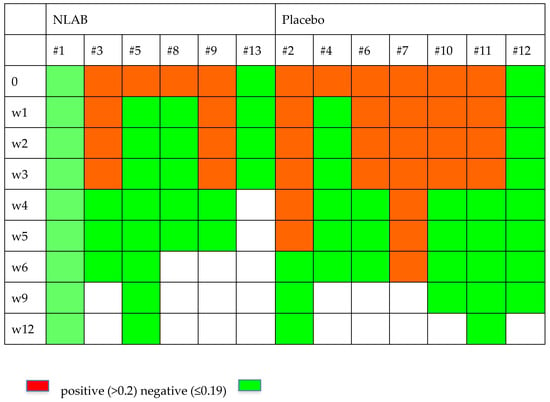
Figure 2.
The serum galactomannan outcomes in both groups.
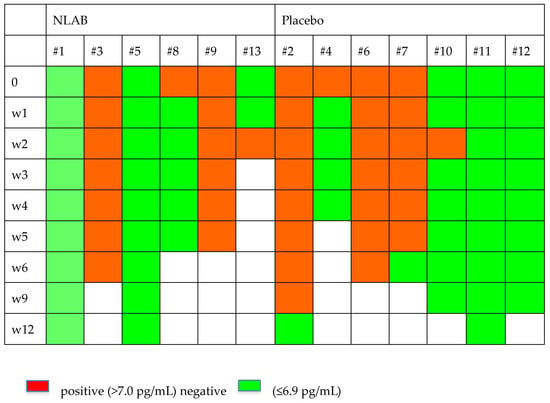
Figure 3.
The serum B-D-Glucan outcomes in both groups.
4. Discussion
This clinical trial shows that NLAB is safe and well tolerated in patients with IPA. Despite the small sample size, it provides data that suggest potential benefits when combined with standard systemic antifungal therapy. No prior randomized clinical trial has analyzed NLAB’s role in treating IPA, and no observational or controlled studies have evaluated its therapeutic efficacy.
In Hagiya’s [10] systematic review, 11 reports administered NLAB therapeutically, with six specifically targeting IPA [17,18,19,20,21,22]. All six reports indicated favorable outcomes. NLAB was also implemented against rare invasive mold infections and Aspergillus infection [23]. Out of 11 observational studies [3,4,5,12,24,25,26,27,28,29], only 1 was multi-centered; they all evaluated the prophylactic use of NLAB, primarily among lung transplant patients.
The majority of patients in the current study had angio-invasive forms of onco-hematological diseases. While nebulized therapies are typically more beneficial in broncho-pulmonary forms that affect mainly non-oncohematologic patients, the effectiveness of Nebulized Liposomal Amphotericin B (NLAB) in preventative care has been demonstrated in patients with hemato-oncological diseases. A rigorously designed, randomized controlled trial supported this, finding that NLAB preventative therapy significantly decreased the incidence of IPA in patients with prolonged neutropenia due to hemato-oncological diseases [6] without negatively impacting their pulmonary function [7]. The widespread use of NLAB as a preventative measure in lung transplant patients was also documented; a recent network meta-analysis suggested that NLAB might be the most effective preventative approach for lung transplant recipients [30].
In the current study, we administered a 25 mg dose three times a week. Earlier studies varied the NALB dosage from 15–25 mg twice daily to 20–50 mg two or three times weekly, frequently for prevention. Our goal was therapy, and we decided on the 25 mg thrice-weekly dosage based on Monforte et al.’s findings [3]. Despite the angio-invasive nature of aspergillosis in hematological patients, the importance of achieving high alveolar concentrations of amphotericin B in these patients is crucial for infection control since amphotericin B is not detected in the serum. Monforte et al. [3] demonstrated that amphotericin B concentration is high on the first day (>11 ug/mL) after the administration of a 25 mg dose of n-LAB and remains elevated one week later (>4 ug/mL) above the common MIC, suggesting that a concentration gradient may result on delivery of effective amounts. A smaller study also concluded that preventive NLAB does not affect the lipid content in pulmonary surfactants [25].
In our study, we used high-performance liquid chromatography with ultra-violet detection (HPLC-UV) to measure amphotericin B levels in the serum. We employed a heat extraction and protein precipitation method, which extracts the amphotericin B from the liposome and measures both liposomal amphotericin B and free amphotericin B concentrations in the serum. This confirmed the absence of serum amphotericin B levels in all patients who received NLAB at their corresponding visits.
Nebulizations were conducted using eFlow® nebulizers, which are vibrating membrane types with ultrasonic frequency. They offer a high dispensed dose rate (63%) and a high flow rate (0.7 mL/min), significantly reducing the inhalation time from the conventional 10–15 min to just 3–5 min. Designed for home use, eFlow® is an ideal choice for domestic applications.
In this study, no substantial decline was observed in FEV1 or FVC among patients. Noteworthy reductions, pre-established as significant if they exceeded 20%, were found only in three incidences involving the same NLAB patient and two incidences involving a patient on the placebo. Additionally, no significant differences in tolerance were observed between the two groups. Prophylactic bronchodilators were required by only three patients (two taking NLAB, one on placebo). In a separate clinical trial conducted using NLAB for prophylaxis, about half of the patients reported induced coughing and a displeasing taste. However, this trial lacked a placebo control group for comparison and to ascertain the true impact of amphotericin B nebulization. Still, there was a reduction in one-year survival rates among patients receiving NLAB prophylaxis [31].
Although long-term use of NLAB prophylaxis is generally tolerable, certain studies have observed a decrease in susceptibility to amphotericin B among both Aspergillus and non-Aspergillus species [5,26]. However, we do not consider this a risk when using NLAB therapeutically. This is due to the low resistance to amphotericin B and high concentrations within the alveolar region. In our study, we identified two isolates of A. terreus and one isolate of A. flavus. These are species with lower sensitivity to amphotericin B, but two resulted in favorable outcomes.
The limited sample size presents a challenge when evaluating clinical responses in these patients. The complete or partial response rates were 67% (four out of six) for the group treated with NLAB and 60% (three out of five) for the placebo group (p: ns). While this study does not definitively confirm a superior clinical response in patients receiving NLAB, some indirect data suggest an enhancement of response without observed toxicity, making NLAB a valuable option to be considered for further analysis in multicenter studies with a larger patient cohort. The incorporation of FDG-PET-CT scans at baseline and at week +6 allowed for an objective assessment of lung inflammation reduction in both groups. This analysis revealed a significant decrease in SUV at week 6 among patients receiving NLAB (−3.6 vs. −0.95, p: 0.039, one-tailed test). The evaluation of invasive fungal infections (IFIs) using FDG-PET-CT has proven to be a valuable diagnostic tool in various studies. A Dutch study demonstrated that FDG-PET-CT added value to the management of 74% of patients with IFI, detecting lesions beyond the scope of anatomy-based imaging methods in 48.6% of cases [32]. An Australian study involving 48 cases of IFI similarly highlighted the utility of FDG-PET-CT in assessing response to antifungal therapy. Compared to computed tomography (CT), FDG-PET-CT detected sites of IFI dissemination in 35% of patients, as opposed to just 5% with CT [33]. Kim et al. further confirmed the progressive resolution of hypermetabolic nodules following antifungal treatment in five patients tracked sequentially with FDG-PET-CT [12].
The study suggests an improved performance of serum IFI markers in patients who received NLAB. However, this conclusion lacks statistical confirmation. By the third week, five out of the seven patients in the placebo group still had positive serum GM, compared with only two out of six patients treated with NLAB. Despite FDA and EMA’s preference for Platelia Aspergillus Ag (Bio-Rad) as a reference for GM detection, this study utilized Ag Virclia Monotest (Vircell S.L.) due to its favorable correlation and greater convenience. One study demonstrated that both methods can automate the process, but Virclia offers the advantage of individual sample processing without the need for additional single-dose strips for controls. This showed a concordance of 288 for 327 analyzed samples (к = 0.722) [34].
A recent study from Barcelona showed a significant decrease in IPA in patients with SARS-CoV-2 (CAPA) who had undergone solid organ transplants following prophylactic treatment with NLAB [35]. Our team also recently verified NLAB’s beneficial effects in an outbreak of CAPA in the intensive care unit of our center [36]. Positive results have also been achieved with the use of nebulized amphotericin B lipid complex (ABLC) [37,38]. The findings of the MUCONAB trial, which compared intravenous liposomal amphotericin B alone (the control group, 3–5 mg/kg/day, n: 15) with the combination of intravenous liposomal amphotericin B and nebulized amphotericin B deoxycholate (NAB, 10 mg twice a day, every other day, n: 17) in patients with mucormycosis, have recently been published. While there was no significant difference in treatment success between the two groups, none of the patients discontinued treatment [39].
Liposomalization has been proven to be a safe and effective method for various antimicrobials, as it increases drug concentration at infection sites while minimizing pharmaceutical toxicity. Future possibilities might involve the use of inhalable amphotericin B proliposomal microparticles formulated with lung-surfactant-like phospholipids. They can easily be administered using an FDA-approved dry powder device named Handihaler® (Boehringer Ingelheim, Ingelheim, Germany), making it practical for clinical settings [40].
In essence, NAIFI is the inaugural randomized, controlled clinical trial to examine the efficacy of liposomal amphotericin B as a supplemental treatment for IPA. A significant limitation is its small sample size; however, this phase I-IIa pilot study was intended to test this treatment’s safety, which has been effectively affirmed by the study. Originally, the design of the study was blind, but the Spanish drug agency (AEMPS) did not approve the use of dyes for placebo masking. The lack of prior trials restricted the determination of optimal treatment dosage, duration, and monitoring times using FDG-PET-CT. The study largely included onco-hematological patients with angio-invasive IPA forms, suggesting that nebulized therapies may be more effective in broncho-pulmonary forms. ICU patients were not included due to initial concerns about the potential risks of amphotericin B precipitating in breathing tubes, although recent research has confirmed this to be a safe procedure. Despite these shortcomings, this study verifies the good tolerance and safety of NLAB. The indirect effectiveness data gathered from image monitoring (FDG-PET-CT) and biomarkers are promising, warranting further, larger-scale multicenter studies.
Author Contributions
Conceptualization, J.F. and S.M.; Methodology, J.F., E.G.-G.d.l.P., A.M.-L., P.P., P.M.-D., A.G.-L., M.J.B., J.L.-J. and S.M.; Software, P.P., R.E., J.M.-G. and N.V.; Validation, P.P., M.J.B., R.E., C.S. and N.V.; Formal analysis, J.F., E.G.-G.d.l.P., P.M.-D., A.G.-L., M.J.B., J.L.-J., F.G., M.E.A.-A. and C.S.; Investigation, J.F., E.G.-G.d.l.P., A.M.-L., P.M.-D., A.G.-L., R.E. and M.E.A.-A.; Resources, E.G.-G.d.l.P., A.M.-L., P.P., F.G., M.E.A.-A., C.S., J.M.-G., D.S.M. and N.V.; Data curation, J.L.-J., F.G., M.E.A.-A., J.M.-G. and D.S.M.; Writing – original draft, J.F.; Writing – review & editing, A.M.-L., P.M.-D., A.G.-L., J.L.-J., F.G., R.E. and S.M.; Supervision, J.F. and S.M.; Funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a ‘Fondo de Investigaciones Sanitarias’ (Health Research Fund) of the Spanish Ministry of Health (FIS grant, PI18/00179).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AEMPS | Spanish Agency of Medicines and Sanitary Products |
| BAL | Bronchoalveolar lavage |
| BDG | Beta-D-Glucan |
| CAPA | COVID-19-associated pulmonary aspergillosis |
| CRF | Case report form |
| CT | Computed tomography |
| DD | Dispensed dose |
| EORTC/MSG | European Organization for Research and Treatment of Cancer/Mycoses Study Group |
| FDG-PET-TC | 18F-Fluorodeoxyglucose positron emission tomography |
| FEV1 | Forced expiratory volume in 1 s |
| FIS | Fondo para la Investigación sanitaria (Spanish Health Ministry) |
| FVC | Forced vital capacity |
| GM | Galactomannan |
| HPLC-UV | High-performance liquid chromatography combined with ultra-violet detection |
| ICU | Intensive care unit |
| IFI | Invasive fungal infection |
| IPA | Invasive pulmonary aspergillosis |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry technique |
| NAIFI | Study analyzing the use of Nebulized Liposomal Amphotercin B in IFI |
| NLAB | Nebulized Liposomal Amphotercin B |
| PCR | Polymerase chain reaction |
| PDA | Photodiode array detector |
| RD | Real decreto |
| SUV | Standardized uptake value |
| TDM | Therapeutical drug monitorization |
References
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Monforte, V.; Ussetti, P.; López, R.; Gavaldà, J.; Bravo, C.; de Pablo, A.; Pou, L.; Pahissa, A.; Morell, F.; Román, A. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: Pharmacokinetics and safety. J. Heart Lung Transplant. 2009, 28, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Monforte, V.; Ussetti, P.; Gavaldà, J.; Bravo, C.; Laporta, R.; Len, O.; García-Gallo, C.L.; Tenorio, L.; Solé, J.; Román, A. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J. Heart Lung Transplant. 2010, 29, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Monforte, V.; Martin-Gomez, M.T.; Ruiz-Camps, I.; Berastegui, C.; Saez, B.; Riera, J.; Ussetti, P.; Solé, J.; Gavaldá, J.; et al. 10 years of prophylaxis with nebulized liposomal amphotericin B and the changing epidemiology of Aspergillus spp. infection in lung transplantation. Transpl. Int. 2016, 29, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rijnders, B.J.; Cornelissen, J.J.; Slobbe, L.; Becker, M.J.; Doorduijn, J.K.; Hop, W.C.; Ruijgrok, E.J.; Lüwenberg, B.; Vulto, A.; Lugtenburg, P.J.; et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: A randomized, placebo-controlled trial. Clin. Infect. Dis. 2008, 46, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Slobbe, L.; Boersma, E.; Rijnders, B.J. Tolerability of prophylactic aerosolized liposomal amphotericin-B and impact on pulmonary function: Data from a randomized placebo-controlled trial. Pulm. Pharmacol. Ther. 2008, 21, 855–859. [Google Scholar] [CrossRef]
- Muthu, V.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Rudramurthy, S.M.; Aggarwal, A.N.; Chakrabarti, A.; Agarwal, R. Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta- analysis. Pulm. Pharmacol. Ther. 2023, 81, 102226. [Google Scholar] [CrossRef]
- Ho, K.M.; Duff, O.; Chambers, D.; Murray, R. Meta-analysis of nebulized amphotericin B to prevent or treat pulmonary aspergillosis in immunosuppressed animals. Transpl. Infect. Dis. 2008, 10, 168–176. [Google Scholar] [CrossRef]
- Hagiya, H.; Nishimura, Y.; Otsuka, F. Safety and usefulness of nebulized liposomal amphotericin B: Systematic scoping review. Pulm. Pharmacol. Ther. 2023, 82, 102233. [Google Scholar] [CrossRef]
- Venanzi, E.; Martín-Dávila, P.; López, J.; Maiz, L.; de la Pedrosa, E.G.G.; Gioia, F.; Escudero, R.; Filigheddu, E.; Moreno, S.; Fortún, J. Aerosolized Lipid Amphotericin B for Complementary Therapy and/or Secondary Prophylaxis in Patients with Invasive Pulmonary Aspergillosis: A Single-Center Experience. Mycopathologia 2019, 184, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yoo, J.W.; Oh, M.; Park, S.H.; Shim, T.S.; Choi, Y.Y.; Ryu, J.S. (18)F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography findings are different between invasive and noninvasive pulmonary aspergillosis. J. Comput. Assist. Tomogr. 2013, 37, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Macapinlac, H.A.; Kontoyiannis, D.P. The use of 18F-fluorodeoxyglucose positron emission tomography for the diagnosis and management of invasive mould infections. Med. Mycol. 2008, 46, 23–29. [Google Scholar] [CrossRef]
- Fernández-Cruz, A.; Lewis, R.E.; Kontoyiannis, D.P. How Long Do We Need to Treat an Invasive Mold Disease in Hematology Patients? Factors Influencing Duration of Therapy and Future Questions. Clin. Infect. Dis. 2020, 71, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Prüfer, N.; Oesterheld, N. Nebulization of active pharmaceutical ingredients with the eFlow(®) rapid: Impact of formulation variables on aerodynamic characteristics. J. Pharm. Sci. 2014, 103, 2585–2589. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Moresco, L.; Cappelli, B.; Cuzzubbo, D.; Moroni, C.; Lanino, E.; Faraci, M. Nebulized liposomal amphotericin B and combined systemic antifungal therapy for the treatment of severe pulmonary aspergillosis after allogeneic hematopoietic stem cell transplant for a fatal mitochondrial disorder. J. Chemother. 2007, 19, 339–342. [Google Scholar] [CrossRef]
- García-Gallo, C.L.; García-Fadul, C.; Laporta-Hernández, R.; Ussetti-Gil, P. Traqueobronquitis aspergilar en paciente sometido a trasplante pulmonar [Aspergillus tracheobronchitis in a lung transplant recipient]. Rev. Iberoam. Micol. 2011, 28, 129–133. [Google Scholar] [CrossRef]
- Hanada, S.; Uruga, H.; Takaya, H.; Miyamoto, A.; Morokawa, N.; Kurosaki, A.; Kishi, K. Nebulized liposomal amphotericin B for treating Aspergillus empyema with bronchopleural fistula. Am. J. Respir. Crit. Care Med. 2014, 189, 607–608. [Google Scholar] [CrossRef]
- Godet, C.; Goudet, V.; Laurent, F.; Le Moal, G.; Gounant, V.; Frat, J.P.; Cateau, E.; Roblot, F.; Cadranel, J. Nebulised liposomal amphotericin B for Aspergillus lung diseases: Case series and literature review. Mycoses 2015, 58, 173–180. [Google Scholar] [CrossRef]
- Hamada, N.; Ishiga, M.; Tanaka, S.; Ooue, Y.; Itano, J.; Tanaka, H.; Yuzurio, S.; Horiuchi, T.; Suwaki, T.; Kimura, G.; et al. Successful Treatment of Antifungal Combination Therapy with Inhaled Liposomal Amphotericin B and Oral Voriconazole for Intractable Chronic Progressive Pulmonary Aspergillosis. Intern. Med. 2021, 60, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, H.; Fernández-Ruiz, M.; Gutiérrez, E.; Sevillano, Á.; Caravaca-Fontán, F.; Morales, E.; López-Medrano, F.; Aguado, J.M.; Praga, M.; Andrés, A. Invasive pulmonary aspergillosis associated with COVID-19 in a kidney transplant recipient. Transpl. Infect. Dis. 2021, 23, e13501. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lu, D.; Zhang, J.; Zhong, X.; Liu, G.; Li, B. Treatment of Disseminated Talaromyces marneffei with Tracheal Infection: Two Case Reports. Mycopathologia 2015, 180, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, E.; Morales, P.; Monte, E.; Vicente, R. A comparision of several formats of amphotericin B as an inhaled antifungal prophylaxis. Transplant. Proc. 2009, 41, 2225–2226. [Google Scholar] [CrossRef] [PubMed]
- Monforte, V.; López-Sánchez, A.; Zurbano, F.; Ussetti, P.; Solé, A.; Casals, C.; Cifrian, J.; de Pablos, A.; Bravo, C.; Román, A. Prophylaxis with nebulized liposomal amphotericin B for Aspergillus infection in lung transplant patients does not cause changes in the lipid content of pulmonary surfactant. J. Heart Lung Transplant. 2013, 32, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Monforte, V.; Martin-Gomez, M.T.; Ruiz-Camps, I.; Berastegui, C.; Saez, B.; Riera, J.; Solé, J.; Gavaldá, J.; Roman, A. Epidemiology of invasive respiratory disease caused by emerging non-Aspergillus molds in lung transplant recipients. Transpl. Infect. Dis. 2016, 18, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.A.; Kauffman, C.A.; Patel, T.S.; Fitzgerald, L.J.; Richards, B.J.; Miceli, M.H. Evaluation of targeted versus universal prophylaxis for the prevention of invasive fungal infections following lung transplantation. Transpl. Infect. Dis. 2021, 23, e13448. [Google Scholar] [CrossRef]
- Van Ackerbroeck, S.; Rutsaert, L.; Roelant, E.; Dillen, K.; Wauters, J.; Van Regenmortel, N. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit. Care 2021, 25, 298. [Google Scholar] [CrossRef]
- Malagola, M.; Turra, A.; Signorini, L.; Corbellini, S.; Polverelli, N.; Masina, L.; Del Fabro, G.; Lorenzotti, S.; Fumarola, B.; Farina, M.; et al. Results of an Innovative Program for Surveillance, Prophylaxis, and Treatment of Infectious Complications Following Allogeneic Stem Cell Transplantation in Hematological Malignancies (BATMO Protocol). Front. Oncol. 2022, 12, 874117. [Google Scholar] [CrossRef]
- Marinelli, T.; Davoudi, S.; Foroutan, F.; Orchanian-Cheff, A.; Husain, S. Antifungal prophylaxis in adult lung transplant recipients: Uncertainty despite 30 years of experience. A systematic review of the literature and network meta-analysis. Transpl. Infect. Dis. 2022, 24, e13832. [Google Scholar] [CrossRef]
- Hullard-Pulstinger, A.; Holler, E.; Hahn, J.; Andreesen, R.; Krause, S.W. Prophylactic application of nebulized liposomal amphotericin B in hematologic patients with neutropenia. Onkologie 2011, 34, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, A.O.; Creemers-Schild, D.; De Keizer, B.; Klein, H.C.; Dierckx, R.A.; Kwee, T.C.; Span, L.F.; De Jong, P.A.; Sathekge, M.M.; Glaudemans, A.W. The Added Value of [18F]FDG PET/CT in the Management of Invasive Fungal Infections. Diagnostics 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.P.; Thursky, K.A.; Worth, L.J.; Drummond, E.; Hogg, A.; Hicks, R.J.; Slavin, M.A. FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: A retrospective comparison to conventional CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Calero, A.L.; Alonso, R.; Gadea, I.; Vega, M.D.M.; García, M.M.; Muñoz, P.; Machado, M.; Bouza, E.; García-Rodríguez, J. Comparison of the Performance of Two Galactomannan Detection Tests: Platelia Aspergillus Ag and Aspergillus Galactomannan Ag Virclia Monotest. Microbiol. Spectr. 2022, 10, e0262621. [Google Scholar] [CrossRef] [PubMed]
- Rombauts, A.; Bodro, M.; Daniel Gumucio, V.; Carbonell, I.; Favà, À.; Llado, L.; González-Costello, J.; Oppenheimer, F.; Castel-Lavilla, M.Á.; Len, O.; et al. Antifungal prophylaxis with nebulized amphotericin-B in solid-organ transplant recipients with severe COVID-19: A retrospective observational study. Front. Cell Infect. Microbiol. 2023, 13, 1165236. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.C.; Narváez-Chávez, G.; López-Olivencia, M.; Fortún, J.; de Pablo, R. Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive pulmonary aspergillosis. Intensive Care Med. 2022, 48, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Rodriguez, G.H. Aerosolized amphotericin B lipid complex as adjunctive treatment for fungal lung infection in patients with cancer-related immunosuppression and recipients of hematopoietic stem cell transplantation. Pharmacotherapy 2013, 33, 1035–1043. [Google Scholar] [CrossRef]
- Husain, S.; Capitano, B.; Corcoran, T.; Studer, S.M.; Crespo, M.; Johnson, B.; Pilewski, J.M.; Shutt, K.; Pakstis, D.L.; Zhang, S.; et al. Intrapulmonary disposition of amphotericin B after aerosolized delivery of amphotericin B lipid complex (Abelcet; ABLC) in lung transplant recipients. Transplantation 2010, 90, 1215–1219. [Google Scholar] [CrossRef]
- Muthu, V.; Gogineni, R.R.; Agarwal, R.; Prasad, K.T.; Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Rudramurthy, S.M.; Singh, H.; Garg, M.; et al. Treatment of pulmonary mucormycosis with adjunctive nebulized amphotericin B (MUCONAB trial): Results of an open-label randomized controlled trial. Mycoses 2023, 66, 688–696. [Google Scholar] [CrossRef]
- Gomez, A.I.; Acosta, M.F.; Muralidharan, P.; Yuan, J.X.J.; Black, S.M.; Hayes, D., Jr.; Mansour, H.M. Advanced spray dried proliposomes of amphotericin B lung surfactant-mimic phospholipid microparticles/nanoparticles as dry powder inhalers for targeted pulmonary drug delivery. Pulm. Pharmacol. Ther. 2020, 64, 101975. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).