Antifungal Effect of Metabolites from Bacterial Symbionts of Entomopathogenic Nematodes on Fusarium Head Blight of Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Fusarium sp. Cultures
2.2. Isolation of EPNs Symbiotic Bacteria
2.3. Production of Bacterial Metabolites
2.4. Preparation of Metabolite Treatments
2.5. Efficacy of Bacterial Metabolite Treatments on F. graminearum Mycelial Growth
2.6. Efficacy of Bacterial Metabolites against F. graminearum Spore Germination
2.7. Statistical Analysis
3. Results
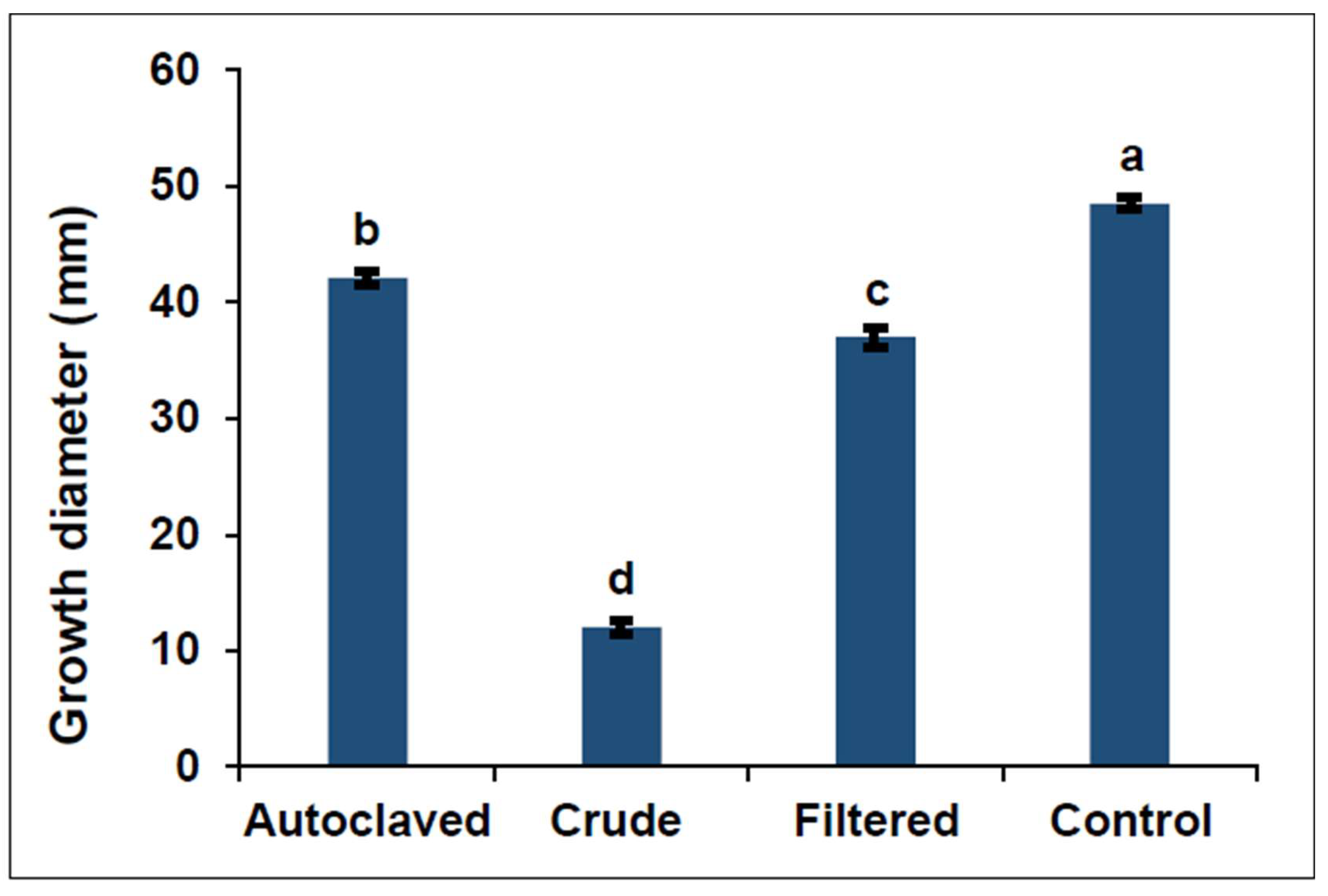
3.1. Efficacy of Bacterial Metabolites on F. graminearum Mycelial Growth
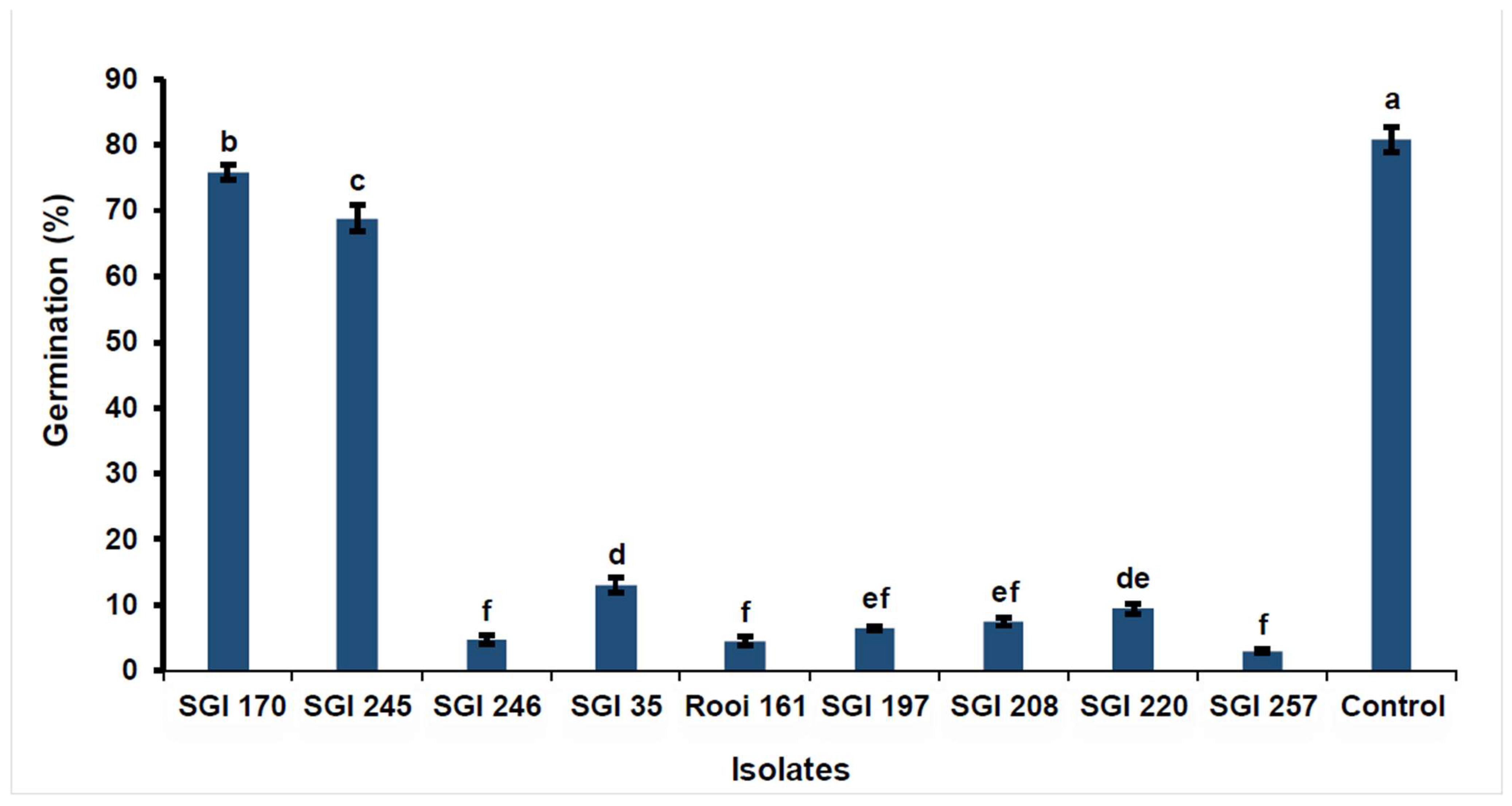
3.2. Efficacy of Bacterial Metabolites on F. graminearum Spore Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Alamah, Z.; Elgammal, W.; Fakih, A. Does twitter economic uncertainty matter for wheat prices? Econ. Lett. 2024, 234, 111463. [Google Scholar] [CrossRef]
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Stein, N.; Choulet, F.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Sun, C.; Hu, H.; Cheng, Y.; Yang, X.; Qiao, Q.; Wang, C.; Zhang, L.; Chen, D.; Zhao, S.; Dong, Z.; et al. Genomics-assisted breeding: The next-generation wheat breeding era. Plant Breed. 2023, 142, 259–268. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2017, 19, 1523–1536. [Google Scholar] [CrossRef]
- Figueroa, M.; Castell-Miller, C.V.; Li, F.; Hulbert, S.H.; Bradeen, J.M. Pushing the boundaries of resistance: Insights from Brachypodium-rust interactions. Front. Plant Sci. 2015, 6, 558. [Google Scholar] [CrossRef][Green Version]
- Jimenez-Garcia, S.N.; Garcia-Mier, L.; Garcia-Trejo, J.F.; Ramirez-Gomez, X.S.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Chapter 3. Fusarium Mycotoxins and Metabolites that Modulate Their Production. In Fusarium Plant Diseases, Pathogen Diversity, Resistance and Molecular Markers; Askun, T., Ed.; IntechOpen: London, UK, 2018; pp. 23–40. [Google Scholar] [CrossRef]
- Dilks, T.; Halsey, K.; De Vos, R.P.; Hammond–Kosack, K.E.; Brown, N.A. Non–canonical fungal G–protein coupled receptors promote Fusarium head blight on wheat. PLoS Pathog. 2019, 15, e1007666. [Google Scholar] [CrossRef]
- Figlan, S.; Mwadzingeni, L. Breeding Tools for Assessing and Improving Resistance and Limiting Mycotoxin Production by Fusarium graminearum in Wheat. Plants 2022, 11, 1933. [Google Scholar] [CrossRef]
- Qi, P.-F.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Wei, Y.-M.; Zheng, Y.-L.; Ouellet, T. Jasmonic acid and abscisic acid play important roles in host–pathogen interaction between Fusarium graminearum and wheat during the early stages of fusarium head blight. Physiol. Mol. Plant Pathol. 2016, 93, 39–48. [Google Scholar] [CrossRef]
- Torres, A.; Palacios, S.; Yerkovich, N.; Palazzini, J.; Battilani, P.; Leslie, J.; Logrieco, A.; Chulze, S. Fusarium head blight and mycotoxins in wheat: Prevention and control strategies across the food chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Haidukowski, M.; Somma, S.; Ghionna, V.; Cimmarusti, M.T.; Masiello, M.; Logrieco, A.F.; Moretti, A. Deoxynivalenol and T-2 Toxin as Major Concerns in Durum Wheat from Italy. Toxins 2022, 14, 627. [Google Scholar] [CrossRef]
- Van der Lee, T.; Zhang, H.; Van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Tini, F.; Beccari, G.; Onofri, A.; Ciavatta, E.; Gardiner, D.M.; Covarelli, L. Fungicides may have differential efficacies towards the main causal agents of Fusarium head blight of wheat. Pest Manag. Sci. 2020, 76, 3738–3748. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, Y.; Bian, C.; Pan, X.; Yao, C.; Wang, J.; Zhou, M. Effects of validamycin in controlling Fusarium head blight caused by Fusarium graminearum: Inhibition of DON biosynthesis and induction of host resistance. Pestic. Biochem. Physiol. 2019, 153, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Feksa, H.R.; Do Couto, H.T.Z.; Garozi, R.; De Almeida, J.L.; Gardiano, C.G.; Tessmann, D.J. Pre- and post infection application of strobilurin-triazole premixes and single fungicides for control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Crop Prot. 2019, 117, 128–134. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, L.; Pan, F.; Deng, Y.; Ding, C.; Liao, M.; Su, X.; Cao, H. Application method affects pesticide efficiency and effectiveness in wheat fields. Pest Manag. Sci. 2020, 76, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.A.; Bradley, C.A.; Madden, L.V.; Lana, F.D.; Bergstrom, G.C.; Dill-Macky, R.; Wise, K.A.; Esker, P.D.; McMullen, M.; Grybauskas, A.; et al. Effects of Pre- and Postanthesis Applications of Demethylation Inhibitor Fungicides on Fusarium Head Blight and Deoxynivalenol in Spring and Winter Wheat. Plant Dis. 2018, 102, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D.W. Fungicide resistance: Facing the challenge—A review. Plant Prot. Sci. 2015, 51, 170–176. [Google Scholar] [CrossRef]
- Dweba, C.; Figlan, S.; Shimelis, H.; Motaung, T.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Muriithi, B.W.; Affognon, H.D.; Diiro, G.M.; Kingori, S.W.; Tanga, C.M.; Nderitu, P.W.; Mohamed, S.A.; Ekesi, S. Impact assessment of Integrated Pest Management (IPM) strategy for suppression of mango-infesting fruit flies in Kenya. Crop Prot. 2016, 81, 20–29. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Nowrin, F.; Yesmin, M.S.; Sultana, N. Entomopathogenic nematode: An effective bio control agent for insect. Eco–Friendly Agric. J. 2018, 11, 119–125. [Google Scholar]
- Lacey, L.A.; Georgis, R. Entomopathogenic Nematodes for Control of Insect Pests Above and Below Ground with Comments on Commercial Production. J. Nematol. 2012, 44, 218–225. [Google Scholar] [PubMed]
- Lu, D.; Baiocchi, T.; Dillman, A.R. Genomics of Entomopathogenic Nematodes and Implications for Pest Control. Trends Parasitol. 2016, 32, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Kepenekci, I.; Hazir, S.; Lewis, E.E. Evaluation of entomopathogenic nematodes and the supernatants of the in vitro culture medium of their mutualistic bacteria for the control of the root-knot nematodes Meloidogyne incognita and M. arenaria. Pest Manag. Sci. 2016, 72, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Chapter 6—Basic and Applied Research: Entomopathogenic Nematodes. Microbial Control of Insect and Mite Pests; Elsevier: London, UK, 2017; pp. 91–105. [Google Scholar]
- Gümüş Askar, A.; Yüksel, E.; Öcal, A.; Özer, G.; Kütük, H.; Dababat, A.; İmren, M. Identification and control potential of entomopathogenic nematodes against the black cutworm, Agrotis ipsilon (Fabricius) (Lepidoptera: Noctuidae), in potato-growing areas of Turkey. J. Plant Dis. Prot. 2022, 129, 911–922. [Google Scholar] [CrossRef]
- Kim, J.; Jaffuel, G.; Turlings, T.C.J. Enhanced alginate capsule properties as a formulation of entomopathogenic nematodes. BioControl 2014, 60, 527–535. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Mollah, M.I.; Kim, Y. Virulent secondary metabolites of entomopathogenic bacteria genera, Xenorhabdus and Photorhabdus, inhibit phospholipase A2 to suppress host insect immunity. BMC Microbiol. 2020, 20, 359. [Google Scholar] [CrossRef]
- Boemare, N. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: New York, NY, USA, 2002; pp. 35–56. [Google Scholar]
- Wang, H.; Dong, H.; Qian, H.; Cong, B. Laboratory assessment of entomopathogenic nematode symbiotic bacteria to control maize pest, Ostrinia furnacalis, and fungi diseases, Bipolaris maydis and Curvularia lunata. J. Asia-Pac. Entomol. 2014, 17, 823–827. [Google Scholar] [CrossRef]
- Engel, Y.; Windhorst, C.; Lu, X.; Goodrich-Blair, H.; Bode, H.B. The Global Regulators Lrp, LeuO, and HexA Control Secondary Metabolism in Entomopathogenic Bacteria. Front. Microbiol. 2017, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Cimen, H.; Ulug, D.; Karagoz, M.; Hazir, S.; Cakmak, I. Acaricidal effect of cell–free supernatants from Xenorhabdus and Photorhabdus bacteria against Tetranychus urticae (Acari: Tetranychidae). J. Invertebr. Pathol. 2019, 160, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.H.; Shapiro-Ilan, D.I.; Wedge, D.E.; Cantrell, C.L. Identification of the antifungal compound, trans-cinnamic acid, produced by Photorhabdus luminescens, a potential biopesticide against pecan scab. J. Pest Sci. 2014, 87, 155–162. [Google Scholar] [CrossRef]
- Sharma, K.; Walia, S.; Ganguli, S.; Kundu, A. Analytical Characterization of Secondary Metabolites from Indian Xenorhabdus Species the Symbiotic Bacteria of Entomopatathogenic Nematode (Steinernema spp.) as Antifungal Agent. Natl. Acad. Sci. Lett. 2016, 39, 175–180. [Google Scholar] [CrossRef]
- Hazir, S.; Shapiro-Ilan, D.I.; Bock, C.H.; Hazir, C.; Leite, L.G.; Hotchkiss, M.W. Relative potency of culture supernatants of Xenorhabdus and Photorhabdus spp. on growth of some fungal phytopathogens. Eur. J. Plant Pathol. 2016, 146, 369–381. [Google Scholar] [CrossRef]
- Matny, O.N. Fusarium Head Blight and Crown Rot on Wheat & Barley: Losses and Health Risks. Adv. Plants Agric. Res. 2015, 2, 38–43. [Google Scholar] [CrossRef]
- Saad, C.A.; Masiello, M.; Habib, W.; Gerges, E.; Sanzani, S.M.; Logrieco, A.F.; Moretti, A.; Somma, S. Diversity of Fusarium Species Isolated from Symptomatic Plants Belonging to a Wide Range of Agri-Food and Ornamental Crops in Lebanon. J. Fungi 2022, 8, 897. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of 12 Techniques in Insect Pathology; Lawrence, A.L., Ed.; Academic Press: Wapato, WA, USA, 1997; pp. 281–324. [Google Scholar]
- Muangpat, P.; Yooyangket, T.; Fukruksa, C.; Suwannaroj, M.; Yimthin, T.; Sitthisak, S.; Chantratita, N.; Vitta, A.; Tobias, N.J.; Bode, H.B.; et al. Screening of the Antimicrobial Activity against Drug Resistant Bacteria of Photorhabdus and Xenorhabdus Associated with Entomopathogenic Nematodes from Mae Wong National Park, Thailand. Front. Microbiol. 2017, 8, 1142. [Google Scholar] [CrossRef]
- Devi, G.; Mishra, H.; Bhattacharyya, B.; Nath, D.J. Occurrence of entomopathogenic nematode (Rhabditida: Heterorhabditidae, Steinernematidae) in white grub infested areas of Majuli, Assam, India. J. Biopestic. 2016, 9, 148–156. [Google Scholar] [CrossRef]
- Akhurst, R.J. Morphological and Functional Dimorphism in Xenorhabdus spp., Bacteria Symbiotically Associated with the Insect Pathogenic Nematodes Neoaplectana and Heterorhabditis. J. Gen. Microbiol. 1980, 121, 303–309. [Google Scholar] [CrossRef]
- Ciche, T. The biology and genome of Heterorhabditis bacteriophora. WormBook 2007, 1–9. [Google Scholar] [CrossRef]
- Bai, X.; Adams, B.J.; Ciche, T.A.; Clifton, S.; Gaugler, R.; Kim, K.-S.; Spieth, J.; Sternberg, P.W.; Wilson, R.K.; Grewal, P.S. A Lover and a Fighter: The Genome Sequence of an Entomopathogenic Nematode Heterorhabditis bacteriophora. PLoS ONE 2013, 8, e69618. [Google Scholar] [CrossRef]
- Cimen, H.; Půža, V.; Nermuť, J.; Hatting, J.; Ramakuwela, T.; Hazir, S. Steinernema biddulphi n. sp., a New Entomopathogenic Nematode (Nematoda: Steinernematidae) from South Africa. J. Nematol. 2016, 48, 148–158. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]
- Cimen, H.; Touray, M.; Gulsen, S.H.; Hazir, S. Natural products from Photorhabdus and Xenorhabdus: Mechanisms and impacts. Appl. Microbiol. Biotechnol. 2022, 106, 4387–4399. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Ma, H.; Li, Y.; Huang, C.; Gu, X.; Jiang, Z.; Sun, B.; Chen, C.; Wei, X.; Shen, G.; et al. Metabolites from symbiotic bacteria of entomopathogenic nematodes have antimicrobial effects against Pythium myriotylum. Eur. J. Plant Pathol. 2020, 158, 35–44. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, M.; Tang, Q.; Wang, Y.; Zhang, X. Inhibitory effect of Xenorhabdus nematophila TB on plant pathogens Phytophthora capsici and Botrytis cinerea in vitro and in planta. Sci. Rep. 2014, 4, 4300. [Google Scholar] [CrossRef] [PubMed]
- Cimen, H.; Touray, M.; Gulsen, S.H.; Erincik, O.; Wenski, S.L.; Bode, H.B.; Shapiro-Ilan, D.; Hazir, S. Antifungal activity of different Xenorhabdus and Photorhabdus species against various fungal phytopathogens and identification of the antifungal compounds from X. szentirmaii. Appl. Microbiol. Biotechnol. 2021, 105, 5517–5528. [Google Scholar] [CrossRef] [PubMed]
- Baiome, B.A.; Ye, X.; Yuan, Z.; Gaafar, Y.Z.A.; Melak, S.; Cao, H. Identification of Volatile Organic Compounds Produced by Xenorhabdus indica Strain AB and Investigation of Their Antifungal Activities. Appl. Environ. Microbiol. 2022, 88, e0015522. [Google Scholar] [CrossRef] [PubMed]
- Otoya-Martinez, N.; Leite, L.G.; Harakava, R.; Touray, M.; Hazir, S.; Chacon-Orozco, J.; Bueno, C.J. Disease caused by Neofusicoccum parvum in pruning wounds of grapevine shoots and its control by Trichoderma spp. and Xenorhabdus szentirmaii. Fungal Biol. 2023, 127, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Lalramchuani, M.; Lalramnghaki, H.; Vanlalsangi, R.; Lalhmingliani, E.; Vanramliana; Lalramliana. Characterization and screening of antifungal activity of bacteria associated with entomopathogenic nematodes from Mizoram, North-Eastern India. J. Environ. Biol. 2020, 41, 942–950. [Google Scholar] [CrossRef]

| Isolate | Nematode | Symbiotic Bacteria |
|---|---|---|
| SGI 35 | Steinernema innovationi | Xenorhabdus sp. |
| SGI 170 | Heterorhabditis bacteriophora | Photorhabdus luminescens [46,47] |
| SGI 197 | Steinernema beitlechemi | Xenorhabdus khoisanae [48] |
| SGI 208 | Steinernema sp. | Xenorhabdus sp. |
| SGI 220 | Steinernema sp. | Xenorhabdus sp. |
| SGI 245 | Heterorhabditis bacteriophora | Photorhabdus sp. |
| SGI 246 | Steinernema biddulphi | Xenorhabdus sp. |
| SGI 257 | Steinernema spp. | Xenorhabdus sp. |
| ROOI 161 | Steinernema khoisanae | Xenorhabdus sp. |
| 3 Days’ Incubation | 7 Days’ Incubation | |||||
|---|---|---|---|---|---|---|
| Isolate | Crude | Filtered | Autoclaved | Crude | Filtered | Autoclaved |
| Xenorhabdus sp. SGI 35 | 77.65 | 58.82 | 14.30 | 47.33 | 29.59 | 4.52 |
| Photorhabdus luminescens SGI 170 | 73.82 | 60.88 | 7.35 | 95.79 | 55.90 | 1.69 |
| Xenorhabdus khoisanae SGI 197 | 83.53 | 42.06 | 21.72 | 96.25 | 15.49 | 5.37 |
| Xenorhabdus sp. SGI 208 | 76.18 | 38.53 | 18.27 | 61.66 | 2.06 | 1.69 |
| Xenorhabdus sp. GI 220 | 68.82 | 39.12 | 11.76 | 42.88 | 2.43 | 4.52 |
| Photorhabdus sp. SGI 245 | 86.76 | 17.65 | 0.00 | 88.39 | 28.37 | 1.69 |
| Xenorhabdus sp. SGI 246 | 64.12 | 20.29 | 0.00 | 54.30 | 0.00 | 0.00 |
| Xenorhabdus sp. SGI 257 | 74.12 | 30.00 | 15.88 | 85.77 | 14.42 | 0.85 |
| Xenorhabdus sp. ROOI 161 | 81.76 | 31.18 | 9.71 | 81.46 | 1.87 | 0.00 |
| Isolate | Spore Germination Percentage | Inhibition Rate |
|---|---|---|
| Xenorhabdus sp. SGI 35 | 13.00 | 83.91 |
| Photorhabdus luminescens SGI 170 | 75.89 | 6.05 |
| Xenorhabdus khoisanae SGI 197 | 6.44 | 92.02 |
| Xenorhabdus sp. SGI 208 | 7.44 | 90.78 |
| Xenorhabdus sp. SGI 220 | 9.50 | 88.23 |
| Photorhabdus sp. SGI 245 | 68.86 | 14.74 |
| Xenorhabdus sp. SGI 246 | 4.78 | 94.09 |
| Xenorhabdus sp. SGI 257 | 3.00 | 96.29 |
| Xenorhabdus sp. ROOI 161 | 4.56 | 94.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgosiemang, J.L.; Ramakuwela, T.; Figlan, S.; Cochrane, N. Antifungal Effect of Metabolites from Bacterial Symbionts of Entomopathogenic Nematodes on Fusarium Head Blight of Wheat. J. Fungi 2024, 10, 148. https://doi.org/10.3390/jof10020148
Kgosiemang JL, Ramakuwela T, Figlan S, Cochrane N. Antifungal Effect of Metabolites from Bacterial Symbionts of Entomopathogenic Nematodes on Fusarium Head Blight of Wheat. Journal of Fungi. 2024; 10(2):148. https://doi.org/10.3390/jof10020148
Chicago/Turabian StyleKgosiemang, Julius Leumo, Tshimangadzo Ramakuwela, Sandiswa Figlan, and Nicolene Cochrane. 2024. "Antifungal Effect of Metabolites from Bacterial Symbionts of Entomopathogenic Nematodes on Fusarium Head Blight of Wheat" Journal of Fungi 10, no. 2: 148. https://doi.org/10.3390/jof10020148
APA StyleKgosiemang, J. L., Ramakuwela, T., Figlan, S., & Cochrane, N. (2024). Antifungal Effect of Metabolites from Bacterial Symbionts of Entomopathogenic Nematodes on Fusarium Head Blight of Wheat. Journal of Fungi, 10(2), 148. https://doi.org/10.3390/jof10020148






