Transcatheter Double Valve Replacement to Treat Aortic Stenosis and Severe Tricuspid Regurgitation with 3D Printing Guidance after Mechanical Mitral Valve Replacement
Abstract
:1. Introduction
2. Case Presentation
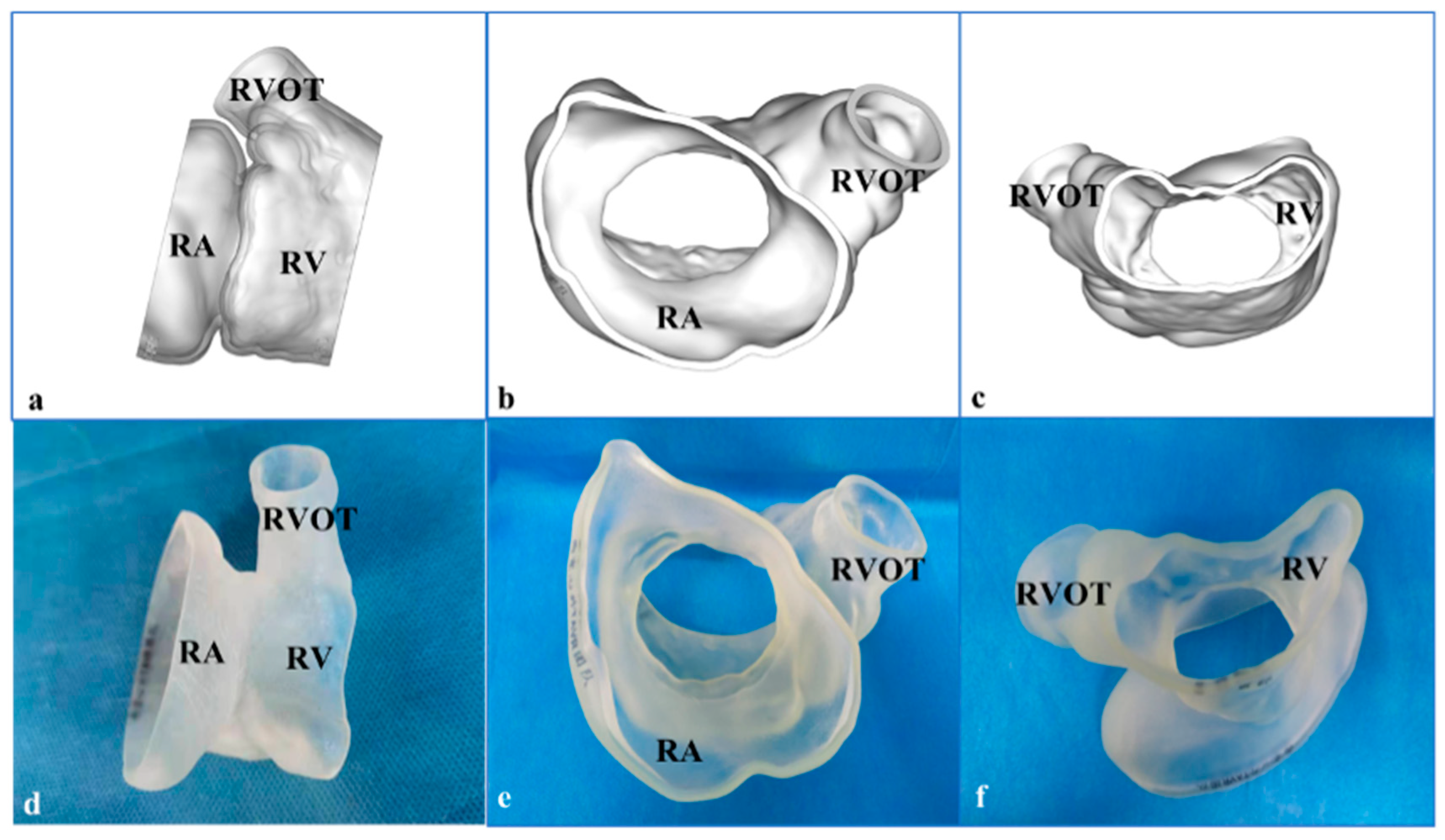
3. 3-Dimensional Printing and Simulation
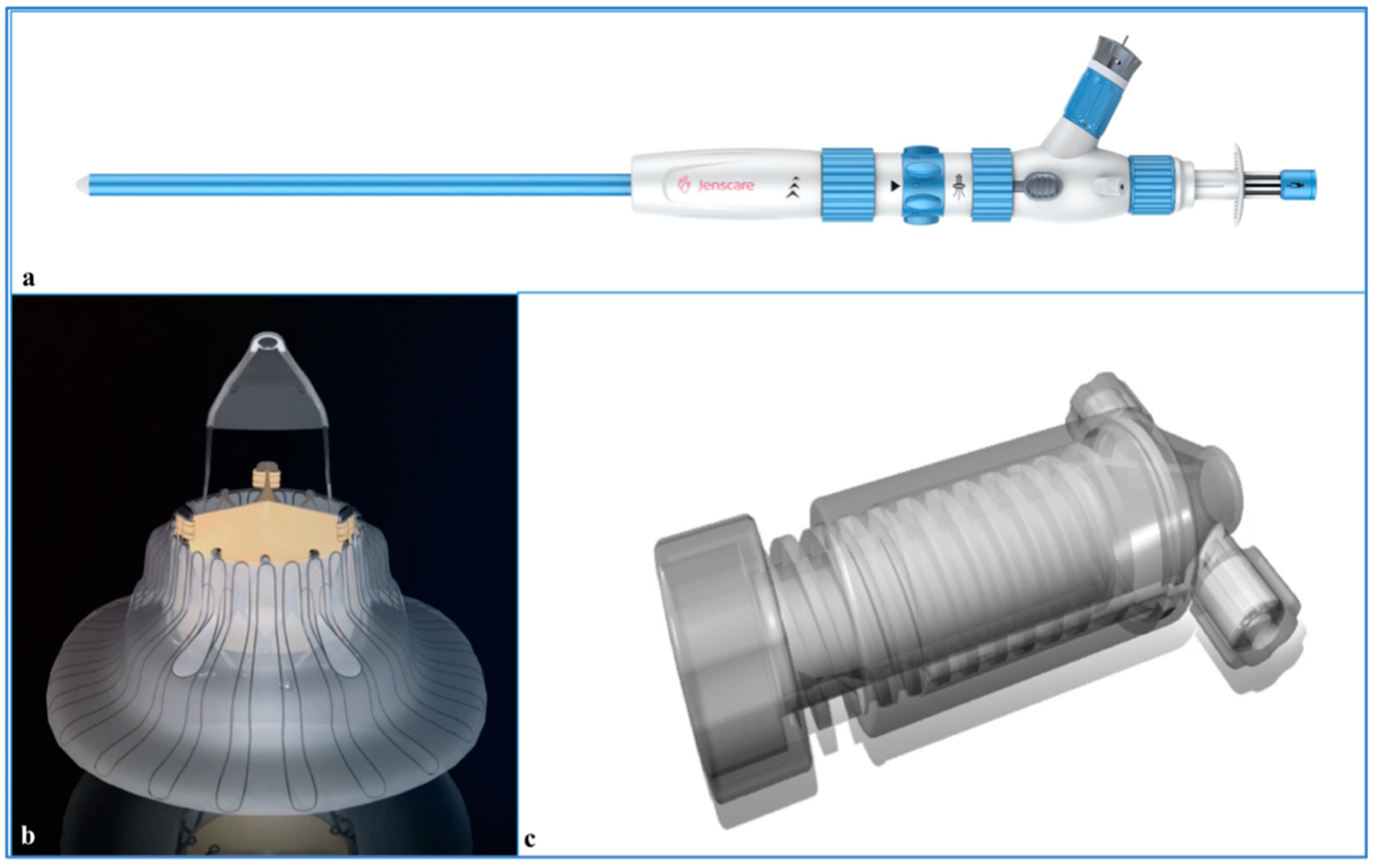
4. Procedures
5. Follow-Up
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodés-Cabau, J.; Hahn, R.T.; Latib, A.; Laule, M.; Lauten, A.; Maisano, F.; Schofer, J.; Campelo-Parada, F.; Puri, R.; Vahanian, A. Transcatheter therapies for treating tricuspid regurgitation. J. Am. Coll. Cardiol. 2016, 67, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.J.; Connolly, H.M. Right-sided valve disease deserves a little more respect. Circulation 2009, 119, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kwon, D.A.; Kim, H.K.; Park, J.S.; Hahn, S.; Kim, K.H.; Kim, K.B.; Sohn, D.W.; Ahn, H.; Oh, B.H.; et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009, 120, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Campelo-Parada, F.; Perlman, G.; Philippon, F.; Ye, J.; Thompson, C.; Bédard, E.; Altisent, O.A.J.; Trigo, M.D.; Leipsic, J.; Blanke, P.; et al. First-in-man experience of a novel transcatheter repair system for treating severe tricuspid regurgitation. J. Am. Coll. Cardiol. 2015, 66, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Navia, J.L.; Kapadia, S.; Elgharably, H.; Maluenda, G.; Bartuś, K.; Baeza, C.; Nair, R.K.; Rodés-Cabau, J.; Beghi, C.; Quijano, R.C. Transcatheter tricuspid valve implantation of navigate bioprosthesis in a preclinical model. JACC Basic Transl. Sci. 2018, 3, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Lausberg, H.F.; Gryszkiewicz, R.; Kuetting, M.; Baumgaertner, M.; Centola, M.; Wendel, H.P.; Nowak-Machen, M.; Schibilsky, D.; Kruger, T.; Schlensak, C. Catheter-based tricuspid valve replacement: First experimental data of a newly designed bileaflet stent graft prosthesis. Eur. J. Cardiothorac. Surg. 2017, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, A.; Montalto, C.; Pagnesi, M.; Jabbour, R.J.; Rodés-Cabau, J.; Moat, N.; Colombo, A.; Latib, A. Mechanism and implications of the tricuspid regurgitation: From the pathophysiology to the current and future therapeutic options. Circ. Cardiovasc. Interv. 2017, 10, e005043. [Google Scholar] [CrossRef] [PubMed]
- Chorin, E.; Rozenbaum, Z.; Topilsky, Y.; Konigstein, M.; Ziv-Baran, T.; Richert, E.; Keren, G.; Banai, S. Tricuspid regurgitation and long-term clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 157–165. [Google Scholar] [CrossRef]
- Pozzoli, A.; Zuber, M.; Reisman, M.; Maisano, F.; Taramasso, M. Comparative anatomy of mitral and tricuspid valve: What can the interventionlist learn from the surgeon. Front. Cardiovasc. Med. 2018, 5, 80. [Google Scholar] [CrossRef]
- Desai, R.R.; Vargas Abello, L.M.; Klein, A.L.; Marwick, T.H.; Krasuski, R.A.; Ye, Y.; Nowicki, E.R.; Rajeswaran, J.; Blackstone, E.H.; Pettersson, G.B. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J. Thorac. Cardiovasc. Surg. 2013, 146, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Desai, R.; Vargas Abello, L.M.; Rajeswaran, J.; Klein, A.L.; Blackstone, E.H.; Pettersson, G.B. Effects of right ventricular morphology and function on outcomes of patients with degenerative mitral valve disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Navia, J.L.; Kapadia, S.; Elgharably, H.; Harb, S.C.; Krishnaswamy, A.; Unai, S.; Mick, S.; Rodriguez, L.; Hammer, D.; Gillinov, A.M.; et al. First-in-human implantations of the navigate bioprosthesis in a severely dilated tricuspid annulus and in a failed tricuspid annuloplasty ring. Circ. Cardiovasc. Interv. 2017, 10, e005840. [Google Scholar] [CrossRef] [PubMed]
- Boudjemline, Y.; Agnoletti, G.; Bonnet, D.; Behr, L.; Borenstein, N.; Sidi, D.; Bonhoeffer, P. Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J. Am. Coll. Cardiol. 2005, 46, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zong, G.J.; Wang, H.R.; Jiang, H.B.; Wang, H.; Wu, H.; Zhao, X.X.; Qin, Y.W. An integrated pericardial valved stent special for percutaneous tricuspid implantation: An animal feasibility study. J. Surg. Res. 2010, 160, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Asmarats, L.; Dagenais, F.; Bédard, E.; Pasian, S.; Hahn, R.T.; Navia, J.L.; Rodés-Cabau, J. Transcatheter tricuspid valve replacement for treating severe tricuspid regurgitation: Initial experience with the navigate bioprosthesis. Can. J. Cardiol. 2018, 34, e5-1370. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Veronesi, F.; Maddalozzo, A.; Dequal, D.; Frajhof, L.; Rabischoffsky, A.; Iliceto, S.; Badano, L.P. 3D printing of normal and pathologic tricuspid valves from transthoracic 3D echocardiography data sets. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 802–808. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, M.; Mao, Y.; Ma, Y.; Liu, Y.; Yang, J. Transcatheter Double Valve Replacement to Treat Aortic Stenosis and Severe Tricuspid Regurgitation with 3D Printing Guidance after Mechanical Mitral Valve Replacement. J. Cardiovasc. Dev. Dis. 2022, 9, 296. https://doi.org/10.3390/jcdd9090296
Zhai M, Mao Y, Ma Y, Liu Y, Yang J. Transcatheter Double Valve Replacement to Treat Aortic Stenosis and Severe Tricuspid Regurgitation with 3D Printing Guidance after Mechanical Mitral Valve Replacement. Journal of Cardiovascular Development and Disease. 2022; 9(9):296. https://doi.org/10.3390/jcdd9090296
Chicago/Turabian StyleZhai, Mengen, Yu Mao, Yanyan Ma, Yang Liu, and Jian Yang. 2022. "Transcatheter Double Valve Replacement to Treat Aortic Stenosis and Severe Tricuspid Regurgitation with 3D Printing Guidance after Mechanical Mitral Valve Replacement" Journal of Cardiovascular Development and Disease 9, no. 9: 296. https://doi.org/10.3390/jcdd9090296
APA StyleZhai, M., Mao, Y., Ma, Y., Liu, Y., & Yang, J. (2022). Transcatheter Double Valve Replacement to Treat Aortic Stenosis and Severe Tricuspid Regurgitation with 3D Printing Guidance after Mechanical Mitral Valve Replacement. Journal of Cardiovascular Development and Disease, 9(9), 296. https://doi.org/10.3390/jcdd9090296



