High Glucose Is a Stimulation Signal of the Salt–Tolerant Yeast Zygosaccharomyces rouxii on Thermoadaptive Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Medium
2.2. Conditions of Z. rouxii Cell Culture
2.3. Transcriptional Sequencing of Z. rouxii under High Temperature Stress
2.4. Real–Time Fluorescence Quantitative Detection
2.5. Detection of Intracellular and Extracellular Metabolites
2.5.1. Preparation of Standard Samples
2.5.2. Extraction of Intracellular Metabolites
2.5.3. Detection of Intracellular Carbohydrate Metabolites
2.5.4. Intracellular Organic Acid and Ethanol Content Detection
2.5.5. Extracellular Glucose, Organic Acid, and Ethanol Content Detection
2.6. Gas Chromatographic Conditions
2.7. Statistical Methods
3. Results
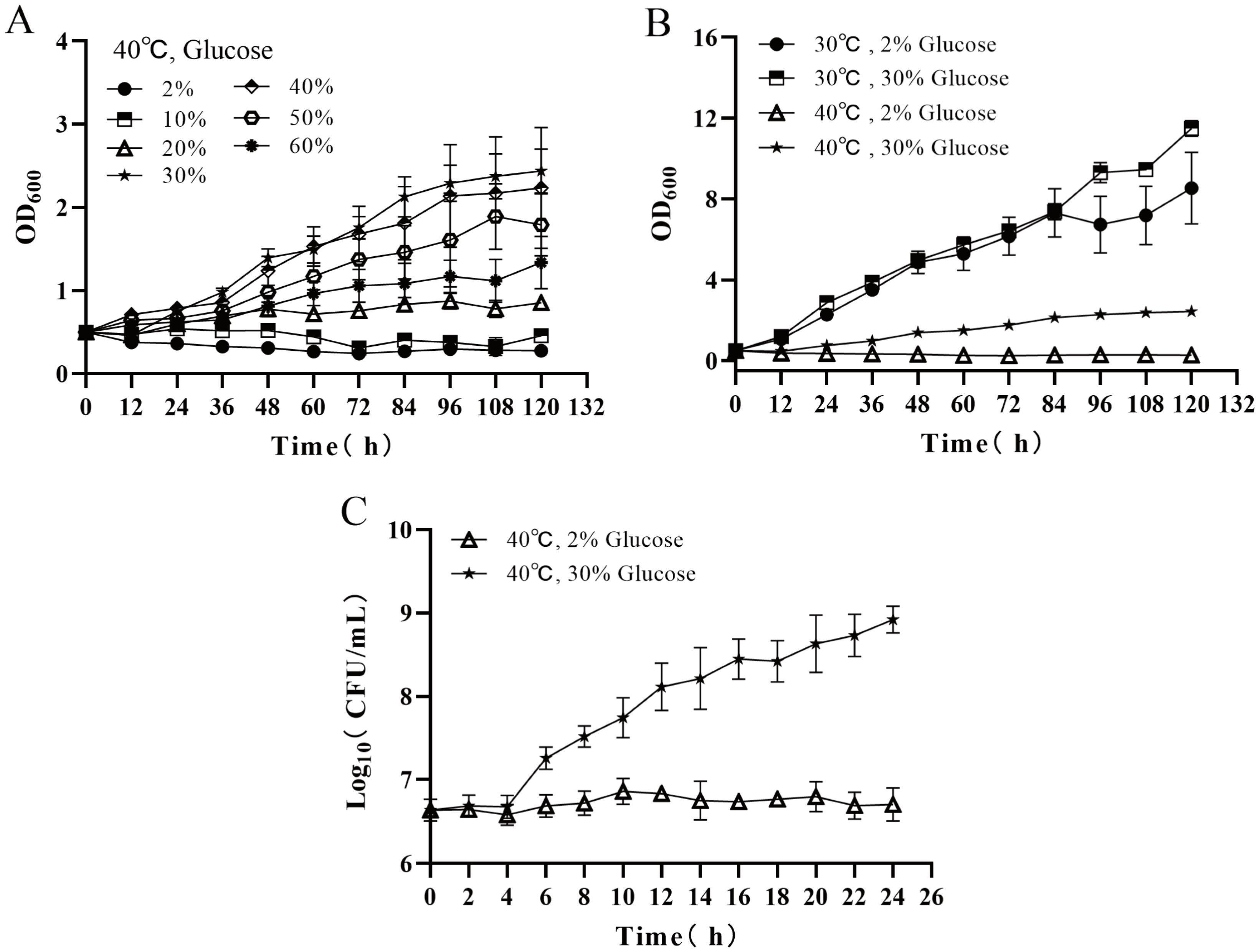
3.1. Effects of Temperature and Glucose Concentration on Cell Proliferation of Z. rouxii
3.2. Micromorphological Changes of Z. rouxii under High Temperature Stress
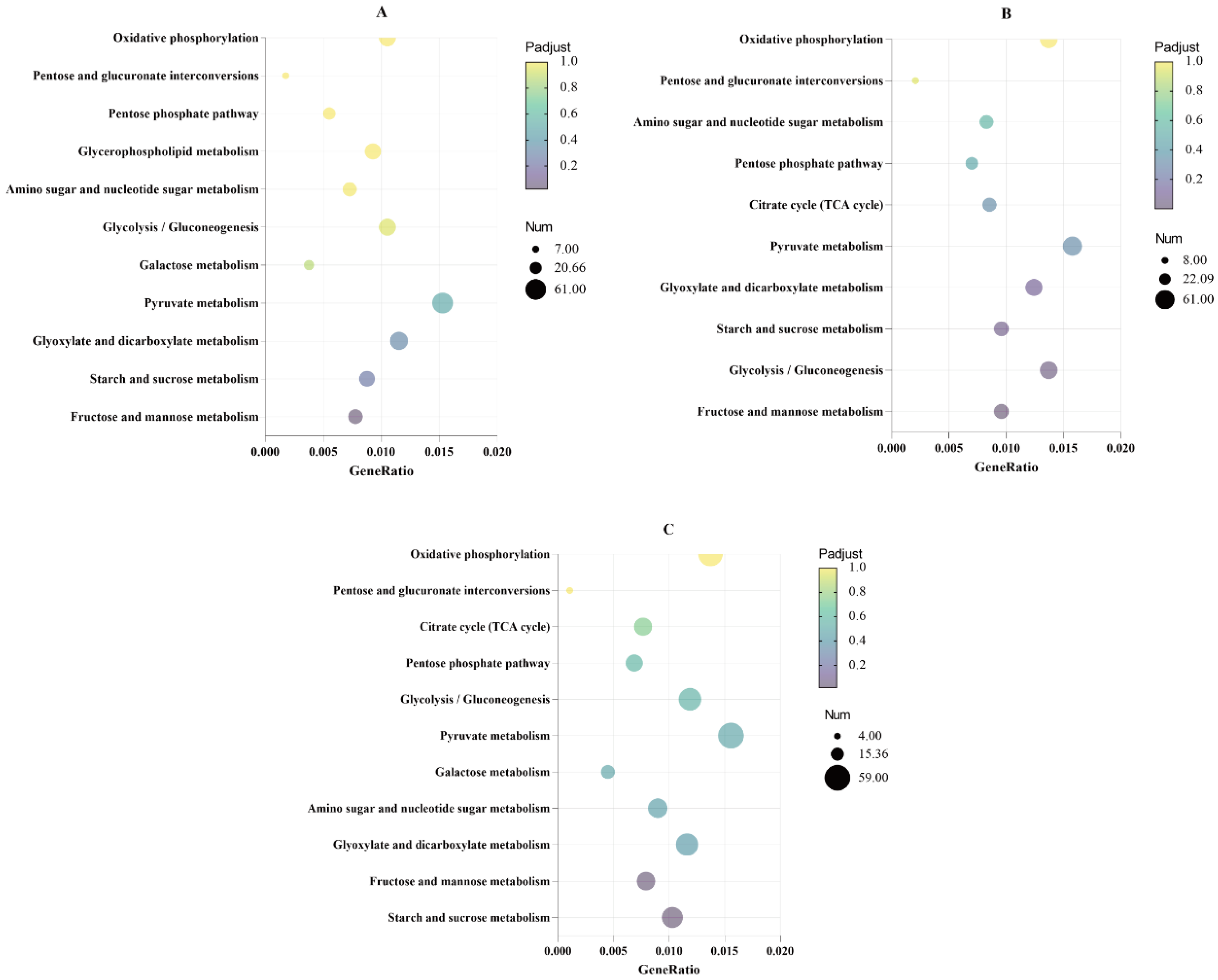
3.3. Analysis of Glucose Metabolism Pathways Significantly Enriched in the KEGG Results of Differentially Expressed Genes
3.4. Transcriptional Timing Expression of Metabolic Pathways of Genes under High Temperature Stress
3.4.1. Glycolytic Pathway
3.4.2. Trehalose Synthesis Pathway
3.4.3. Xylitol Synthesis Pathway
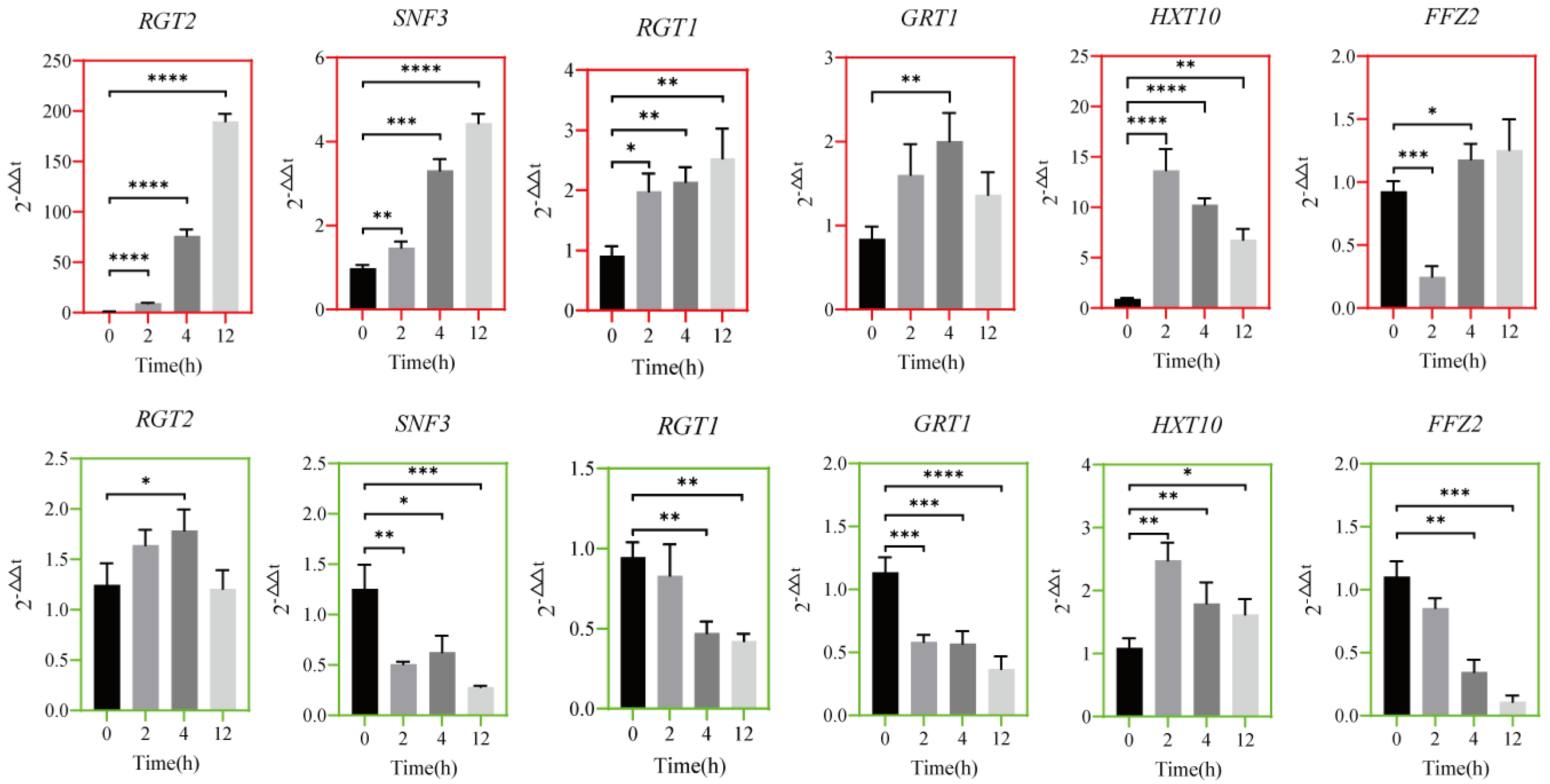
3.5. Transcriptional Timing Expression of Transport Regulatory Protein Genes
3.6. Metabolic Characteristics of Z. rouxii during the Early Stage of Thermoadaptive Growth
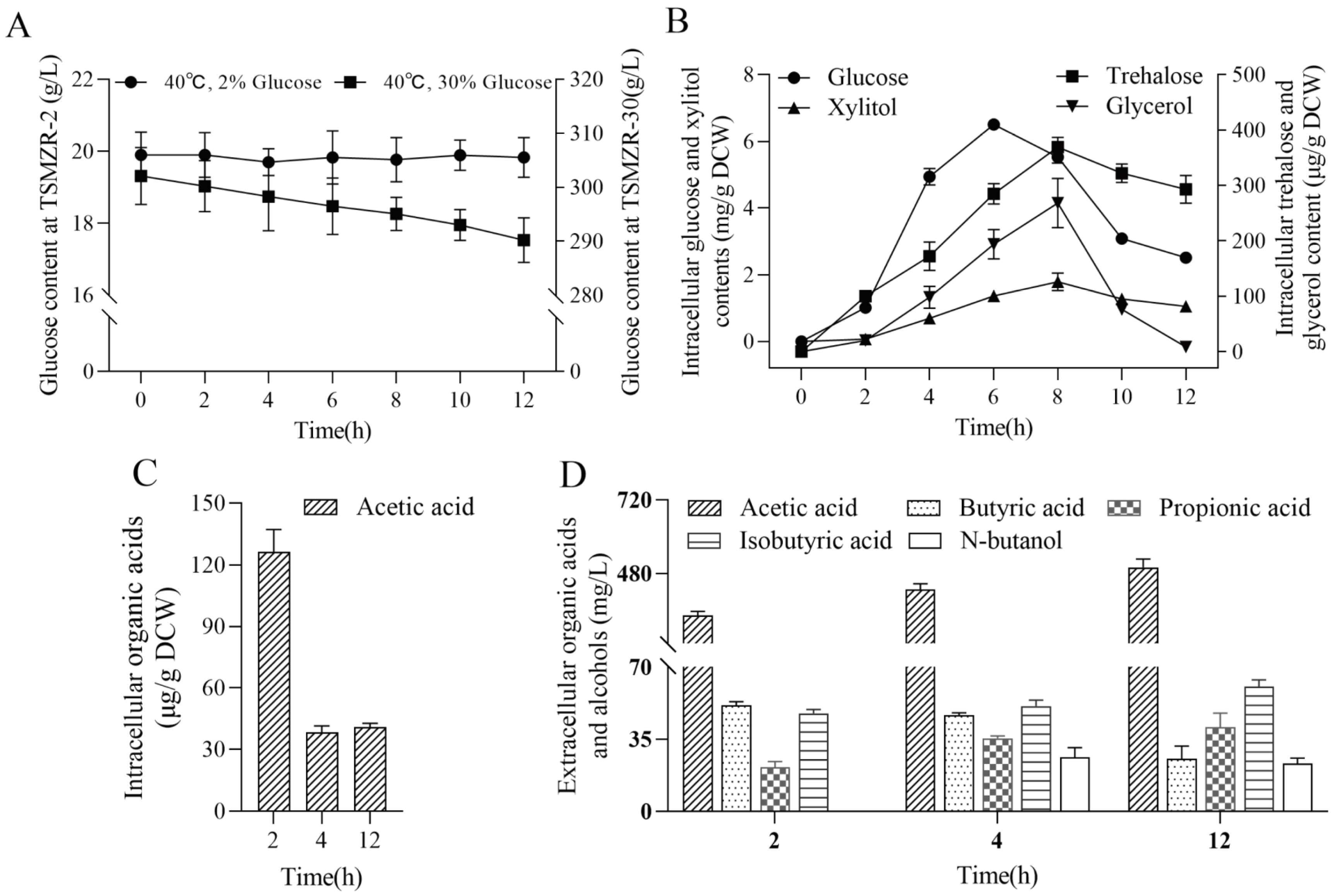
3.6.1. Intracellular/Extracellular Glucose Metabolism Analysis
3.6.2. Intracellular/Extracellular Organic Acid Metabolite Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Niu, C.; Liu, B.; Wei, J.; Wang, H.; Yuan, Y.; Yue, T. Protein abundance changes of Zygosaccharomyces rouxii in different sugar concentrations. Int. J. Food Microbiol. 2016, 233, 44–51. [Google Scholar] [CrossRef]
- Qian, Y.; Song, N.; Gao, Z. Advances in salt stress adaptation mechanism and omics research methods of Zygosaccharomyces rouxii. Biotechnology 2022, 32, 779–786. [Google Scholar]
- Garcia-Rios, E.; Guillamon, J.M. Mechanisms of Yeast Adaptation to Wine Fermentations. Prog. Mol. Subcell. Biol. 2019, 58, 37–59. [Google Scholar]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Andreu, C.; Zarnowski, R.; Del Olmo, M.L. Recent developments in the biology and biotechnological applications of halotolerant yeasts. World J. Microbiol. Biotechnol. 2022, 38, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; He, X.; Weng, P.; Liu, Y.; Wu, Z. A review of yeast: High cell–density culture, molecular mechanisms of stress response and tolerance during fermentation. FEMS Yeast Res. 2022, 22, foac050. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Solieri, L.; Giudici, P. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014, 185, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Goossens, A.; Dever, T.E.; Pascual-Ahuir, A.; Serrano, R. The protein kinase gcn2p mediates sodium toxicity in yeast. J. Biol. Chem. 2001, 276, 30753–30760. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, C.; Wang, M.; Cao, X.; Hou, L. Comparative analysis of salt–tolerant gene HOG1 in a Zygosaccharomyces rouxii mutant strain and its parent strain. J. Sci. Food Agric. 2013, 93, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Du, W.; Meng, F.B.; Rao, J.W.; Liu, D.Y.; Peng, L.X. Tartary buckwheat protein hydrolysates enhance the salt tolerance of the soy sauce fermentation yeast Zygosaccharomyces rouxii. Food Chem. 2021, 342, 128382. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mi, T.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Metabolomics analysis of salt tolerance of Zygosaccharomyces rouxii and guided exogenous fatty acid addition for improved salt tolerance. J. Sci. Food Agric. 2022, 102, 6263–6272. [Google Scholar] [CrossRef]
- Song, N.; Xia, H.; Yang, Q.; Zhang, X.; Yao, L.; Yang, S.; Chen, X.; Dai, J. Differential analysis of ergosterol function in response to high salt and sugar stress in Zygosaccharomyces rouxii. FEMS Yeast Res. 2022, 22, foac040. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Zygosaccharomyces rouxii combats salt stress by maintaining cell membrane structure and functionality. J. Microbiol. Biotechnol. 2020, 30, 62–70. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhu, X.; Yuan, Y.; Gao, Z.; Yue, T. Application of electrical discharge plasma on the inactivation of Zygosaccharomyces rouxii in apple juice. LWT Food Sci. Technol. 2020, 121, 108974. [Google Scholar] [CrossRef]
- Qi, Y.; Qin, Q.; Wang, B.; Jin, C.; Fang, W. Research progress of molecular mechanism of thermo–tolerance in yeast. Guangxi Sci. 2022, 29, 13–22. [Google Scholar]
- Hauck, T.; Brühlmann, F.; Schwab, W. 4–Hydroxy–2,5–dimethyl–3(2H)–furanone formation by Zygosaccharomyces rouxii: Effect of the medium. J. Agric. Food Chem. 2003, 51, 4753–4756. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Voon, M.K.W.; Huang, D.; Lee, P.; Liu, S. Combined effects of fermentation temperature and pH on kinetic changes of chemical constituents of durian wine fermented with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 3005–3014. [Google Scholar] [CrossRef]
- Tobias, H.; Fredi, B.; Wilfried, S. Formation of 4–hydroxy–2,5–dimethyl–3[2H]–furanone by Zygosaccharomyces rouxii: Identification of an intermediate. Appl. Environ. Microbiol. 2003, 69, 3911–3918. [Google Scholar]
- Martinez-Matias, N.; Chorna, N.; Gonzalez-Crespo, S.; Villanueva, L.; Montes-Rodriguez, I.; Melendez-Aponte, L.M.; Roche-Lima, A.; Carrasquillo-Carrion, K.; Santiago-Cartagena, E.; Rymond, B.C.; et al. Toward the discovery of biological functions associated with the mechanosensor Mtl1p of Saccharomyces cerevisiae via integrative multi–OMICs analysis. Sci. Rep. 2021, 11, 7411. [Google Scholar] [CrossRef]
- Veri, A.O.; Robbins, N.; Cowen, L.E. Regulation of the heat shock transcription factor Hsf1 in fungi: Implications for temperature–dependent virulence traits. FEMS Yeast Res. 2018, 18, foy041. [Google Scholar] [CrossRef]
- Xu, L.; Nitika; Hasin, N.; Cuskelly, D.D.; Wolfgeher, D.; Doyle, S.; Moynagh, P.; Perrett, S.; Jones, G.W.; Truman, A.W. Rapid deacetylation of yeast Hsp70 mediates the cellular response to heat stress. Sci. Rep. 2019, 9, 16260. [Google Scholar] [CrossRef]
- Auesukaree, C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J. Biosci. Bioeng. 2017, 124, 133–142. [Google Scholar] [CrossRef]
- Pallapati, A.R.; Prasad, S.; Roy, I. Glycerol 3–phosphate dehydrogenase regulates heat shock response in Saccharomyces cerevisiae. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119238. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Sun, H.; Li, C.; Zhao, Z.; Liu, G. Advances in mechanisms and modifications for rendering yeast thermotolerance. J. Biosci. Bioeng. 2016, 121, 599–606. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Mursula, A.M.; Rottensteiner, H.; Wierenga, R.K.; Kastaniotis, A.J.; Gurvitz, A. The biochemistry of peroxisomal β–oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2003, 27, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Liu, C.; Liu, S.; Xue, Y. Advances in response mechanism of Saccharomyces cerevisiae to oxidative stress. Biol. Resour. 2019, 41, 298–304. [Google Scholar]
- Chowdhary, S.; Kainth, A.S.; Gross, D.S. Heat shock protein genes undergo dynamic alteration in their three–dimensional structure and genome organization in response to thermal stress. Mol. Cell Biol. 2018, 37, e00292-17. [Google Scholar] [CrossRef]
- Matsumoto, I.; Arai, T.; Nishimoto, Y.; Leelavatcharamas, V.F.M.; Kishida, M. Thermotolerant yeast Kluyveromyces marxianus reveals more tolerance to heat shock than the brewery yeast Saccharomyces cerevisiae. Biocontrol Sci. 2018, 23, 133–138. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Masser, A.E.; Kaimal, J.M.; Planells, J.; Andreasson, C. Genetic inactivation of essential HSF1 reveals an isolated transcriptional stress response selectively induced by protein misfolding. Mol. Biol. Cell 2023, 34, ar101. [Google Scholar] [CrossRef]
- Kainth, A.S.; Chowdhary, S.; Pincus, D.; Gross, D.S. Primordial super–enhancers: Heat shock–induced chromatin organization in yeast. Trends Cell Biol. 2021, 31, 801–813. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Huang, J.; Zhou, R.; Jin, Y.; Zhao, D.; Zheng, J.; Wu, C. Heat preadaptation improved the ability of Zygosaccharomyces rouxii to salt stress: A combined physiological and transcriptomic analysis. Appl. Microbiol. Biotechnol. 2021, 105, 259–270. [Google Scholar] [CrossRef]
- Huang, C.; Lu, M.; Chang, Y.; Li, W.; Zhang, J. Experimental evolution of yeast for high–temperature tolerance. Mol. Biol. Evol. 2018, 35, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Lu, X.; Zhuge, B.; Zong, H. Selection and application of novel high temperature inducible promoters in the tolerant yeast Candida glycerinogenes. J. Biosci. Bioeng. 2020, 130, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.A.; Beuchat, L.R. Interactive effects of solutes, potassium sorbate and incubation temperature on growth, heat resistance and tolerance to freezing of Zygosaccharomyces rouxii. J. Appl. Microbiol. 1992, 73, 524–530. [Google Scholar] [CrossRef]
- Jermini, M.F.G.; Schmidt-Lorenz, W. Cardinal Temperatures for Growth of Osmotolerant Yeasts in Broths at Different Water Activity Values. J. Food Prot. 1987, 50, 473–478. [Google Scholar] [CrossRef]
- Martorell, P.; Stratford, M.; Steels, H.; Fernández-Espinar, M.T.; Querol, A. Physiological characterization of spoilage strains of Zygosaccharomyces bailii and Zygosaccharomyces rouxii isolated from high sugar environments. Int. J. Food Microbiol. 2007, 114, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Goldberg, J.F. Scanning Electron Microscopy (SEM) and Immuno–SEM of Nuclear Pore Complexes from Amphibian Oocytes, Mammalian Cell Cultures, Yeast, and Plants. Methods Mol. Biol. 2022, 2502, 417–437. [Google Scholar]
- Zhou, X.; Ren, L.; Meng, Q. The next–generation sequencing technology and application. Protein Cell 2010, 1, 520–536. [Google Scholar] [CrossRef]
- Dai, J.; Li, K.; Song, N.; Yao, W.; Xia, H.; Yang, Q.; Zhang, X.; Li, X.; Wang, Z.; Yao, L.; et al. Zygosaccharomyces rouxii, an Aromatic Yeast Isolated from Chili Sauce, Is Able to Biosynthesize 2–Phenylethanol via the Shikimate or Ehrlich Pathways. Front. Microbiol. 2020, 11, 597454. [Google Scholar] [CrossRef]
- Putri, S.P.; Nakayama, Y.; Matsuda, F.; Uchikata, T.; Kobayashi, S.; Matsubara, A.; Fukusaki, E. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013, 115, 579–589. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, S.; Li, H. Changes in intracellular metabolism underlying the adaptation of Saccharomyces cerevisiae strains to ethanol stress. Ann. Microbiol. 2017, 67, 195–202. [Google Scholar] [CrossRef]
- Khatri, D.; Chhetri, S.B.B. Reducing Sugar, Total Phenolic Content, and Antioxidant Potential of Nepalese Plants. Biomed. Res. Int. 2020, 2020, 7296859. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, M.; Yan, Z.; Chen, D.; Chen, X.; Li, X. Transcriptome difference analysis of trehalose coupled fermentation process in Zygosaccharomyces rouxii. Biotechnology 2023, 33, 150–156, 163. [Google Scholar]
- Alam, S.; Gu, Y.; Reichert, P.; Bähler, J.; Oliferenko, S. Optimization of energy production and central carbon metabolism in a non–respiring eukaryote. Curr. Biol. 2023, 33, 2175–2186. [Google Scholar] [CrossRef]
- Planqué, R.; Bruggeman, F.J.; Teusink, B.; Hulshof, J. Understanding bistability in yeast glycolysis using general properties of metabolic pathways. Math. Biosci. 2014, 255, 33–42. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, C.; Qiu, J. Metabolic Control Strategy of Increasing Spinosad Biosynthesis Precursor–Acetyl Coa Flow. Sci. Technol. Bull. 2018, 34, 9–15. [Google Scholar]
- Babazadeh, R.; Lahtvee, P.J.; Adiels, C.B.; Goksor, M.; Nielsen, J.B.; Hohmann, S. The yeast osmostress response is carbon source dependent. Sci. Rep. 2017, 7, 990. [Google Scholar] [CrossRef]
- Magalhaes, R.; Popova, B.; Braus, G.H.; Outeiro, T.F.; Eleutherio, E. The trehalose protective mechanism during thermal stress in Saccharomyces cerevisiae: The roles of Ath1 and Agt1. FEMS Yeast Res. 2018, 18, foy066. [Google Scholar] [CrossRef]
- Jules, M.; Beltran, G.; François, J.; Parrou, J.L. New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p–dependent trehalose mobilization. Appl. Environ. Microb. 2008, 74, 605–614. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, Z.; Liu, M.; Chen, D.; Chen, X.; Li, X. Metabolic characteristics of intracellular trehalose enrichment in salt–tolerant Zygosaccharomyces rouxii. Front. Microbiol. 2022, 13, 935756. [Google Scholar] [CrossRef]
- Zaman, S.; Lippman, S.I.; Zhao, X.; Broach, J.R. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 2008, 42, 27–81. [Google Scholar] [CrossRef]
- Scharff-Poulsen, P.; Moriya, H.; Johnston, M. Genetic analysis of signal generation by the Rgt2 glucose sensor of Saccharomyces cerevisiae. G3 Genes Genomes Genet. 2018, 8, 2685–2696. [Google Scholar] [CrossRef]
- Solieri, L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J. Microb. Biotechnol. 2021, 37, 96. [Google Scholar] [CrossRef]
- Santiago, A.M.; Gonçalves, D.L.; Morano, K.A. Mechanisms of sensing and response to proteotoxic stress. Exp. Cell Res. 2020, 395, 112240. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Kim, J.; Bloor, D.; Rodriguez, R.; Mohler, E.; Mailloux, L.; Melton, S.; Jung, D. Casein kinases are required for the stability of the glucose–sensing receptor Rgt2 in yeast. Sci. Rep. 2022, 12, 1598. [Google Scholar] [CrossRef]
- Snowdon, C.; Johnston, M. A novel role for yeast casein kinases in glucose sensing and signaling. Mol. Biol. Cell 2016, 27, 3369–3375. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A.; Wang, D.; Hong, J. The novel properties of Kluyveromyces marxianus glucose sensor/receptor repressor pathway and the construction of glucose repression–released strains. Microb. Cell Factories 2023, 22, 123. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Wang, Y.; Yang, X.; Chen, S.; Zhao, Y.; Wu, Y.; Li, L. Salt stress improves thermotolerance and high–temperature bioethanol production of multi–stress–tolerant Pichia kudriavzevii by stimulating intracellular metabolism and inhibiting oxidative damage. Biotechnol. Biofuels 2021, 14, 222. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, W.; Sui, Y.; Ding, R.; Yi, W.; Hu, Y.; Liu, H.; Zhu, C. Sugar protectants improve the thermotolerance and biocontrol efficacy of the biocontrol yeast, Candida oleophila. Front. Microbiol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Lin, N.; Xu, Y.; Yu, X. Overview of yeast environmental stress response pathways and the development of tolerant yeasts. Syst. Microbiol. Biomanufacturing 2022, 2, 232–245. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, Y.; Fan, S.; Nobile, C.J.; Guan, G.; Huang, G. Integration of the tricarboxylic acid (TCA) cycle with cAMP signaling and Sfl2 pathways in the regulation of CO2 sensing and hyphal development in Candida albicans. PLoS Genet. 2017, 13, e1006949. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, H. Regulation of trehalose, a typical stress protectant, on central metabolisms, cell growth and division of Saccharomyces cerevisiae. Food Microbiol. 2020, 89, 103459. [Google Scholar] [CrossRef]
- Lei, S.; Zavala-Flores, L.; Garcia-Garcia, A.; Nandakumar, R.; Huang, Y.; Madayiputhiya, N.; Stanton, R.C.; Dodds, E.D.; Powers, R.; Franco, R. Alterations in energy/redox metabolism induced by mitochondrial and environmental toxins: A specific role for glucose–6–phosphate–dehydrogenase and the pentose phosphate pathway in paraquat toxicity. ACS Chem. Biol. 2014, 9, 2032–2048. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Han, D.; Mo, X.; Zhao, C. Metabolic flux analysis of Saccharomyces cerevisiae under salt stress conditions. J. Jilin Univ. (Sci. Ed.) 2010, 48, 699–703. [Google Scholar]
- Guaragnella, N.; Bettiga, M. Acetic acid stress in budding yeast: From molecular mechanisms to applications. Yeast 2021, 38, 391–400. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, W.; Xu, S.; Zhou, J. Metabolism and strategies for enhanced supply of acetyl–CoA in Saccharomyces cerevisiae. Bioresour. Technol. 2021, 342, 125978. [Google Scholar] [CrossRef]
- Wang, J.; Ledesma-Amaro, R.; Wei, Y.; Ji, B.; Ji, X.J. Metabolic engineering for increased lipid accumulation in Yarrowia lipolytica—A Review. Bioresour. Technol. 2020, 313, 123707. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Xiao, X.; Liu, Q.; Wei, Y.; Cai, D.; Chen, X.; Li, X. High Glucose Is a Stimulation Signal of the Salt–Tolerant Yeast Zygosaccharomyces rouxii on Thermoadaptive Growth. J. Fungi 2024, 10, 185. https://doi.org/10.3390/jof10030185
Yan Z, Xiao X, Liu Q, Wei Y, Cai D, Chen X, Li X. High Glucose Is a Stimulation Signal of the Salt–Tolerant Yeast Zygosaccharomyces rouxii on Thermoadaptive Growth. Journal of Fungi. 2024; 10(3):185. https://doi.org/10.3390/jof10030185
Chicago/Turabian StyleYan, Zhenzhen, Xiong Xiao, Quan Liu, Yangjian Wei, DongBo Cai, Xiong Chen, and Xin Li. 2024. "High Glucose Is a Stimulation Signal of the Salt–Tolerant Yeast Zygosaccharomyces rouxii on Thermoadaptive Growth" Journal of Fungi 10, no. 3: 185. https://doi.org/10.3390/jof10030185
APA StyleYan, Z., Xiao, X., Liu, Q., Wei, Y., Cai, D., Chen, X., & Li, X. (2024). High Glucose Is a Stimulation Signal of the Salt–Tolerant Yeast Zygosaccharomyces rouxii on Thermoadaptive Growth. Journal of Fungi, 10(3), 185. https://doi.org/10.3390/jof10030185




