The Endoplasmic Reticulum–Plasma Membrane Tethering Protein Ice2 Controls Lipid Droplet Size via the Regulation of Phosphatidylcholine in Candida albicans
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Nile Red Staining and Fluorescence Microscopy
2.3. Triacylglycerol (TG) Level Analysis
2.4. Phosphatidylcholine Level Analysis Using Thin-Layer Chromatography
2.5. Detection of the Morphogenetic Ability and Drug Sensitivity of C. albicans
2.6. LD Autophagy Assessment
2.7. Statistical Analysis
3. Results
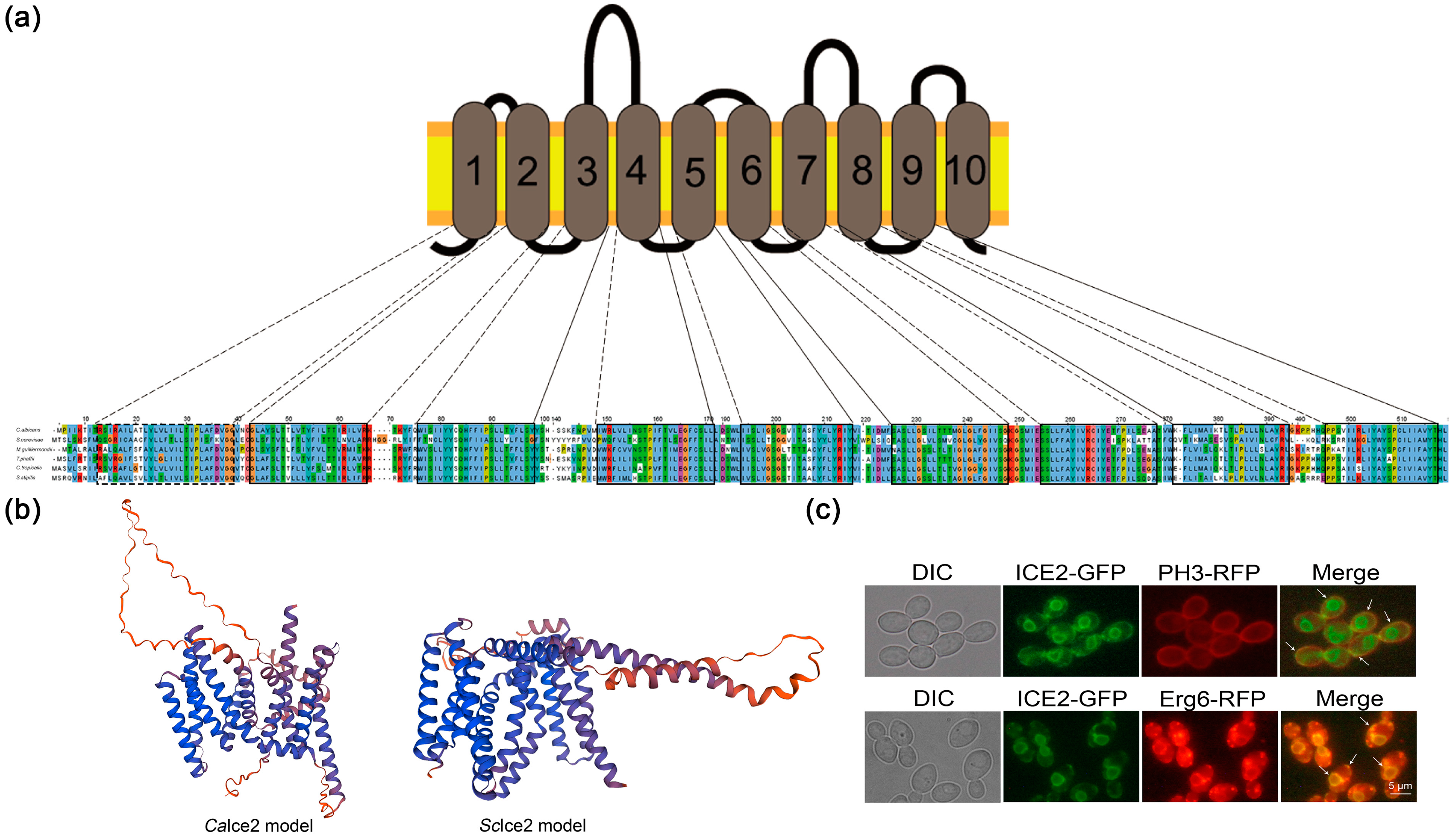
3.1. Ice2 Is an ER–PM Tethering Protein in C. albicans
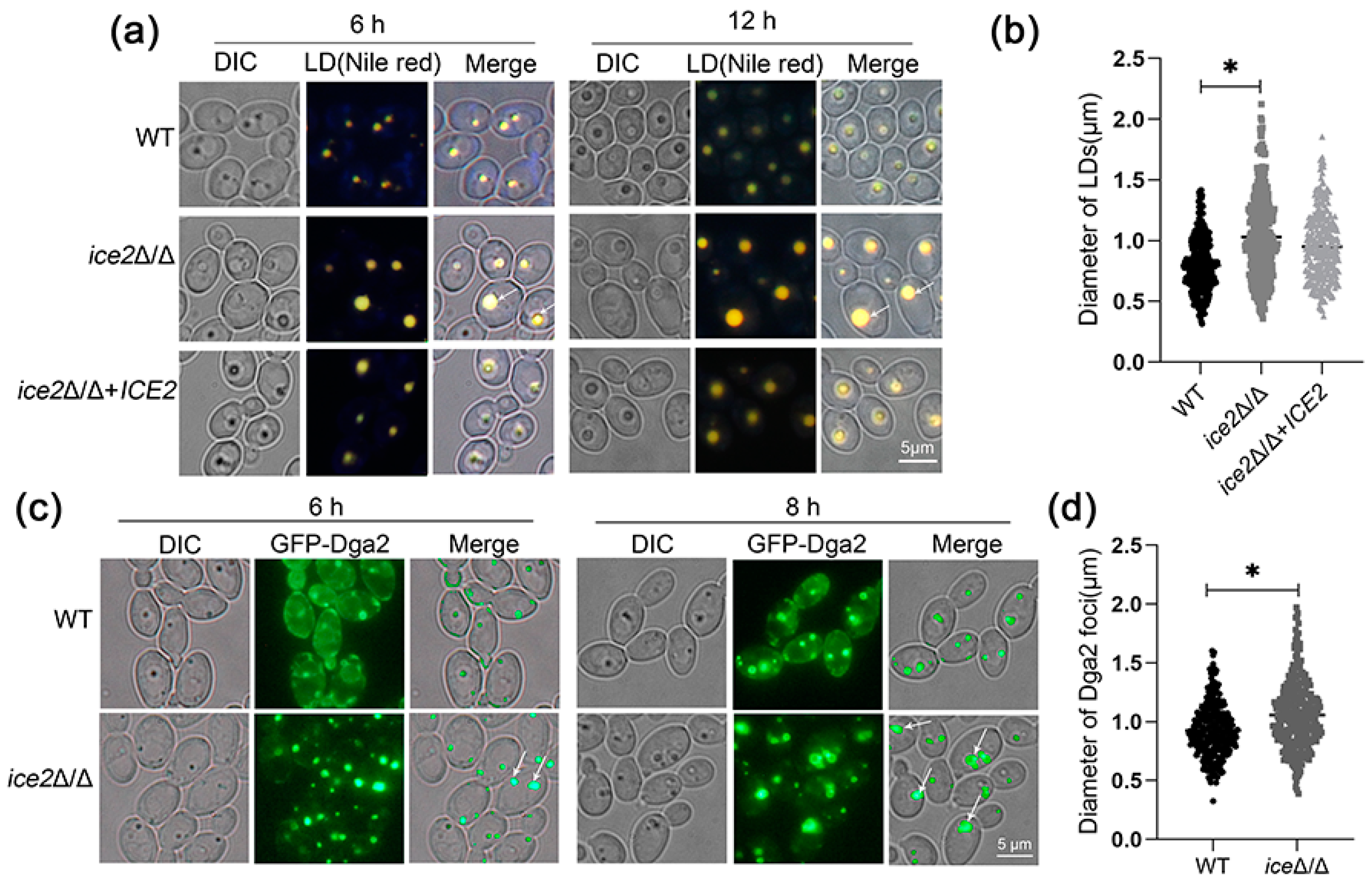
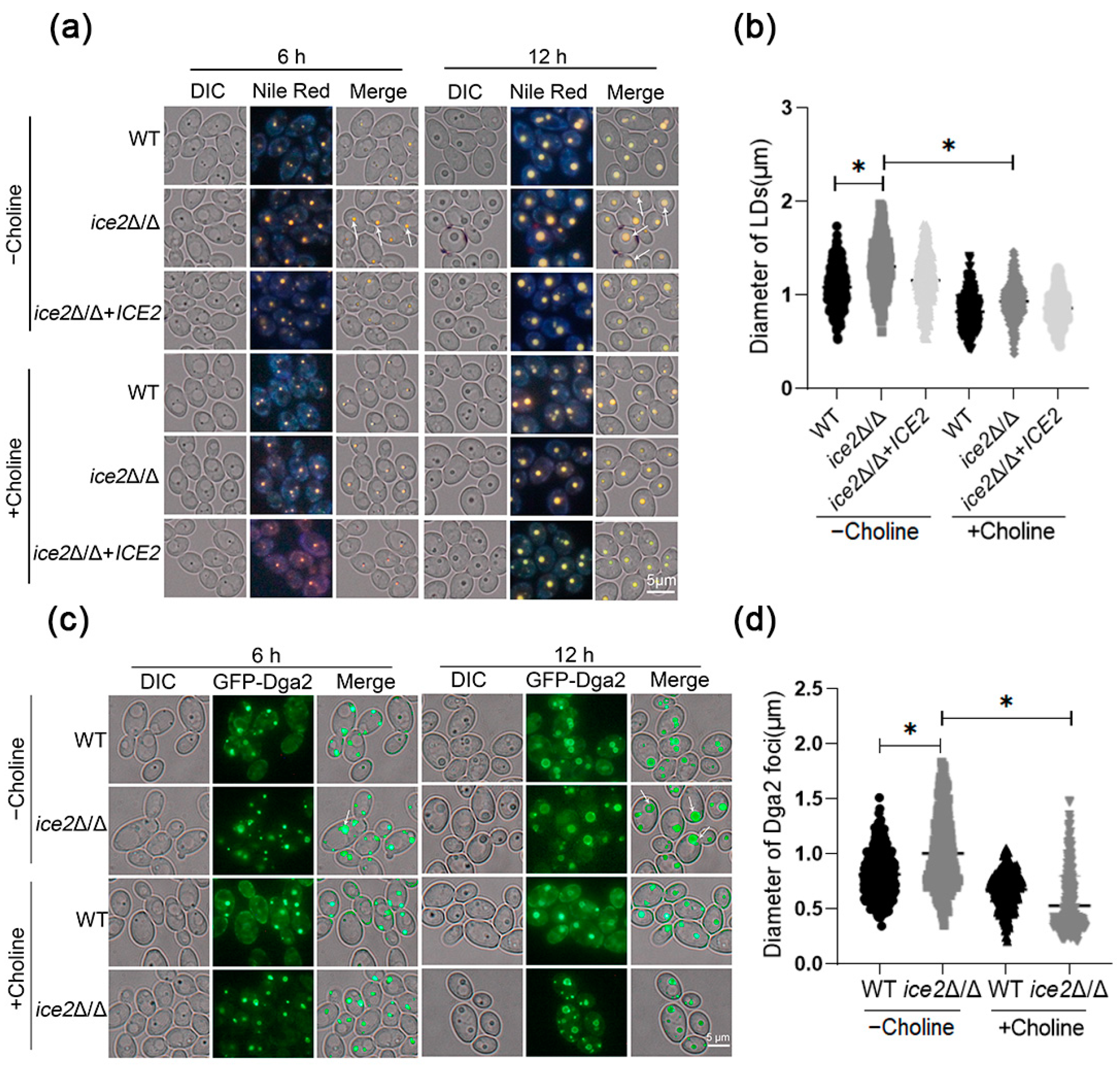
3.2. Deletion of ICE2 Leads to the Accumulation of Large Lipid Droplets
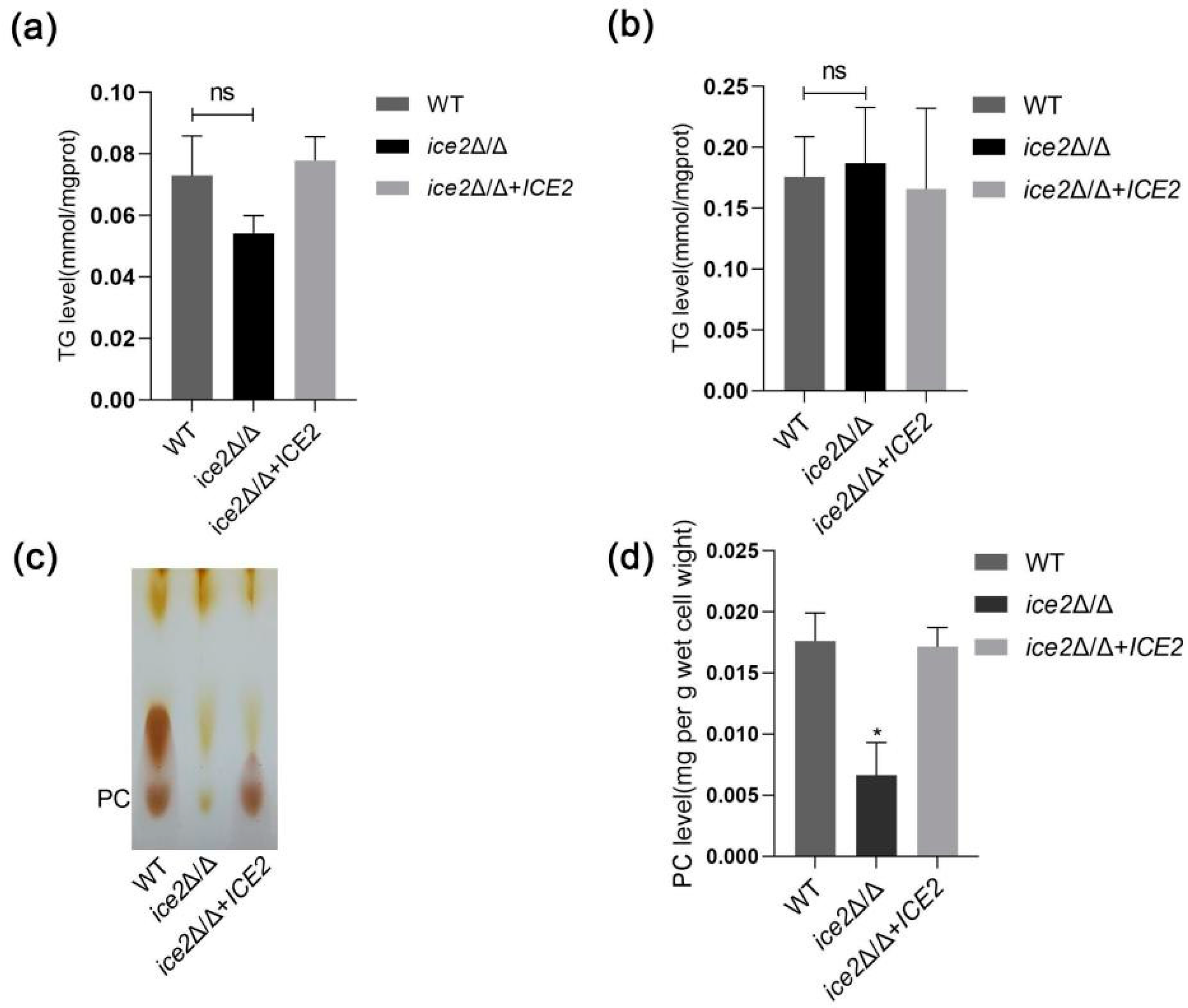
3.3. Deletion of ICE2 Impairs the Synthesis of Phosphatidylcholine
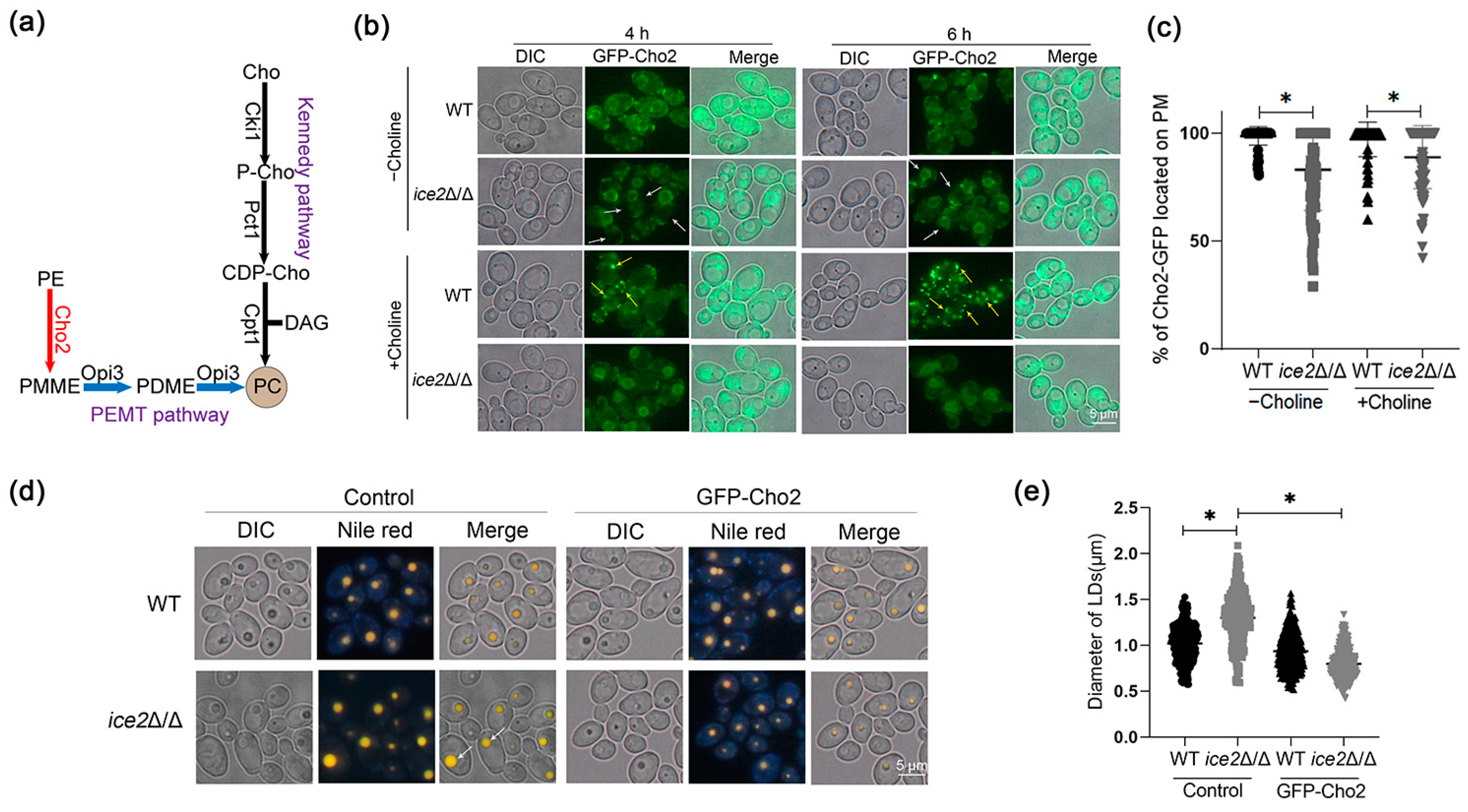
3.4. Deletion of ICE2 Attenuates the Plasma Membrane Localization of Cho2
3.5. The Addition of Choline Reduces the Accumulation of Large Lipid Droplets in the ice2Δ/Δ Mutant
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tauchi-Sato, K.; Ozeki, S.; Houjou, T.; Taguchi, R.; Fujimoto, T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 2002, 277, 44507–44512. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Krahmer, N.; Farese, R.V., Jr.; Walther, T.C. Balancing the fat: Lipid droplets and human disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef]
- Wilfling, F.; Wang, H.; Haas, J.T.; Krahmer, N.; Gould, T.J.; Uchida, A.; Cheng, J.X.; Graham, M.; Christiano, R.; Fröhlich, F.; et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 2013, 24, 384–399. [Google Scholar] [CrossRef]
- Poppelreuther, M.; Rudolph, B.; Du, C.; Großmann, R.; Becker, M.; Thiele, C.; Ehehalt, R.; Füllekrug, J. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J. Lipid Res. 2012, 53, 888–900. [Google Scholar] [CrossRef]
- Brasaemle, D.L.; Dolios, G.; Shapiro, L.; Wang, R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 2004, 279, 46835–46842. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Itabe, H.; Kinoshita, T.; Homma, K.J.; Onoduka, J.; Mori, M.; Yamaguchi, S.; Makita, M.; Higashi, Y.; Yamashita, A.; et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J. Lipid Res. 2007, 48, 1280–1292. [Google Scholar] [CrossRef]
- Yang, H.; Galea, A.; Sytnyk, V.; Crossley, M. Controlling the size of lipid droplets: Lipid and protein factors. Curr. Opin. Cell Biol. 2012, 24, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Shui, G.; Zhang, Y.; Krahmer, N.; Ferguson, C.; Kapterian, T.S.; Lin, R.C.; Dawes, I.W.; Brown, A.J.; Li, P.; et al. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 2011, 7, e1002201. [Google Scholar] [CrossRef]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011, 14, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Shui, G.; Gaeta, B.; Du, X.; Kuerschner, L.; Li, P.; Brown, A.J.; Wenk, M.R.; Parton, R.G.; Yang, H. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 2008, 180, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Huang, P.; Jenkins, G.M.; Chan, D.C.; Schiller, J.; Frohman, M.A. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat. Cell Biol. 2006, 8, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Vitale, N.; Caumont, A.S.; Chasserot-Golaz, S.; Du, G.; Wu, S.; Sciorra, V.A.; Morris, A.J.; Frohman, M.A.; Bader, M.F. Phospholipase D1: A key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 2001, 20, 2424–2434. [Google Scholar] [CrossRef]
- Oelkers, P.; Cromley, D.; Padamsee, M.; Billheimer, J.T.; Sturley, S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002, 277, 8877–8881. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Daum, G. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 23317–23323. [Google Scholar] [CrossRef]
- Gong, J.; Sun, Z.; Wu, L.; Xu, W.; Schieber, N.; Xu, D.; Shui, G.; Yang, H.; Parton, R.G.; Li, P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 2011, 195, 953–963. [Google Scholar] [CrossRef]
- Kadereit, B.; Kumar, P.; Wang, W.J.; Miranda, D.; Snapp, E.L.; Severina, N.; Torregroza, I.; Evans, T.; Silver, D.L. Evolutionarily conserved gene family important for fat storage. Proc. Natl. Acad. Sci. USA 2008, 105, 94–99. [Google Scholar] [CrossRef]
- Grippa, A.; Buxó, L.; Mora, G.; Funaya, C.; Idrissi, F.Z.; Mancuso, F.; Gomez, R.; Muntanyà, J.; Sabidó, E.; Carvalho, P. The seipin complex Fld1/Ldb16 stabilizes ER-lipid droplet contact sites. J. Cell Biol. 2015, 211, 829–844. [Google Scholar] [CrossRef]
- Wang, C.W.; Miao, Y.H.; Chang, Y.S. Control of lipid droplet size in budding yeast requires the collaboration between Fld1 and Ldb16. J. Cell Sci. 2014, 127, 1214–1228. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Tang, Y.; Liu, Y.; Zhao, L.; Deng, J.; Xu, G.; Peng, X.; Ju, S.; Liu, G.; et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum. Mol. Genet. 2011, 20, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, K.M.; Binns, D.; Bartz, R.; Grishin, N.V.; Li, W.P.; Agarwal, A.K.; Garg, A.; Anderson, R.G.; Goodman, J.M. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl. Acad. Sci. USA 2007, 104, 20890–20895. [Google Scholar] [CrossRef]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 2017, 546, 162–167. [Google Scholar] [CrossRef]
- Shai, N.; Yifrach, E.; van Roermund, C.W.T.; Cohen, N.; Bibi, C.; Ijlst, L.; Cavellini, L.; Meurisse, J.; Schuster, R.; Zada, L.; et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat. Commun. 2018, 9, 1761. [Google Scholar] [CrossRef] [PubMed]
- Prinz, W.A. Bridging the gap: Membrane contact sites in signaling, metabolism, and organelle dynamics. J. Cell Biol. 2014, 205, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Chen, F.J.; Zhou, L.; Su, L.; Xu, D.; Xu, L.; Li, P. Control of lipid droplet fusion and growth by CIDE family proteins. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, L.; Aw, Y.C.; Mak, H.Y.; Xu, Y.; Rae, J.; Wang, W.; Zadoorian, A.; Hancock, S.E.; Osborne, B.; et al. ORP5 localizes to ER-lipid droplet contacts and regulates the level of PI(4)P on lipid droplets. J. Cell Biol. 2020, 219, e201905162. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Q.; Liang, C.; Zhang, B.; Li, M. Lipid homeostasis is involved in plasma membrane and endoplasmic reticulum stress in Pichia pastoris. Biochem. Biophys. Res. Commun. 2016, 478, 777–783. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Vevea, J.D.; Garcia, E.J.; Chan, R.B.; Zhou, B.; Schultz, M.; Di Paolo, G.; McCaffery, J.M.; Pon, L.A. Role for lipid droplet biogenesis and microlipophagy in adaptation to lipid imbalance in yeast. Dev. Cell 2015, 35, 584–599. [Google Scholar] [CrossRef]
- Quon, E.; Sere, Y.Y.; Chauhan, N.; Johansen, J.; Sullivan, D.P.; Dittman, J.S.; Rice, W.J.; Chan, R.B.; Di Paolo, G.; Beh, C.T.; et al. Endoplasmic reticulum-plasma membrane contact sites integrate sterol and phospholipid regulation. PLoS Biol. 2018, 16, e2003864. [Google Scholar] [CrossRef]
- Estrada de Martin, P.; Du, Y.; Novick, P.; Ferro-Novick, S. Ice2p is important for the distribution and structure of the cortical ER network in Saccharomyces cerevisiae. J. Cell Sci. 2005, 118, 65–77. [Google Scholar] [CrossRef]
- Pichler, H.; Gaigg, B.; Hrastnik, C.; Achleitner, G.; Kohlwein, S.D.; Zellnig, G.; Perktold, A.; Daum, G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur. J. Biochem. 2001, 268, 2351–2361. [Google Scholar] [CrossRef]
- Markgraf, D.F.; Klemm, R.W.; Junker, M.; Hannibal-Bach, H.K.; Ejsing, C.S.; Rapoport, T.A. An ER protein functionally couples neutral lipid metabolism on lipid droplets to membrane lipid synthesis in the ER. Cell Rep. 2014, 6, 44–55. [Google Scholar] [CrossRef]
- Garbarino, J.; Padamsee, M.; Wilcox, L.; Oelkers, P.M.; D’Ambrosio, D.; Ruggles, K.V.; Ramsey, N.; Jabado, O.; Turkish, A.; Sturley, S.L. Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid-mediated cell death. J. Biol. Chem. 2009, 284, 30994–31005. [Google Scholar] [CrossRef]
- Binns, D.; Januszewski, T.; Chen, Y.; Hill, J.; Markin, V.S.; Zhao, Y.; Gilpin, C.; Chapman, K.D.; Anderson, R.G.; Goodman, J.M. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 2006, 173, 719–731. [Google Scholar] [CrossRef]
- Haider, A.; Wei, Y.C.; Lim, K.; Barbosa, A.D.; Liu, C.H.; Weber, U.; Mlodzik, M.; Oras, K.; Collier, S.; Hussain, M.M.; et al. PCYT1A regulates phosphatidylcholine homeostasis from the inner nuclear membrane in response to membrane stored curvature elastic stress. Dev. Cell 2018, 45, 481–495. [Google Scholar] [CrossRef]
- Kent, C. Regulatory enzymes of phosphatidylcholine biosynthesis: A personal perspective. Biochim. Biophys. Acta 2005, 1733, 53–66. [Google Scholar] [CrossRef]
- Howe, A.G.; Zaremberg, V.; McMaster, C.R. Cessation of growth to prevent cell death due to inhibition of phosphatidylcholine synthesis is impaired at 37 degrees C in Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 44100–44107. [Google Scholar] [CrossRef] [PubMed]
- Morash, S.C.; McMaster, C.R.; Hjelmstad, R.H.; Bell, R.M. Studies employing Saccharomyces cerevisiae cpt1 and ept1 null mutants implicate the CPT1 gene in coordinate regulation of phospholipid biosynthesis. J. Biol. Chem. 1994, 269, 28769–28776. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- McMaster, C.R. From yeast to humans—Roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2018, 592, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, O.; Ren, J.; Wu, C.; Zhao, Y. Aromaticity/Bulkiness of surface ligands to promote the interaction of anionic amphiphilic gold nanoparticles with lipid bilayers. Langmuir ACS J. Surf. Colloids 2016, 32, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Papagiannidis, D.; Bircham, P.W.; Lüchtenborg, C.; Pajonk, O.; Ruffini, G.; Brügger, B.; Schuck, S. Ice2 promotes ER membrane biogenesis in yeast by inhibiting the conserved lipin phosphatase complex. EMBO J. 2021, 40, e107958. [Google Scholar] [CrossRef]
- Quon, E.; Beh, C.T. Membrane contact sites: Complex zones for membrane association and lipid exchange. Lipid Insights 2015, 8, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg-Bord, M.; Shai, N.; Schuldiner, M.; Bohnert, M. A tether is a tether is a tether: Tethering at membrane contact sites. Dev. Cell 2016, 39, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Kagiwada, S.; Hashimoto, M. The yeast VAP homolog Scs2p has a phosphoinositide-binding ability that is correlated with its activity. Biochem. Biophys. Res. Commun. 2007, 364, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Temmerman, K.; Ercan, E.; Nickel, W.; Seedorf, M. Binding of plasma membrane lipids recruits the yeast integral membrane protein Ist2 to the cortical ER. Traffic 2009, 10, 1084–1097. [Google Scholar] [CrossRef]
- Raiborg, C.; Wenzel, E.M.; Pedersen, N.M.; Stenmark, H. Phosphoinositides in membrane contact sites. Biochem. Soc. Trans. 2016, 44, 425–430. [Google Scholar] [CrossRef]
- Dickson, E.J.; Jensen, J.B.; Vivas, O.; Kruse, M.; Traynor-Kaplan, A.E.; Hille, B. Dynamic formation of ER-PM junctions presents a lipid phosphatase to regulate phosphoinositides. J. Cell Biol. 2016, 213, 33–48. [Google Scholar] [CrossRef]
- Alli-Balogun, G.O.; Levine, T.P. Fungal Ice2p is in the same superfamily as SERINCs, restriction factors for HIV and other viruses. Proteins 2021, 89, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.S.; Taneva, S.G.; Lee, J.M.; Cornell, R.B. The curvature sensitivity of a membrane-binding amphipathic helix can be modulated by the charge on a flanking region. Biochemistry 2014, 53, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Bogan, M.J.; Agnes, G.R.; Pio, F.; Cornell, R.B. Interdomain and membrane interactions of CTP:phosphocholine cytidylyltransferase revealed via limited proteolysis and mass spectrometry. J. Biol. Chem. 2005, 280, 19613–19624. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.B. Membrane lipid compositional sensing by the inducible amphipathic helix of CCT. Biochim. Biophys. Acta 2016, 1861, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; Curwin, A.J.; Stefan, C.J.; McMaster, C.R. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc. Natl. Acad. Sci. USA 2007, 104, 15352–15357. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.A.; McMaster, C.R. Lipid binding requirements for oxysterol-binding protein Kes1 inhibition of autophagy and endosome-trans-Golgi trafficking pathways. J. Biol. Chem. 2010, 285, 33875–33884. [Google Scholar] [CrossRef]
- Xie, M.; Smith, J.L.; Ding, Z.; Zhang, D.; Cornell, R.B. Membrane binding modulates the quaternary structure of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 2004, 279, 28817–28825. [Google Scholar] [CrossRef]
- Thiam, A.R.; Antonny, B.; Wang, J.; Delacotte, J.; Wilfling, F.; Walther, T.C.; Beck, R.; Rothman, J.E.; Pincet, F. COPI buds 60-nm lipid droplets from reconstituted water-phospholipid-triacylglyceride interfaces, suggesting a tension clamp function. Proc. Natl. Acad. Sci. USA 2013, 110, 13244–13249. [Google Scholar] [CrossRef]
- Chen, Z.; Rand, R.P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 1997, 73, 267–276. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Kozlov, M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef]
- Leikin, S.; Kozlov, M.M.; Fuller, N.L.; Rand, R.P. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys. J. 1996, 71, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.P.; Ulrich, K.M.; Johnson, J.R.; Newton, B.W.; Vashisht, A.A.; Wohlschlegel, J.A.; Krogan, N.J.; Toczyski, D.P. The yeast DNA damage checkpoint Kinase Rad53 targets the exoribonuclease, Xrn1. G3 2018, 8, 3931–3944. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, S.M.; Nuriel, T. Intracellular cholesterol visualization in brain tissue using D4H. STAR Protoc. 2023, 5, 102779. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zhu, H.; Wang, Y.; Dong, Y.; Du, J.; Yu, Q.; Li, M. The Endoplasmic Reticulum–Plasma Membrane Tethering Protein Ice2 Controls Lipid Droplet Size via the Regulation of Phosphatidylcholine in Candida albicans. J. Fungi 2024, 10, 87. https://doi.org/10.3390/jof10010087
Deng Y, Zhu H, Wang Y, Dong Y, Du J, Yu Q, Li M. The Endoplasmic Reticulum–Plasma Membrane Tethering Protein Ice2 Controls Lipid Droplet Size via the Regulation of Phosphatidylcholine in Candida albicans. Journal of Fungi. 2024; 10(1):87. https://doi.org/10.3390/jof10010087
Chicago/Turabian StyleDeng, Ying, Hangqi Zhu, Yanting Wang, Yixuan Dong, Jiawen Du, Qilin Yu, and Mingchun Li. 2024. "The Endoplasmic Reticulum–Plasma Membrane Tethering Protein Ice2 Controls Lipid Droplet Size via the Regulation of Phosphatidylcholine in Candida albicans" Journal of Fungi 10, no. 1: 87. https://doi.org/10.3390/jof10010087
APA StyleDeng, Y., Zhu, H., Wang, Y., Dong, Y., Du, J., Yu, Q., & Li, M. (2024). The Endoplasmic Reticulum–Plasma Membrane Tethering Protein Ice2 Controls Lipid Droplet Size via the Regulation of Phosphatidylcholine in Candida albicans. Journal of Fungi, 10(1), 87. https://doi.org/10.3390/jof10010087






